Abstract
The tendency of amyloid β (Aβ42) peptide to misfold and aggregate into insoluble amyloid fibrils in Alzheimer's disease (AD) has been well documented. Accumulation of Aβ42 fibrils has been correlated with abnormal apoptosis and unscheduled cell division which can also trigger the death of neuronal cells, while oligomers can also exhibit similar activities. While investigations using chemically-synthesized Aβ42 peptide have become common practice, there appear to be differences in outcomes from different preparations. In order to resolve this inconsistency, we report 2 separate methods of preparing chemically-synthesized Aβ42 and we examined their effects in yeast. Hexafluoroisopropanol pretreatment caused toxicity while, ammonium hydroxide treated Aβ42 induced cell proliferation in both C. glabrata and S. cerevisiae. The hexafluoroisopropanol prepared Aβ42 had greater tendency to form amyloid on yeast cells as determined by thioflavin T staining followed by flow cytometry and microscopy. Both quiescent and non-quiescent cells were analyzed by these methods of peptide preparation. Non-quiescent cells were susceptible to the toxicity of Aβ42 compared with quiescent cells (p < 0.005). These data explain the discrepancy in the previous publications about the effects of chemically-synthesized Aβ42 on yeast cells. The effect of Aβ42 on yeast cells was independent of the size of the peptide aggregates. However, the Aβ42 pretreatment determined whether the molecular conformation of peptide resulted in proliferation or toxicity in yeast based assays.
Introduction
Soluble oligomers of amyloid β (Aβ42) peptide have been found to be highly neurotoxic and important in the etiology of AD in comparison to fibril forms.Citation1 However, it is still unknown which form of the aggregates are more dangerous; oligomers and protofibrils or fibrils and filaments.Citation2-4 Despite these uncertainties aggregation studies in search for chemo-preventatives that block Aβ42 oligomer and fibril formation using synthetic forms of Aβ42 have become common practice.Citation5-8
The preparation of Aβ42 peptide can affect its amyloidogenicityCitation4 yet pretreatments are required in order to produce monomers.Citation9 Lioudyno et al.Citation10 reported that pretreatment with 1,1,1,3,3,3-hexafluoroisopropanol (HFIP) enhanced the amyloidogenicity of the Aβ42 peptide as well as its interaction with biological membranes.
Complex mechanisms contribute to development of AD. Synaptic loss due to plaque formationCitation11 and neuronal death due to oligomeric forms of Aβ42 are all hallmarks of the disease.Citation12 It has been reported that different conformations of Aβ42 induce neurotoxicity by distinct mechanisms in human cortical neurons.Citation13,14 The longer variants have higher propensity to aggregation than the shorter form.Citation13 The mechanism of Aβ42 toxicity is still unknown, although it is suggested that amyloidogenic protein aggregates on neuronal cells cause permeabilisation of lipid membranes,Citation15-17 and increase oxidative stress,Citation18,19 mitochondrial dysfunction,Citation20-22 and endoplasmic reticulum stressCitation23,24 which ultimately leads to apoptosis.
Yeast exhibit some responses to Aβ42 peptide that indicate they are good models for studying the effects of on neuronal cells.Citation25 However, some limitations are expected since yeast lack receptors that have been proposed for Aβ42 binding. Putative receptors include the insulin receptor,Citation26 the alpha7 nicotinic receptor,Citation27 the metabotrophic glutamate receptor,Citation28 and cellular prion protein.Citation29 Chacińska and colleaguesCitation30 showed an induction of proliferation in the presence of synthetic Aβ42 in exponentially growing Saccharomyces cerevisiae, while Bharadwaj et al.Citation31 showed toxicity of synthetic Aβ42 with a different preparation method on Candida glabrata cells. In this study we report on the preparation of Aβ42 peptide with 2 different methods and treatments of identical yeast cell cultures. The two pretreatments resulted in totally opposite outcomes.
