Abstract
Eukaryotic GCN2 (general control nonderepressible 2) is a serine/threonine protein kinase that plays an essential role in modulating amino acid metabolism in response to nutrient deprivation. A wide spectrum of GCN2 functions in yeast and mammals has been characterized that spans from responses to amino acid deficiency, development, differentiation and proper functions of mammalian organs to organism's life span, tumor cell survival and immune responses. Here we demonstrate that Arabidopsis thaliana GCN2 (AtGCN2) plays crucial roles in plant growth and development. We present evidence that AtGCN2 negatively regulates seed germination under diverse environmental conditions. Our genetic data supported the notion that AtGCN2 is required for leaf morphology and normal cellular physiology by controlling chlorophyll contents. Our gene expression analyses revealed that AtGCN2 negatively regulates several transcription factor genes that play important roles in plant gibberellic acid-related crosstalk. We concluded that AtGCN2 plays pivotal roles in various cellular processes essential for normal growth and development, hence expanding the functions of this general regulator beyond being merely a stress player.
Introduction
Plant growth is governed by intricate interactions between fundamental elements such as light and chemical energy, water, carbon as well as mineral nutrients, and dynamic environmental conditions. In response to both biotic and abiotic stresses, a plant makes elastic and plastic adjustments by implying signaling pathways that convert environmental cues into discrete intracellular events.Citation1-4 While transcriptional and post-transcriptional gene regulatory mechanisms actively participate in this regulation, translational control of gene expression that globally alters protein synthesis rate has emerged as a crucial and very effective mechanism to rapidly mediate signaling in cellular stress pathways.Citation5 Intriguingly, mechanism of amino acids sensing is conserved across evolutionarily diverged species. The protein kinase GCN2 (general control non-derepressible 2) is highly conserved in eukaryotes at both the structural and functional levels. Extensive research on yeast Gcn2p and human GCN2 signaling in response to amino acid deprivation allowed understanding the molecular underpinnings of GCN2-mediated translational control.Citation6 GCN2 is a multidomain protein that contains a domain with homology to HisRS (histidyl-tRNA synthetase). Uncharged tRNAs bind to HisRS, which induces a conformational change in GCN2 resulting in kinase activation through homodimerization and autophosphorylation. This tRNAs-mediated stimulation of GCN2 directs the phosphorylation of its substrate, α subunit of eukaryotic initiation factor (eIF2α).Citation5,7-9 This series of events leads to reduced global protein synthesis but selectively increases the translation of specific mRNAs such as yeast Gcn4 and mammalian ATF4 (Activating Transcription Factor 4) that contain specific regulatory elements in their 5′ UTRs (untranslated regions). This phenomenon of selective translational up-regulation of key transcriptional activators is known as translational derepression.Citation5,7-9 Translational derepression in response to various abiotic stresses is also an emerging field of research in plant molecular biology.Citation10-12
GCN2 has been demonstrated to be involved in a wide-spectrum of biological processes, some of which are conserved from yeast to human. In mammals, it has been implicated in aging, developmental-related diseases such as cancer and Alzheimer's disease, long-term memory formation and the immune system.Citation6,7,13-15 Arabidopsis thaliana (Arabidopsis) genome also contains an ortholog of GCN2 (AtGCN2) that shares 48% sequence homology with yeast Gcn2p kinase.Citation16-18 Intriguingly, despite rather limited homology levels, AtGCN2 was demonstrated to functionally complement yeast gcn2 mutant under stress conditions.Citation19 AtGCN2 is widely expressed in a range of Arabidopsis tissues/organs including roots, leaves, stems, buds, flowers, siliques and seedlings.Citation19 AtGCN2 contains functional kinase and HisRS domains, displays direct physical interaction with uncharged tRNA and phosphorylates Arabidopsis eIF2α in response to various stresses.Citation16,17 In addition, a heat shock transcription factor TBF1 (HsfB1), functionally reminiscent of mammalian ATF4, was recently discovered in Arabidopsis that harbors 2 uORFs (upstream Open Reading Frames) in its 5′ UTR.Citation20 Moreover, it was also shown that the translational derepression of TBF1 is dependent on eIF2α phosphorylation.Citation20 While several functions of AtGCN2 under stress conditions have been revealed, it remains to be understood how this key regulatory protein is implicated in plant-specific processes such as seed germination, control of leaf shape and maintaining appropriate amount of foliar chlorophyll. We also set out to identify whether AtGCN2 can influence plant hormone signaling pathways, with a specific focus on gibberellic acid. Our findings provide a unique, plant-specific perspective on some of the previously unknown GCN2 functions. Our data indicate that AtGCN2 is an essential molecule for plant growth and development and suggest a possible involvement of AtGCN2 in the gibberellic acid signaling pathway.
Materials and Methods
Arabidopsis lines and growth conditions
Wild-type and atgcn2 Arabidopsis thaliana plants used in this study are from the Landsberg erecta (Ler) ecotype. The Ler seeds were obtained from the Arabidopsis Biological Resource Center (ABRC; Ohio State University, OH, USA). The atgcn2 Genetrap insertion line GT8359 was obtained from Cold Spring Harbor Laboratory, New York (http://genetrap.cshl.org). The Genetrap lines carry a transposable element insertion (Ds) in a Ler background. Arabidopsis seeds were incubated for 72 h at 4°C (unless otherwise stated for germination assays) and grown on MetroMix 360 soil under 12 h light/12 h dark cycle at 65% humidity for 4 weeks. Seed viability was tested by germinating seeds in sterile water and was found to be between 97 and 100%.
