Abstract
The effect of drought on plant isoprene emission varies tremendously across species and environments. It was recently shown that an increased ratio of photosynthetic electron transport rate (ETR) to net carbon assimilation rate (NAR) consistently supported increased emission under drought. In this commentary, we highlight some of the physiological aspects of drought tolerance that are central to the observed variability. We briefly discuss some of the issues that must be addressed in order to refine our understanding of plant isoprene emission response to drought and increasing global temperature.
Increasing and irregular droughts induced by global warming will affect plant responses and could potentially define land plant evolution during the Anthropocene.Citation1 Terrestrial plant ecosystems are a major sink for CO2 and constitute a central component of global carbon cycle. Net primary production (NPP; ∼50 PgC/yr) is sensitive to changes in global temperatures and could decline in future due to unprecedented warm temperatures and extended periods of drought.Citation2-3 The emission of isoprene (∼0.5 PgC/yr) by forests releases carbon to the atmosphere in a form that influences oxidation reactions in the troposphere.Citation4 Emissions increase in plants under oxidative stress caused by high light, low CO2, drought and heat; such observations have led to various hypotheses concerning the biological function and evolutionary role of isoprene emission in ecosystems.Citation5-8 While photosynthesis clearly declines under abiotic stresses (particularly drought), isoprene emission responses are far less predictable.Citation9
Isoprene biosynthesis and emission by plants depends on carbon, energy and reducing power supplied by photosynthesis.Citation10-12 One of the hypotheses that attempts to explain variation in isoprene emission states that secondary metabolism and volatile emissions increase under abiotic stresses because the net carbon assimilation declines but the supply of reducing equivalents remains high.Citation13-15 We have successfully tested the hypothesis that photosynthetic carbon reduction competes with non-photosynthetic sinks for reducing power under stress-free conditions. A claim is made that residual reducing power unused by carbon assimilation drives increased isoprene emission under various abiotic stressesCitation16,17 ().
Figure 1. A notional view of the relationship between net assimilation rates (green line in all images) and electron transport rate, photorespiration rate and isoprene emission rate (top to bottom) as eucalypts are to exposed diminishing water supply.
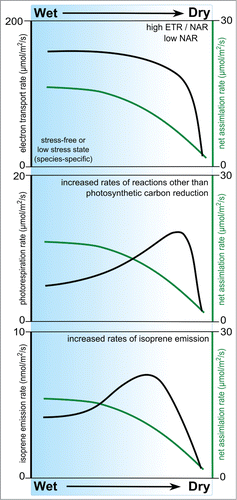
Exploiting the contrasting drought tolerance of 2 Eucalyptus species, we providedCitation16 an exhaustive experimental data-set showing that an increased ETR-to-NAR ratio sustained increased isoprene emission rates under drought, which appears to follow a species-specific threshold for drought tolerance (). When placed in an ecological context, aggregate isoprene emission depends on the mix of species in plant communities, each emitting at a characteristic range of rates.Citation13 While vegetation maps define species distributions reasonably well, there is still very scarce information about the physiological determinants of emission rates. Improving global emission algorithms requires a broad consensus of species’ responses to drought, which so far has proven a challenge. In this addendum to the paper by Dani et al.,Citation16 we highlight issues that are yet to be addressed.
We have monitored photosynthesis (at leaf-level) and isoprene emission (both at leaf and whole plant-level) under glasshouse conditions in many species of genetically diverse eucalypts selected from different regions across Australia. While any relationship between emission rates obtained through canopy sampling (of young seedlings) and leaf-level photosynthesis gas exchange measurements has epistemological problems, and is at best only indicative, we wish to highlight some pertinent observations that we believe are relevant to future studies involving isoprene emission and abiotic stress.
The emission rates observed in all of our experiments involving eucalypts, including both multiple species and the pairwise comparison cited above,Citation16 were low (0 to 10 nmol/m2/s) compared with those from more widely studied poplars and oaks (20 to 100 nmol/m2/s).Citation6 Many native Australian plants have abundant Rubisco and eucalypts in particular are notable for their extremely high photosynthetic capacities among trees, operating as they generally do in high-light environments.Citation18 While a large carboxylation capacity could make full use of high energy status of the leaves of eucalypts, it could also leave limited reducing power for the MEP pathway under stress-free conditions. These characteristics, along with perennial monoterpene storing, may explain why evergreen eucalypts emit less isoprene than deciduous poplars or oaks, which go through annual cycles of senescence and periodic drought. A strong positive correlation between photosynthetic electron transport and isoprene emission is knownCitation19 and based on published recent empirical dataCitation16,17 we project that plants with low photosynthetic rates should be high isoprene emitter if they posses high electron transport rates relative to carbon assimilation rates.