Results
Pretreatment determines the activity of Aβ42 peptide
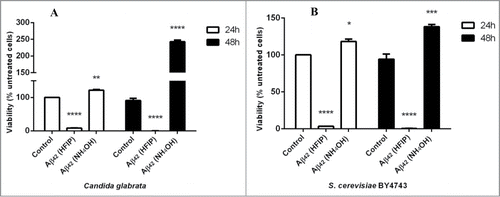
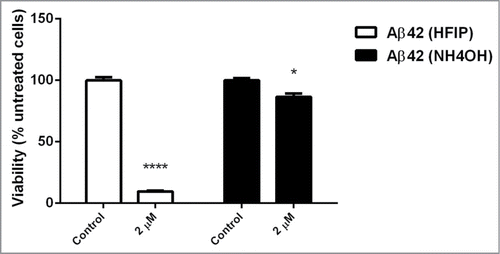
HFIP pretreatment of Aβ42 was previously shown to cause toxicity to C. glabrata cells.Citation31 This study confirms significant toxicity of HFIP pretreated Aβ42 to C. glabrata () and to S. cerevisiae (). There was even more cell killing after 48 h of incubation. Conversely, NH4OH pretreatment resulted in a significant increase in the number of yeast cells. C. glabrata () more than doubled in number (P < 0.001), and S. cerevisiae () numbers significantly increased over the time (P < 0.005).
Figure 1. Effect of HFIP and NH4OH pretreated synthetic Aβ42 on the yeast cells. 2 μM peptide was added to exponential phase C. glabrata (A) and S. cerevisiae (B) cells, suspended in water. Samples were incubated at 30°C for 48 h. Viability was determined as percentage of untreated cells (control). (* P < 0.05, ** P < 0.01, *** P < 0.005, and **** P < 0.001). Data are shown as Mean ± SEM.
Susceptibility of quiescent and non-quiescent cells to toxic Aβ42
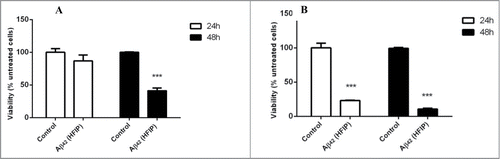
Stationary phase fractions of C. glabrata were tested for their susceptibility to 2 μM Aβ42 generated by HFIP pretreatment method. Results indicated that quiescent daughter cells () are more resistant to the toxic effect of Aβ42 than older non-quiescent mother cells (). The older cells lost their viability within 24 h in the presence of amyloid peptide whereas younger cells were affected by the toxicity after 48 h of incubation (see ).
Figure 2. Effect of Aβ42 on the quiescent and non-quiescent fractions of C. glabrata. The quiescent daughter cells (A) were more resistant to Aβ42 prepared with HFIP method than non-quiescent mother cells (B) in the first 24 h of incubation. After 48 h of incubation both cell fractions were susceptible to the toxicity effect of Aβ42 (*** P < 0.005).
Amyloid staining on the yeast cell surface and aggregation propensity
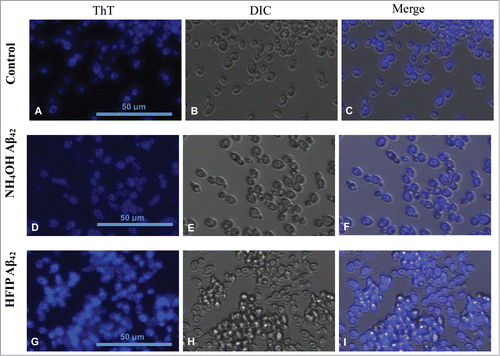
In order to examine the presence of amyloidogenic proteins on the surface of yeast cells ThT treated cells were visualised for fluorescence. S. cerevisiae exhibited presence of a high level of ThT stained proteins on their surface (). Yeast treated with HFIP prepared Aβ42 on the other hand have more aggregation propensity, causing the cells to adhere and attach to each other (see ). Cell aggregation appears absent in those cells treated with NH4OH prepared Aβ42 (). This result indicates that peptide produced by each method of preparation interacts differently with the yeast cell walls.
Figure 3. Staining of amyloid proteins present on S. cerevisiae cell walls by thioflavin T. First row (A–C) shows untreated S. cerevisiae cells in presence of ThT in comparison with cells treated with 5 μM NH4OH (D–F) and HFIP (G–I) prepared Aβ42 for 24 h in H2O. ThT staining comparison of untreated (control), NH4OH and HFIP pretreated cells are shown in panels A, D and G respectively. The HFIP pretreatment of Aβ42 results in higher fluorescence intensity and adhesion of peptide to the S. cerevisiae yeast cell wall protein. Scale bar = 50 μm.