Germination assays
For germination experiments, seeds were sterilized by soaking for 2 min in 70% ethanol, followed by 2 min in 100% ethanol and plated on half-strength MS plates as described previously.Citation21 The MS salts were purchased from Phytotechnology Laboratories, KS, USA. Subsequently, seeds were subjected to extended (5 days) cold treatment at 4°C in the dark or transferred directly into growth chamber at 22°C with no additional imbibition time. Next, the seeds were exposed to constant light illumination for a period of 200 hours (hrs). Numbers of germinated seedlings were recorded initially every 2 hrs, and the reading frequency was gradually reduced to every 4–10 hrs at later stages of the experiment when the differences were less dramatic. A total of 30 and 32 time points were included for cold stratified and non-stratified seeds, respectively. To establish the quantitative difference between the cold treatment effects on atgcn2 germination, differences in percentage germination between Ler and atgcn2 were calculated for both stratification and no stratification conditions.
For the pharmacological GA sensitivity experiments, seeds were sterilized and placed on MS plates containing half-strength MS media supplemented with various concentrations of GA4+7 (0 μM, 0.5 μM, and 5 μM), each combined with 0 or 100 μM Paclobutrazol (Pac). GA4+7 and Pac were obtained from Phytotechnology Laboratories, KS, USA.
Leaf shape measurements
Leaf measurement was conducted using soil-grown 4-week-old Ler and atgcn2 mutants, with 6 individual plants per genotype. The leaf number 1 through number 7 was detached and the leaf blade length and width measurement was taken using a ruler. The length to width ratio for each leaf number was calculated. Significance was evaluated using one-way ANOVA.
Chlorophyll measurements
Total chlorophyll was extracted and quantified as described previouslyCitation22 using soil-grown 4-week-old Arabidopsis rosettes, with 5 individual plants per genotype. Chlorophyll concentrations were calculated using the equations: total chlorophyll (mg/L) = 17.90A647 + 8.08A664.5. Chlorophyll a and b were determined spectrophotometrically at wavelengths of 647 and 664.5 nm as described previously.Citation22,23
Gene expression analyses
For gene expression analyses, 3 detached Arabidopsis leaves harvested from individual 4-week-old soil-grown plants (200 mg of tissue per sample) were flash frozen in liquid nitrogen. RNA was extracted from each sample using TRIzol reagent (Invitrogen) and treated with DNase I (Ambion). cDNA was synthesized using a SuperScript III first-strand RT-PCR kit (Invitrogen). Transcript abundance was quantified using the GoTaq qPCR Master Mix (Promega) in a RealPlex S MasterCycler (Eppendorf). The primers used in the qPCR reactions were: UBQ5-F 5′-GACGCTTCATCTCGTCC-3′, UBQ5-R 5′-GTAAACGTAGGTGAGTCC-3′, GNC-F 5′-CTCCATCTTTCTCAACCCCTC-3′, GNC-R 5′-TCGTTAGTTTCAGTCTTGTCTCC-3′, CGA1- F 5′-CTCGCTCAAGTGGATATCTTCG-3′, CGA1-R 5′-GCTTAGGTTTGAGGATTGGTCG-3′, PRE1-F 5′-GATTGACCTCGTATCTAAGCTCC-3′, PRE1-R 5′-TCGCTCAGATTGTCAACTTCTC-3′, PRE5-F 5′-AGGTGTCAGCATCAAAGGTAC-3′, PRE5-R 5′-TCTAATCACGGCAGCTTCAG-3′, XER-F 5′-GCAAGAAACAGGCAGACAATG-3′, XER-R 5′-CAATGGGCAAGTGATGTTCC-3′.