CO2 compensation point (Γ*) is an important indicator of photorespiratory capacity of a species and it takes an appreciable range of values even among C3 plants (30 to 70 ppm).20 Drought exacerbates oxidative stress by decreasing stomatal conductance and as a result increasing leaf temperature and CO2 compensation point. Γ* also conforms to a diurnal cycle (40 ppm to 160 ppm), with a daily maximum coinciding with midday depression in carboxylation efficiency.Citation21 The well-known diurnal peak of isoprene emission just after midday is consistent with the mechanistic link between carboxylation efficiency and emission rates. We also observed a large range in Γ* values (40 ppm to 80 ppm) across many species of the genus Eucalyptus under stress-free conditions. This diversity in photorespiration among eucalypts has important implications for the way drought-induced photorespiration affects isoprene emission in trees. Photorespiration directly competes with the MEP pathway for reducing power and the competition becomes acute under drought stress as photorespiration rate increases and carbon supply for the MEP pathway decreases.Citation16 The large range in Γ* could be due to diversity in leaf structural traits (mesic vs. xeric) that in turn reflect rainfall and nutrient availability in their respective habitats. Many C3 plants are known to recapture photorespiratory CO2 and the energy requirements of such processes are completely unknown.
It is recently reported that drought-induced increase in concentration of soluble sugars in the cytoplasm has no bearing on isoprene emission.Citation22 These findings rule out import of cytosolic carbon (sugars) into chloroplasts under severe drought. It further reinforces the claim that isoprene emission behavior is most strongly influenced by photorespiration and other pathways occurring within chloroplasts, which therefore compete directly with the co-located MEP pathway for both photosynthetic carbon and reducing power.
The interactive effects of CO2, heat and drought on emission are quite complex.Citation16 Even closely related eucalypts exhibit significant differences in their photorespiratory sink strengths for reducing power, indicating that differences between more distantly related taxa could be even more striking and could make the assessment of their emission responses to drought far more difficult using existing models and mechanistic assumptions. Elevated CO2 levels for long periods are suggested to cause a significant decrease in maximum carboxylation capacity in many C3 plants (due to less RubiscoCitation23), although the response of electron transport rate is less clearly defined.Citation24 Acclimation of plants to elevated CO2 and its impact on carbon gain, plant water use efficiency uncertain.Citation25 Changes in carboxylation and RUBP regeneration (contents of chlorophyll and Rubisco)Citation26 cannot be generalized across many taxa that characterize important isoprenoid-emitting ecosystems. Our results suggest that obtaining a reliable global atlas of tolerances for abiotic stresses among major plant biomes is crucial to understanding the future of plant isoprene emission in a high-CO2 world.
Acknowledgments
We thank Macquarie Engineering and Technical Services, Dr Christopher McRae, Mr Md Masood and the students of BIOL313 (Plants: Cells to Ecosystems) for their support; Dr Vincent Maire and Dr Roger Hiller for discussions; the Australian Commonwealth for IPRS scholarship to KGSD and Macquarie University Higher Degrees Research grant 40310819.
References
- Hartmann H. Will a 385 million year-struggle for light become a struggle for water and for carbon?–How trees may cope with more frequent climate change-type drought events. Global Change Biol 2011; 17:642-55; http://dx.doi.org/10.1111/j.1365-2486.2010.02248.x
- Melillo JM, McGuire AD, Kicklighter DW, Moore B, Vorosmarty CJ, Schloss AL. Global climate change and terrestrial net primary production. Nature 1993; 363:234-40; http://dx.doi.org/10.1038/363234a0
- Zhao M, Running SW. Drought-induced reduction in global terrestrial net primary production from 2000 through 2009. Science 2010; 329:940-3; PMID:20724633; http://dx.doi.org/10.1126/science.1192666
- Guenther A, Karl T, Harley P, Wiedinmyer C, Palmer PI, Geron C. Estimates of global terrestrial isoprene emissions using MEGAN (Model of Emissions of Gases and Aerosols from Nature). Atmos Chem Phys 2006; 6:3181-210; http://dx.doi.org/10.5194/acp-6-3181-2006
- Vickers CE, Gershenzon J, Lerdau MT, Loreto F. A unified mechanism of action for volatile isoprenoids in plant abiotic stress. Nature Chemical Biol. 2009; 5:283-91; PMID:19377454; http://dx.doi.org/10.1038/nchembio.158
- Dani KGS, Jamie IM, Prentice IC, Atwell BJ. Evolution of isoprene emission capacity in plants. Trends Plant Sci. 2014; 19:439-46; PMID:24582468; http://dx.doi.org/10.1016/j.tplants.2014.01.009