Flow cytometry for analysis of fluorescence intensity associated with peptide
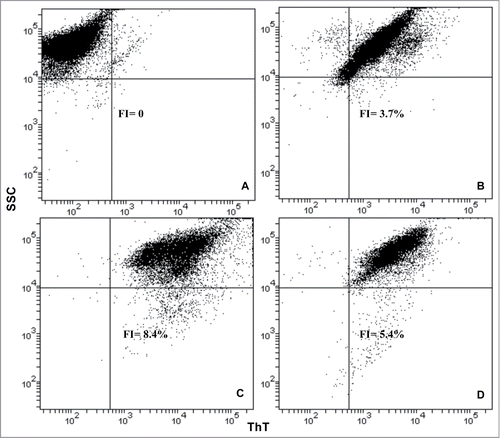
The fluorescence intensity associated with ThT level on the surface of the yeast cells treated with HFIP- and NH4OH-prepared amyloid peptide was measured by flow cytometry. Data showed an increase in the fluorescence intensity (FI) in the presence of HFIP pretreated peptide () compared with the NH4OH method (), and untreated controls (): fluorescence intensity was measured to be 8.4%, 5.4% and 3.7% respectively (see ). It seems the peptide conformation generated from the HFIP method has a greater propensity to induce aggregation and may cause amyloid deposition on the surface of yeast cells.
Figure 4. Flow cytometry analysis of S. cerevisiae treated with HFIP and NH4OH prepared Aβ42. The fluorescence intensity (FI) of ThT in the experimental samples was determined as a percentage of unstained cells. Yeast cells were grow to exponential phase, washed and resuspended in H2O. The cells were then treated with HFIP and NH4OH pretreated Aβ42 and incubated overnight along with control (untreated samples) at 30°C with shaking. The next day, cells were treated with 20 μM ThT and were analyzed by flow cytometry. Pacific blue filter was chosen to measure fluorescence. Unstained control cells (A), ThT stained control cells (B) and those treated with 5 μM of HFIP (C), and NH4OH pretreated Aβ42 have been shown.
TEM analysis of peptide conformers generated by pretreatment
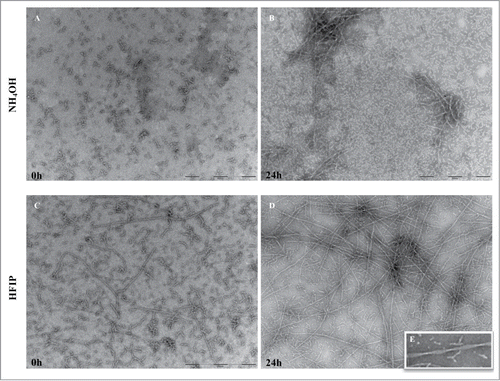
The Aβ42 prepared for viability counts was analyzed by TEM. The NH4OH method resulted in more uniform aggregate free peptide solution () compared with HFIP (). The latter showed the presence of protofibrils structures at 0 h. After 24 h incubation at 37°C short fibrils were observed in NH4OH prepared peptide () while, the HFIP method () resulted in long and twisted fibrils ().
Figure 5. TEM analysis of peptide pretreated by NH4OH and HFIP at 37°C. The NH4OH method of oligomer formation (A) results in a higher amount of monomeric peptide compared with HFIP method in which still contain long fibrils (B). After 24 h of incubation the NH4OH method of pretreatment (C) induced a lower tendency form fibrils and those generated were shorter fibrils. The HFIP method (D) on the other hand resulted in a higher level of twisted and longer fibrils. The HFIP twisted fibril is shown (E). Scale bar = 200 nm.
Effect of fibrils on the yeast cells
The toxicity of fibrils, formed after prolonged incubation, was examined. Fibrils generated from HFIP and NH4OH pretreatment were both toxic to yeast cells (). However, HFIP prepared Aβ42 fibrils exhibited greater toxicity compared to those fibrils prepared with NH4OH.
Figure 6. Effect of aged peptide on yeast cells. The NH4OH and HFIP pretreated Aβ42 peptide was incubated for 6 d at 37°C with no shaking. Exponentially growing S. cerevisiae were washed and suspended in H2O and then treated with 2 μM of these aged peptides and incubated overnight. Viability was determined the next day by transferring 100 μl of treated and untreated samples onto YEPD solid agar. (* P < 0.05 and **** P < 0.001).