Results and Discussion
Seed germination is delayed in atgcn2 mutant plants
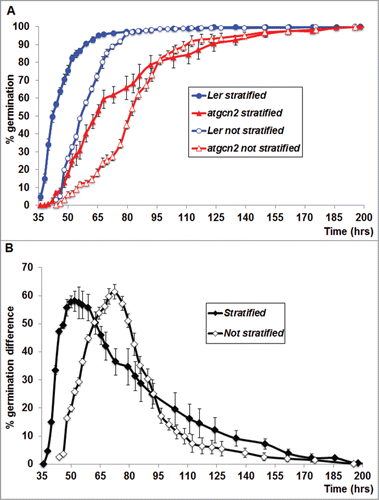
The activity of AtGCN2 was shown to result in a strong reduction of global protein synthesis under diverse biotic and abiotic stress conditions.Citation16,17 To gain further insights into possible functions of AtGCN2 in normal plant physiology, we examined the effect of AtGCN2 on seed germination. We compared the percentage of germinated seeds with and without stratification between wild type plants (accession Landsberg erecta, Ler) and atgcn2 mutant seeds over the course of time. We observed that Ler seeds that were stratified for 5 days at 4°C in the dark began to germinate at around 35 hrs after transferring them to growth room (22°C, constant illumination), and nearly all of the Ler seeds were germinated by 65 hrs (based on presence of initial radicle protrusion). In contrast, the atgcn2 mutant seeds displayed a significantly delayed germination pattern. Germination of atgcn2 seeds started at approximately 42 hrs after transfer to growth room and only around 50% of seeds were germinated by 65 hrs. However, the atgcn2 mutant seeds continued to germinate and finally over 90% of seeds were germinated by 155 hrs. This finding demonstrates that disruption of AtGCN2 delays seed germination but does not completely inhibit it (). To gain further insights into the role of AtGCN2 in the control of seed germination, we analyzed seed germination patterns of Ler and the atgcn2 mutant under constant light but without stratification. It is known that light-grown non-stratified Ler seed display a significant delay in germination compared to stratified seed.Citation24 In our experiment, we observed a consistent delay in germination for both Ler and atgcn2 mutant compared to stratified seeds. Germination of Ler seeds started at 44 hrs and was completed by 80 hrs (). In contrast, the atgcn2 mutant seeds exhibited significantly slower germination pattern. Germination of atgcn2 seeds started at 46 hrs, continued at slow pace and completed at 155 hrs. We next calculated the difference in percentage of germinated seedlings between Ler and atgcn2 grown with and without stratification, and discovered that the difference, although slightly bigger without the prior cold treatment (61% in non-stratified seedlings vs. 58% in stratified seedlings), actually declines earlier than in stratified plants, and is statistically significant starting at 95 hrs (). These data demonstrated that AtGCN2 may play a role in seed germination of Arabidopsis and this process might be regulated by the duration of cold exposure. It is well established that seed germination is tightly controlled by environmental conditions including light, temperature and osmotic stress.Citation24 In addition, it was also demonstrated that a phytohormone gibberellic acid (GA) can break seed dormancy and partially compensate for cold or light treatments.Citation24 GA sensitivity was shown to be directly controlled by temperature. Cold treatments can stimulate biosynthesis of specific gibberellins in Arabidopsis seeds as well as increase their sensitivity to exogenously applied GAs.Citation24-26 Thus, it's likely that AtGCN2 might participate in modulation of the GA signaling in Arabidopsis.
Figure 1. (A) Germination of Ler and atgcn2 seeds was tested with no prior stratification (open circles and triangles) or following an extended (5 days) stratification treatment (filled circles and triangles). Data are means (±SE) of 5 replicates (each with 100 seeds for each line). (B) Differences in percentage germination between Ler and atgcn2 were calculated for each stratification condition. Error bars represent standard error.
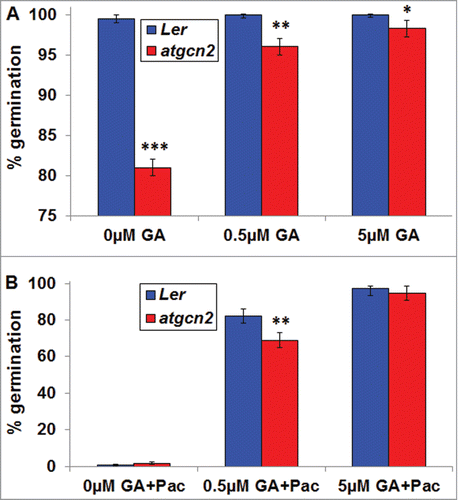
To test the possible involvement of GA in atgcn2 germination, we grew Ler and atgcn2 seeds in presence of various concentrations of biologically active gibberellins GA4+7 (). We subjected the seeds of both genotypes to 5 days stratification at 4°C and subsequently followed the germination in the growth room at 22°C over the period of 96 hrs, at which time point germination of Ler seeds was completed. In the absence of exogenous GA4+7 we observed a significant delay in the germination of the atgcn2 seeds, consistent with our previous observations. Presence of increasing concentrations of GA4+7 dramatically improved atgcn2 germination rates but did not fully complement the mutant deficiency. This result indicates that the germination delay in the atgcn2 mutant might be caused by a combination of defective GA biosynthesis and downstream signal transduction. To gain a deeper insight into the nature of this deficiency in the atgcn2 seeds, we next conducted a germination assay on media supplemented with various concentrations of gibberellins GA4+7 and 100 µM Paclobutrazol (Pac)Citation24 (). Pac inhibits the ent-kaurene oxidation reactions of GA biosynthesis.Citation27 Previous work involving Pac showed that GAs are synthesized de novo during germination in Arabidopsis seeds.Citation24 While mutants with enhanced GA signaling or decreased abscisic acid content are more resistant to inhibition of germination by Pac, opposite is expected from mutants displaying a decrease in GA signaling or elevated ABA levels in the seeds.Citation28 In Ler seeds, presence of Pac blocks the endogenous GA production, resulting in complete inhibition of germination.Citation24 Exogenously supplied GA4+7 can rescue this phenotype in a dose-dependent manner as demonstrated by scoring the germination at 148 hrs post-transfer to growth room (). The atgcn2 mutant grown in the presence of Pac also exhibited a nearly-complete arrest of seed germination, indicating that the GA signaling is not elevated and abscisic acid content in the seeds is not expected to be different from Ler plants. Simultaneous exposure to GA4+7 and Pac caused a marked increase in the atgcn2 germination and a full complementation of the previously observed germination defect was observed at the highest GA4+7 dose tested (5 µM) ().