- Monson RK, Jones RT, Rosenstiel TN, Schnitzler JP. Why only some plants emit isoprene. Plant Cell Environ. 2013; 36:503-16; PMID:22998549; http://dx.doi.org/10.1111/pce.12015
- Sharkey TD, Monson RK. The future of isoprene emission from leaves, canopies and landscapes. Plant Cell Environ. 2014; 37:1727-40; PMID:24471530; http://dx.doi.org/10.1111/pce.12289
- Loreto F, Schnitzler J-P. Abiotic stresses and induced BVOCs. Trends Plant Sci. 2010; 15:154-66; PMID:20133178; http://dx.doi.org/10.1016/j.tplants.2009.12.006
- Delwiche CF, Sharkey TD. Rapid appearance of 13C in biogenic isoprene when 13CO2 is fed to intact leaves. Plant Cell Environ. 1993; 16:587-91; http://dx.doi.org/10.1111/j.1365-3040.1993.tb00907.x
- Loreto F, Sharkey TD. On the relationship between isoprene emission and photosynthetic metabolites under different environmental conditions. Planta, 1993; 189:420-4; PMID:24178500; http://dx.doi.org/10.1007/BF00194440
- Seemann M, Tse Sum Bui B, Wolff M, Miginiac-Maslow M, Rohmer M. Isoprenoid biosynthesis in plant chloroplasts via the MEP pathway: direct thylakoid/ferredoxin-dependent photoreduction of GcpE/IspG. FEBS Lett. 2006; 580:1547-52; PMID:16480720; http://dx.doi.org/10.1016/j.febslet.2006.01.082
- Niinemets Ü, Tenhunen JD, Harley PC, Steinbrecher R. A model of isoprene emission based on energetic requirements for isoprene synthesis and leaf photosynthetic properties for Liquidambar and Quercus. Plant Cell Environ. 1999; 22:1319-35; http://doi/10.1046/j.1365-3040.1999.00505.x/abstract
- Harrison SP, Morfopoulos C, Dani KGS, Prentice IC, Arneth A, Atwell BJ, Barkley, P, Leishman M, Loreto F, Medlyn B, et al, Wright IJ. Volatile isoprenoid emission from plastids to planet. New Phytol. 2013; 197:49-57; PMID:23145556; http://dx.doi.org/10.1111/nph.12021/full
- Selmar D, Kleinwächter M. Stress enhances the synthesis of secondary plant products: the impact of the stress-related over-reduction on the accumulation of natural products. Plant Cell Physiol 2013; 54:817-826; PMID:23612932; http://dx.doi.org/10.1093/pcp/pct054
- Dani KGS, Jamie IM, Prentice IC, Atwell BJ. Increased ratio of electron transport to net assimilation rate supports elevated isoprenoid emission rate in eucalypts under drought. Plant Physiol 2014; 166:1-14; http://dx.doi.org/10.1104/pp.114.900492
- Morfopoulos C, Sperlich D, Peñuelas J, Filella I, Llusià J, Medlyn BE, Niinemets U, Possell M, Sun Z, Prentice IC. A model of plant isoprene emission based on available reducing power captures responses to atmospheric CO2. New Phytol 2014; 203:125-139; PMID:24661143; http://dx.doi.org/10.1111/nph.12770/full
- Niinemets Ü, Hauff K, Bertin N, Tenhunen JD, Steinbrecher R, Seufert G. Monoteprene emissions in relation to foliar photosynthetic and structural variables in Mediterranean evergreen Quercus species. New Phytologist 2002; 153:243-56; http://dx.doi.org/10.1046/j.0028-646X.2001.00323.x/full
- Warren CR, Adams MA, Chen Z. Is photosynthesis related to concentrations of nitrogen and Rubisco in leaves of Australian native plants? Aust J Plant Physiol 2000; 27:407-16; http://dx.doi.org/10.1071/PP98162
- Black CC Jr. Photosynthetic carbon fixation in relation to net CO2 uptake. Annu Rev Plant Physiol 1973; 24:253-86; http://dx.doi.org/10.1146/annurev.pp.24.060173.001345
- Tenhunen JD, Lange OL, Gebel J, Beyschlag W, Weber JA. Changes in photosynthetic capacity, carboxylation efficiency, and CO2 compensation point associated with midday stomatal closure and midday depression of net CO2 exchange of leaves of Quercus suber. Planta 1984; 162:193-203; PMID:24253090
- Rodríguez-Calcerrada J, Buatois B, Chiche E, Shahin O, Staudt M. Leaf isoprene emission declines in Quercus pubescens seedlings experiencing drought – Any implication of soluble sugars and mitochondrial respiration? Environ Expt Bot 2013; 85:36-42; http://dx.doi.org/10.1016/j.envexpbot.2012.08.001; PMID:Can't
- Norby RJ, Wullschleger SD, Gunderson CA, Johnson DW, Ceulemans R. Tree responses to rising CO2 in field experiments: implications for the future forest. Plant Cell Environ 1999; 22:683-714; http://dx.doi.org/10.1046/j.1365-3040.1999.00391.x
- Medlyn BE, Badeck F-W, De Pury DGG, Barton CVM, Broadmeadow M, Ceulemans R, DeAngelis P, Forstreuter M, Jach ME, Kellomachi S et al. Effects of elevated [CO2] on photosynthesis in European forest species: a meta-analysis of model parameters. Plant Cell Environ 1999; 22:1475-1495; http://dx.doi.org/10.1046/j.1365-3040.1999.00523.x
- Haxeltine A, Prentice IC. A general model for the light-use efficiency of primary production. Funct. Ecol 1996; 10:551-61; http://www.jstor.org/stable/2390165; http://dx.doi.org/10.2307/2390165
- Maire V, Martre P, Kattge J, Gastal F, Esser G, Fontaine S, Soussana J-F. The coordination of leaf photosynthesis links C and N fluxes in C3 plant species. PLoS One 2012; 7:e38345; http://dx.doi.org/10.1371/journal.pone.0038345