Discussion
The current study indicates that different preparations of Aβ42 peptide can lead to different biological activity toward yeast. The recently introduced NH4OH pretreatment of synthetic Aβ42 reportedly results in formation of higher levels of monomeric peptideCitation32 and has not previously been tested on yeast. Soluble monomers of the Aβ42 prepared by NH4OH caused cell proliferation and an increase in the number of colonies in both S. cerevisiae and C. glabrata, regardless of the type of solvent (H2O or dilute NaOH). With prolonged incubation more fibrils were formed and these were toxic to S. cerevisiae cells.
In contrast, the Aβ42 peptide prepared by the HFIP method contained high levels of aggregates and caused cell death within the first 24 h as well as after this time. Older cells were shown to be more susceptible to the effects of Aβ42. The Aβ42 peptide conformation appears to be the major determinant of its effect on yeast cells. Previous studies showed that Aβ42 prepared using HFIP tends to be more potent in the electrophysiological tests and has a greater tendency to modulate biological membrane properties.Citation4,9,10 To further investigate the different outcomes caused by HFIP, it would be worth examining if HFIP affects the toxicity of additional fibril forming peptides.
The attachment of the Aβ42 peptide to the yeast cell surface has been demonstrated by Bharadwaj (2011) who demonstrated direct binding of fluorescein-labeled Aβ42 to the cell surface by fluorescence microscopy.Citation33 This finding was further confirmed by electron microscopy and western blotting of sub-cellular fractions.Citation33 In this study we showed that the Aβ42 peptide caused increased ThT staining of the yeast cell surface. Without Aβ42 peptide treatment, there was some ThT staining which may be attributed to proteins such as Flo1p, Als1p, Als5p, Muc1p and Bgl2p.Citation34-37 While there is clear evidence for peptides from Flo1p, Als1p, Als5p, and Muc1p to be amyloid forming, the evidence for these proteins forming amyloid on the yeast surface is currently incomplete and limited to the birefringence and ThT staining that they cause.Citation36 While it is tempting to speculate that Aβ42 may be forming amyloid on the yeast cell surface, proof of amyloid formation requires analysis of the resistance to an ionic detergent. This evidence is still lacking for other yeast cell surface proteins.
HFIP-treated Aβ42 resulted in peptide with long strand fibril morphology compared with the NH4OH method. It is known that HFIP pretreatment method does not efficiently generate aggregate free solutions. This HFIP pretreatment results in peptide that has a high propensity to adhere to the yeast cell wall. In this form the peptide in any size, monomeric, oligomeric and even fibrils are toxic to yeast cells. On the other hand, Aβ42 that is freshly pretreated with NH4OH caused cell proliferation due to monomeric and oligomeric forms while prolonged incubation led to a fibrillar conformation which was toxic to S. cerevisiae cells.
The data presented here show that different methods for the preparation of Aβ42 can lead to different biological activities in in vitro and in yeast biological assays. This work should serve as a caution in working with and interpreting the outcomes of biological assays.
Materials and Methods
Yeast strains and growth conditions
C. glabrata (ATCC90030) and S. cerevisiae BY4743 (a/α his3Δ/his3Δ leu2Δ/leu2Δ +/lys2Δ met15Δ/+ ura3Δ/ura3Δ) were utilised for experiments and were grown in YEPD (1% yeast extract, 2% peptone, and 2% D-glucose) with aeration at 30°C. For fractionation of quiescent and non-quiescent cells, growth in the YEPD media continued for 7 d with shaking (200 rpm). Cells were subsequently pelleted and washed for further analysis. For exponential phase of growth, yeast cells were grown overnight and were harvested by centrifugation the next day and resuspended in 20 ml fresh media. Then one ml was inoculated into 80 ml of fresh media and incubated at 30°C with shaking (200 rpm) for another 2-3 h to reach an OD600 of 1.0.
Fractionation of stationary phase C. glabrata and S. cerevisiae
Percoll density gradients (GE Healthcare, United Kingdom) were prepared according to the manufacturer's preformed gradient protocol and method described by Allen et al.Citation40 with some modification. Briefly, Percoll was diluted 9:1 (v/v) with 1.5 M NaCl. To form a gradient 10 ml of the Percoll solution was added to 15 ml Corex® tubes and centrifuged for 15 min at 20°C in a Beckman Coulter centrifuge (SW41Ti rotor). Stationary phase yeast cells which were grown for 7 d at 30°C with shaking were then pelleted and resuspended in 1 ml Tris buffer (50 mM, pH 7.5). Around 2 ml of cell suspension was overlaid onto preformed gradient, and centrifuged at 400 g for 60 min in a Beckman Coulter centrifuge. Fractions of quiescent (lower fraction) and non-quiescent cells were collected and washed twice in 40 ml Tris buffer to remove any Percoll residue, pelleted and then resuspended in ddH2O.