Figure 2. (A) Germination of Ler and atgcn2 seeds on plates containing increasing concentrations of GA4+7 was assayed at 96 hrs after transfer to growth room. (B) The germination assay was conducted in the same manner as in (A) but the media was supplemented with 100 µM of GA biosynthesis inhibitor Paclobutrazol (Pac) and germination was assayed at 148 hrs after transfer to growth room. Error bars represent standard error. Student's t-test was conducted to determine statistical relevance of observed differences. * P < 0.05, ** P < 0.01, *** P < 0.001. The experiment was conducted in 3 biological replications.
atgcn2 mutant exhibits altered leaf shape
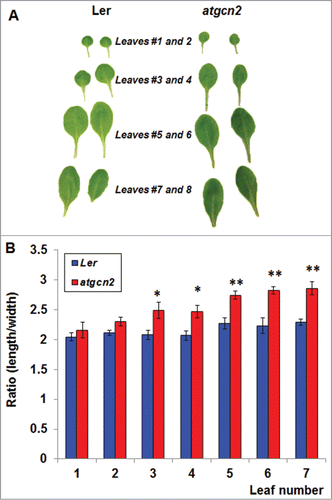
Since GA influences a number of processes related to plant growth and development, we visually inspected the atgcn2 mutants for any obvious morphological differences. Analysis of an array of representative true leaves sampled from Ler and atgcn2 plants revealed a remarkable difference in leaf blade morphology (). To quantify this difference, we examined the leaf length and width of 4-week-old Ler and atgcn2 mutant plants. We followed standard leaf numbering guidelines as described previously.Citation29 Leaves number 1 and 2 of atgcn2 were indistinguishable from Ler. However, the subsequently emerging leaves appeared to be more elongated. In order to determine a statistical difference between Ler and atgcn2 leaves, if any, we systematically measured length/width ratio of leaves from 1 through 7, which is an established method for quantification of altered leaf shape in plants.Citation30 While we did not find any difference between leaf 1 or leaf 2 of Ler and atgcn2, statistically significant different length/width ratios were recorded on leaves from 3 to 7 (). Based on these findings, we concluded that AtGCN2 plays a vital role in regulating Arabidopsis leaf morphology. In accordance with our data, it is known that gibberellins can cause alteration of leaf size and shape in many plants, including pea, corn, tomato and beans, and it was previously demonstrated that reduced levels of GA1 or gibberellic acid-insensitive (gai) mutation in Arabidopsis results in smaller and narrower leaflets.Citation30-33
Figure 3. (A) Representative leaves # 1-8 from 4-week-old Ler and atgcn2 plants were sampled and photographed. (B) Leaves 1-7 were sampled from 6 individual Ler and atgcn2 plants and their length and width were measured. Length-to-width ratios were calculated for each leaf and plotted. Error bars represent standard error. One-way ANOVA was conducted to determine statistical relevance of observed differences. * P < 0.05, ** P < 0.01.
Plants lacking functional AtGCN2 display increased chlorophyll contents
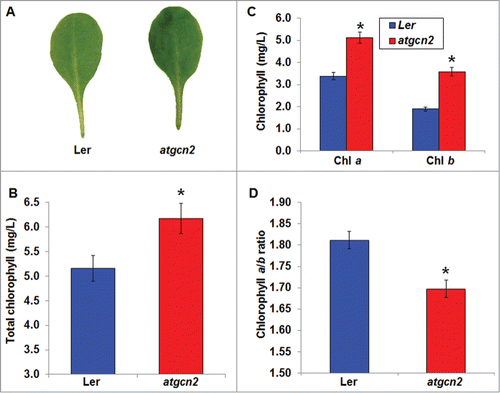
In yeast and mammals, GCN2 is able to sense and respond to amino acid starvation and fluctuations in dietary nutrients, and is implicated in playing a pivotal role in growth and development.Citation6,14 Another key question concerns a possible role of AtGCN2 in plant nutrition-related physiological processes. In plants, the proportion and amount of chlorophyll in leaves are closely related to plant nutrient status.Citation34 In addition, leaf color can be used as an indicator of chlorophyll contents in plants. Thus, leaf color and chlorophyll contents can be used as an index to diagnose nutrient status in plants. To further enhance our understanding of the roles of AtGCN2 in plant physiology, we investigated the leaf pigmentation of Ler and atgcn2 mutant plants. We visually examined the leaf color of 4-week-old plants and noted that leaves of atgcn2 mutant plants display darker pigmentation compared to Ler (). To corroborate these data and detect the quantitative differences, we measured the total chlorophyll in 4-week-old Ler and atgcn2 mutants under normal growth conditions. We demonstrated that Ler plants harbor an average of 5.3 mg/L total chlorophyll. In contrast, plants lacking functional AtGCN2 contain a significantly higher chlorophyll contents, averaging at 6.2 mg/L (). Given the difference in total chlorophyll accumulation, we next conducted measurements of chlorophylls a and b. Because the chlorophyll a/b ratio correlates with functional maturity of the photosynthetic apparatus and with the photosynthetic activity,Citation35 the observed increase of the ratio indicates that photosynthesis may be enhanced in the atgcn2 plants. Taken together, we concluded that AtGCN2 negatively regulates leaf chlorophyll accumulation and is required for the normal Arabidopsis growth and development. Our result is in agreement with the previous findings showing that a gibberellin-insensitive gai mutant has higher chlorophyll contentsCitation31,36 and exogenous application of GA leads to decreased chlorophyll contents and chlorosis.Citation37 This notion strengthens the hypothesis that AtGCN2 is implicated in the GA signaling pathway.
Figure 4. (A) Representative leaves from 4-week-old Ler and atgcn2 plants were sampled and photographed to document visual difference in pigmentation intensity. (B) Total chlorophyll amounts were quantified in Ler and atgcn2 plants. (C) Amounts of chlorophylls a and b were quantified in Ler and atgcn2 plants. (D) Chlorophyll a to b ratios were calculated in Ler and atgcn2 plants. Four technical replicates were averaged. Error bars represent standard error. Statistical analysis was performed using Student's t-test. * P < 0.05. Experiment was repeated 4 times with similar results.
Gibberellin-related genes are differentially expressed in atgcn2 mutant
It has been already reported that GA-mediated signaling transduction pathways plays essential roles in determining plant organ size, chlorophyll levels and flower development.Citation38 To understand the molecular mechanisms underlying AtGCN2 involvement in seed germination, leaf morphology and chlorophyll contents, we performed a quantitative reverse transcription polymerase chain reaction (q-RT-PCR) on Ler and atgcn2 mutant plants. We selected 2 recently characterized paralogs of GATA transcription factors, GNC (GATA, Nitrate-inducible, Carbon-metabolism-involved) and CGA1/GNL (Cytokinin-responsive GATA 1/GNC-like). GNC and CGA1 were previously shown to be induced by several environmental stimuli such as light, circadian clock, nitrogen sources as well as cytokinin, another phytohormone that exerts antagonistic effect on the GA-mediating signaling.Citation39,40 In addition, differential expression levels of both GNC and CGA1 were also demonstrated to be linked with chlorophyll contents, germination and flowering time in Arabidopsis.Citation40 In our qRT-PCR experiments, we showed that transcript levels of both GNC and CGA1 are markedly up-regulated in the atgcn2 mutant compared to Ler plants (). Intriguingly, it has been demonstrated that plants exhibiting reduced levels of GNC and CGA display significantly reduced chlorophyll content compared to wild type plants, thus substantiating our findings. To provide additional support for the AtGCN2 role in GA signaling, we also assayed transcript accumulation of 3 additional GA marker genes, PRE1, PRE5 and XERICO (). PRE1 (Paclobutrazol Resistance 1) and PRE5 code for bHLH transcription factors that mediate brassinosteroid regulation of cell elongation and are repressed by DELLA proteins,Citation38 negative regulators of gibberellin signaling that act immediately downstream of the GA receptors.Citation41,42 Thus, PRE1 and PRE5 are hypothesized to link GAs with growth in certain circumstances.Citation41 XERICO (XER) encodes a small RING finger protein whose expression is induced by salt, osmotic stress and DELLA proteins and repressed by GA.Citation43,44 In our qRT-PCR experiments, XERICO transcript levels were markedly increased in the atgcn2 mutant plants, consistent with the hypothesis that this mutant displays phenotypes reminiscent of diminished GA signaling. Conversely, levels of PRE1 and PRE5 were decreased in the atgcn2 mutant plants, which is in agreement with their transcriptional repression by the DELLAs, reported previously.Citation41
Figure 5. (A) The transcript accumulation of GNC and CGA1 in leaf tissues collected from 4-week-old Ler and atgcn2 plants was measured by real-time RT-PCR. Data represent the mean and SE of 3 technical replicates per genotype. Experiments with at least 3 independent biological replications yielded similar results. (B) The transcript accumulation of PRE1, PRE5 and XERICO (XER) in Ler and atgcn2 plants was measured by real-time RT-PCR. Data represent the mean and SE of 3 technical replicates per genotype. Experiments with at least 3 independent biological replications yielded similar results. (C) A model for AtGCN2 role in suppression of GA-mediated plant physiological processes, such as chlorophyll production, leaf blade morphogenesis and seed germination. GNC, CGA1, PRE1, PRE5 and XER, known to act as modulators of GA signaling, may be implicated in some of the regulatory steps proposed.
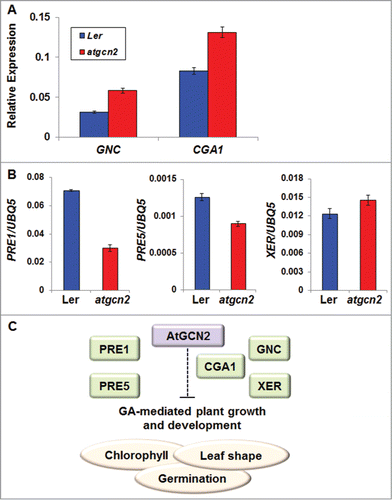
Given that the atgcn2 mutant maintains high basal levels of GNC and CGA1, the 2 GATA-related transcription factors known to repress GA (), we hypothesized that AtGCN2 negatively regulates GNC and CGA1 steady-state levels, in turn affecting GA signaling, and leading to changes in leaf morphology, leaf pigmentation and germination. Consistent with the proposed defect in the GA signaling, transcript levels of PRE1 and PRE5 are repressed and expression of XER is derepressed in the atgcn2 plants (). Thus, we propose that AtGCN2 is required for effective GA signal transduction in plant growth and developmental processes.
Conclusion
Our genetic data unequivocally demonstrate the role of AtGCN2 in diverse biological processes in Arabidopsis. We demonstrated that plants lacking functional AtGCN2 exhibit elongated leaf morphology and possess higher total chlorophyll contents and a lower chlorophyll a/b ratio. The atgcn2 mutants also display slow germination patterns under different stratification conditions and experiments conducted with exogenous GA4+7 and paclobutrazol indicated that this germination defect might be partly explained by defective GA biosynthesis. However, the downstream GA signaling pathway is also likely affected in the atgcn2 plants, given that they harbor increased levels of GNC, CGA1 and XER transcripts while accumulating lower levels of PRE1 and PRE5. It is well known that GAs are important regulators of the skotomorphogenic developmental program.Citation38 Intriguingly, a number of phenotypes observed in the atgcn2 plants, such as increases in specific leaf areas and reduced chlorophyll a/b ratio are reminiscent of shade-acclimation responses.Citation45 Downregulation of PRE1 and PRE5, which encode bHLH transcription factors that impair cell expansion,Citation46 could link AtGCN2 with growth during skotomorphogenic development. Future studies will be focused on understanding the mechanistic underpinnings of how AtGCN2 plays crucial roles in rewiring plant hormonal signaling networks and modulating plant growth and development under both steady-state as well as nutrient-deficient conditions.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by NSF-CAREER (IOS‐1350244) and UAB Faculty Development Grant to KPM.
References
- Chaves MM, Flexas J, Pinheiro C. Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann Bot 2009; 103:551–60; PMID:18662937; http://dx.doi.org/10.1093/aob/mcn125
- Osakabe Y, Osakabe K, Shinozaki K, Tran LS. Response of plants to water stress. Front Plant Sci 2014; 5:86; PMID:24659993; http://dx.doi.org/10.3389/fpls.2014.00086
- Ni FT, Chu LY, Shao HB, Liu ZH. Gene expression and regulation of higher plants under soil water stress. Curr Genomics 2009; 10:269-80; PMID:19949548; http://dx.doi.org/10.2174/138920209788488535
- Floris M, Mahgoub H, Lanet E, Robaglia C, Menand B. Post-transcriptional regulation of gene expression in plants during abiotic stress. Int J Mol Sci 2009; 10:3168-85; PMID:19742130; http://dx.doi.org/10.3390/ijms10073168
- Wek R, Jiang H, Anthony T. Coping with stress: eIF2 kinases and translational control. Biochem Soc Tran 2006; 34:7-11; PMID:16246168; http://dx.doi.org/10.1042/BST0340007
- Murguía JR, Serrano R. New functions of protein kinase Gcn2 in yeast and mammals. IUBMB Life 2012; 64:971-4; http://dx.doi.org/10.1002/iub.1090
- Guillem H, Rafael A-S, Consuelo M, Silvia L, Jose RM, Ramon S. A novel role for protein kinase Gcn2 in yeast tolerance to intracellular acid stress. Biochem J 2012; 441:255-64; PMID:21919885; http://dx.doi.org/10.1042/BJ20111264
- Yang R, Wek SA, Wek RC. Glucose limitation induces GCN4Translation by activation of Gcn2 protein kinase. Mol Cell Biol 2000; 20:2706-17; PMID:10733573; http://dx.doi.org/10.1128/MCB.20.8.2706-2717.2000
- Zhang P, McGrath BC, Reinert J, Olsen DS, Lei L, Gill S, Wek SA, Vattem KM, Wek RC, Kimball SR. The GCN2 eIF2α kinase is required for adaptation to amino acid deprivation in mice. Mol Cell Biol 2002; 22:6681-8; PMID:12215525; http://dx.doi.org/10.1128/MCB.22.19.6681-6688.2002
- Zoschke R, Watkins KP, Barkan A. A rapid ribosome profiling method elucidates chloroplast ribosome behavior in vivo. Plant Cell 2013; 25:2265-75; PMID:23735295; http://dx.doi.org/10.1105/tpc.113.111567
- Juntawong P, Girke T, Bazin J, Bailey-Serres J. Translational dynamics revealed by genome-wide profiling of ribosome footprints in Arabidopsis. Proc Natl Acad Sci U S A 2014; 111:E203-12; PMID:24367078; http://dx.doi.org/10.1073/pnas.1317811111
- Liu MJ, Wu SH, Wu JF, Lin WD, Wu YC, Tsai TY, Tsai HL, Wu SH. Translational landscape of photomorphogenic Arabidopsis. Plant Cell 2013; 25:3699-710; PMID:24179124; http://dx.doi.org/10.1105/tpc.113.114769
- Ashe MP, Susan K, Sachs AB. Glucose depletion rapidly inhibits translation initiation in yeast. Mol Biol Cell 2000; 11:833-48; PMID:10712503; http://dx.doi.org/10.1091/mbc.11.3.833
- Dever TE, Feng L, Wek RC, Cigan AM, Donahue TF, Hinnebusch AG. Phosphorylation of initiation factor 2α by protein kinase GCN2 mediates gene-specific translational control of GCN4 in yeast. Cell 1992; 68:585-96; PMID:1739968; http://dx.doi.org/10.1016/0092-8674(92)90193-G
- Tattoli I, Sorbara MT, Vuckovic D, Ling A, Soares F, Carneiro LA, Yang C, Emili A, Philpott DJ, Girardin SE. Amino acid starvation induced by invasive bacterial pathogens triggers an innate host defense program. Cell Host Microbe 2012; 11:563-75; PMID:22704617; http://dx.doi.org/10.1016/j.chom.2012.04.012
- Lageix S, Lanet E, Pouch-Pélissier M-N, Espagnol M-C, Robaglia C, Deragon J-M, Pélissier T. Arabidopsis eIF2α kinase GCN2 is essential for growth in stress conditions and is activated by wounding. BMC Plant Biol 2008; 8:134; PMID:19108716; http://dx.doi.org/10.1186/1471-2229-8-134
- Li MW, AuYeung WK, Lam HM. The GCN2 homologue in Arabidopsis thaliana interacts with uncharged tRNA and uses Arabidopsis eIF2α molecules as direct substrates. Plant Biol 2013; 15:13-8; PMID:22672016; http://dx.doi.org/10.1111/j.1438-8677.2012.00606.x
- Zhang Y, Wang Y, Kanyuka K, Parry MA, Powers SJ, Halford NG. GCN2-dependent phosphorylation of eukaryotic translation initiation factor-2α in Arabidopsis. J Exp Bot 2008; 59:3131-41; PMID:18603615; http://dx.doi.org/10.1093/jxb/ern169
- Zhang Y, Dickinson JR, Paul MJ, Halford NG. Molecular cloning of an Arabidopsis homologue of GCN2, a protein kinase involved in co-ordinated response to amino acid starvation. Planta 2003; 217:668-75; PMID:12905023; http://dx.doi.org/10.1007/s00425-003-1025-4
- Pajerowska-Mukhtar KM, Wang W, Tada Y, Oka N, Tucker CL, Fonseca JP, Dong X. The HSF-like transcription factor TBF1 is a major molecular switch for plant growth-to-defense transition. Curr Biol 2012; 22:103-12; PMID:22244999; http://dx.doi.org/10.1016/j.cub.2011.12.015
- Valvekens D, Montagu MV, Van Lijsebettens M. Agrobacterium tumefaciens-mediated transformation of Arabidopsis thaliana root explants by using kanamycin selection. Proc Natl Acad Sci U S A 1988; 85:5536-40; PMID:16593964; http://dx.doi.org/10.1073/pnas.85.15.5536
- Inskeep WP, Bloom PR. Extinction coefficients of chlorophyll a and B in n,n-dimethylformamide and 80% acetone. Plant Physiol 1985; 77:483-5; PMID:16664080; http://dx.doi.org/10.1104/pp.77.2.483
- Schwarzauer-Rockett K, Al-Hamdani SH, Rayburn JR, Mwebi NO. Utilization of kudzu as a lead phytoremediator and the impact of lead on selected physiological responses. Canadian J Plant Sci 2013; 93:951-9; http://dx.doi.org/10.4141/cjps10191
- Debeaujon I, Koornneef M. Gibberellin requirement for Arabidopsis seed germination is determined both by testa characteristics and embryonic abscisic acid. Plant Physiol 2000; 122:415-24; PMID:10677434; http://dx.doi.org/10.1104/pp.122.2.415
- Derkx MPM, Karssen CM. Effects of light and temperature on seed dormancy and gibberellin-stimulated germination in arabidopsis-thaliana – studies with gibberellin-deficient and gibberellin-insensitive mutants. Physiol Plantarum 1993; 89:360-8; http://dx.doi.org/10.1111/j.1399-3054.1993.tb00167.x
- Yamauchi Y, Ogawa M, Kuwahara A, Hanada A, Kamiya Y, Yamaguchi S. Activation of gibberellin biosynthesis and response pathways by low temperature during imbibition of Arabidopsis thaliana seeds. Plant Cell 2004; 16:367-78; PMID:14729916; http://dx.doi.org/10.1105/tpc.018143
- Swain SM, Singh DP, Helliwell CA, Poole AT. Plants with increased expression of ent-kaurene oxidase are resistant to chemical inhibitors of this gibberellin biosynthesis enzyme. Plant Cell Physiol 2005; 46:284-91; PMID:15695465; http://dx.doi.org/10.1093/pcp/pci027
- Blazquez M. Phenotypic analysis of Arabidopsis mutants: gibberellin/abscisic acid/paclobutrazol hormone response. CSH Protocols 2008; 2008:pdb prot4964
- Farmer E, Mousavi S, Lenglet A. Leaf numbering for experiments on long distance signalling in Arabidopsis. Protocol Exchange 2013; http://dx.doi.org/10.1038/protex.2013.071
- Gray RA. Alteration of leaf size and shape and other changes caused by gibberellins in plants. Am J Bot 1957; 44:674-82; http://dx.doi.org/10.2307/2438632
- Peng J, Harberd NP. Gibberellin deficiency and response mutations suppress the stem elongation phenotype of phytochrome-deficient mutants of Arabidopsis. Plant Physiol 1997; 113:1051-8; PMID:9112768; http://dx.doi.org/10.1104/pp.113.4.1051
- Ross JJ, Murfet IC, Reid JB. Distribution of gibberellins in Lathyrus odoratus L. and their role in leaf growth. Plant Physiol 1993; 102:603-8; PMID:12231850
- Yanai O, Shani E, Russ D, Ori N. Gibberellin partly mediates LANCEOLATE activity in tomato. Plant J 2011; 68:571-82; PMID:21771122; http://dx.doi.org/10.1111/j.1365-313X.2011.04716.x
- Munoz-Huerta RF, Guevara-Gonzalez RG, Contreras-Medina LM, Torres-Pacheco I, Prado-Olivarez J, Ocampo-Velazquez RV. A review of methods for sensing the nitrogen status in plants: advantages, disadvantages and recent advances. Sensors 2013; 13:10823-43; PMID:23959242; http://dx.doi.org/10.3390/s130810823
- Kourill R, Ilik P, Naus J, Schoefs B. On the limits of applicability of spectrophotometric and spectrofluorimetric methods for the determination of chlorophyll a/b ratio. Photosyn Res 1999; 62:107-16; http://dx.doi.org/10.1023/A:1006359213151
- Cheminant S, Wild M, Bouvier F, Pelletier S, Renou JP, Erhardt M, Hayes S, Terry MJ, Genschik P, Achard P. DELLAs regulate chlorophyll and carotenoid biosynthesis to prevent photooxidative damage during seedling deetiolation in Arabidopsis. Plant Cell 2011; 23:1849-60; PMID:21571951; http://dx.doi.org/10.1105/tpc.111.085233
- Das G. Possible mechanism of gibberellin-induced chlorosis in lettuce seedlings. Canadian J Bot 1973; 51:175-84; http://dx.doi.org/10.1139/b73-025
- Sun TP. The molecular mechanism and evolution of the GA-GID1-DELLA signaling module in plants. Curr Biol 2011; 21:R338-45; PMID:21549956; http://dx.doi.org/10.1016/j.cub.2011.02.036
- Richter R, Behringer C, Muller IK, Schwechheimer C. The GATA-type transcription factors GNC and GNL/CGA1 repress gibberellin signaling downstream from DELLA proteins and PHYTOCHROME-INTERACTING FACTORS. Genes Dev 2010; 24:2093-104; PMID:20844019; http://dx.doi.org/10.1101/gad.594910
- Hudson D, Guevara D, Yaish MW, Hannam C, Long N, Clarke JD, Bi YM, Rothstein SJ. GNC and CGA1 modulate chlorophyll biosynthesis and glutamate synthase (GLU1/Fd-GOGAT) expression in Arabidopsis. PloS One 2011; 6:e26765; PMID:22102866; http://dx.doi.org/10.1371/journal.pone.0026765
- Gallego-Bartolome J, Alabadi D, Blazquez MA. DELLA-induced early transcriptional changes during etiolated development in Arabidopsis thaliana. PloS One 2011; 6:e23918; PMID:21904598; http://dx.doi.org/10.1371/journal.pone.0023918
- Wang H, Zhu Y, Fujioka S, Asami T, Li J, Li J. Regulation of Arabidopsis brassinosteroid signaling by atypical basic helix-loop-helix proteins. Plant Cell 2009; 21:3781-91; PMID:20023194; http://dx.doi.org/10.1105/tpc.109.072504
- Ariizumi T, Hauvermale AL, Nelson SK, Hanada A, Yamaguchi S, Steber CM. Lifting della repression of Arabidopsis seed germination by nonproteolytic gibberellin signaling. Plant Physiol 2013; 162:2125-39; PMID:23818171; http://dx.doi.org/10.1104/pp.113.219451
- Ko JH, Yang SH, Han KH. Upregulation of an Arabidopsis RING-H2 gene, XERICO, confers drought tolerance through increased abscisic acid biosynthesis. Plant J 2006; 47:343-55; PMID:16792696; http://dx.doi.org/10.1111/j.1365-313X.2006.02782.x
- Vandenbussche F, Pierik R, Millenaar FF, Voesenek LA, Van Der Straeten D. Reaching out of the shade. Curr Opin Plant Biol 2005; 8:462-8; PMID:16040269; http://dx.doi.org/10.1016/j.pbi.2005.07.007
- Lee S, Lee S, Yang KY, Kim YM, Park SY, Kim SY, Soh MS. Overexpression of PRE1 and its homologous genes activates Gibberellin-dependent responses in Arabidopsis thaliana. Plant Cell Physiol 2006; 47:591-600; PMID:16527868; http://dx.doi.org/10.1093/pcp/pcj026