Preparation of Aβ42 peptide
Synthetic human Aβ42 peptide was purchased from Keck laboratories (Yale University, New Haven, CT). The peptide was synthesized and purified using tBOC chemistry with DCC and HOBT coupling reagents in the form of lyophilized powder. All solvents used for the preparation of Aβ42 solutions were filtered and centrifuged to minimise the presence of debris that could induce aggregation of the peptide.
Preparation of Aβ42 using NH4OH
Peptide solutions were prepared according to the protocol of Ryan et al.Citation32 Briefly, 20 mg peptide was dissolved in 40 ml of 10% NH4OH to final concentration of 0.5 mg/ml solution (w/v). The solution was then left at room temperature for 10 min and then sonicated for 5 min. For yeast treatments, the peptide was prepared immediately prior to use. Each 0.5 mg aliquot of Aβ pellet was dissolved in 60 μl of ddH2O and then vortexed to promote the dissolving of the peptide. The solution was then sonicated for 5 min and centrifuged at 14,000 g for 5 min to pellet any undissolved peptide. The supernatant was carefully recovered for use in subsequent assays. Peptide concentration was determined from the A214nm measurement of the solution in a quartz cuvette (BioMate™ Spec, Thermo Scientific, UK).
Preparation of Aβ42 using HFIP
Aβ42 was dissolved in 1,1,1,3,3,3-hexafluoroisopropanol (HFIP) according to the method described by Crescenzi and colleagues.Citation39 Briefly the peptide film was dissolved in HFIP to a concentration of 1 mg/ml, sonicated for 5 min on ice and dried overnight to form a clear film. The film was subsequently dissolved in 60 μl of ddH2O, sonicated for 5 min and centrifuged to remove insoluble aggregates. Peptide concentrations were determined described above.
Effect of prolonged incubation of Aβ42 on the survival of yeast
The Aβ42 samples from each pretreatment method were incubated at 35°C for 6 d. Exponentially growing S. cerevisiae were washed and suspended in H2O and then were treated with 5 μM of HFIP and NH4OH pretreated peptide and incubated overnight. Treatment and viability were determined as follows.
Exponentially growing yeast cells were washed twice and resuspended in ddH2O. After estimation of cell numbers by a haemocytometer, cells were transferred into 96-well culture plates at a concentration of 10Citation3 cells/ml, treated with 2-5 μM of Aβ42 peptide in a final volume of 200 μl per microtiter well and incubated overnight at 30°C with shaking. The next day 100 μl aliquots of cell suspension were plated onto YEPD plates and incubated for 2 days at 30°C. Colonies were counted for each treatment and the viability was estimated as percentage of untreated cells. All treatments were performed in triplicate.
Flow cytometry analyses after Thioflavin T staining of treated cells
Exponentially growing S. cerevisiae were washed and incubated in H2O in the presence and absence of pretreated HFIP and NH4OH peptide. After 16 h of incubation, Thioflavin T (ThT) was added to each sample to a final concentration of 20 μM and incubated for 10 min.Citation40 Around 10,000 cells per sample were analyzed by flow cytometry (FACS, Canto II- BD™) using a pacific blue filter with 351 nm excitation and collecting fluorescent emission at 450/65. Data were recorded and saved as FCS3 files. All data were analyzed by WEASEL (WEHI, Melbourne, Australia) software. ThT fluorescence intensity of multiple experimental samples was calculated as percentage of unstained yeast cells.
Confocal microscopy after Thioflavin T staining of treated cells
Confocal fluorescence and differential interference contrast (DIC) microscopy was performed using a Nikon® Eclipse Ti confocal laser-scanning microscope. Treated and untreated yeast cells with Aβ42 peptide in H2O in a 96 well microtiter plate were stained with ThT to a final concentration of 20 μM. ThT fluorescence was observed using a DAPI filter following excitation of the sample with light of 405 nm wavelength. Images were acquired using a Nikon camera and NIS-Elements imaging software.
Transmission electron microscopy
Freshly prepared aliquots of Aβ42 peptide with both pretreatment methods were used for imaging the starting peptide conformation. The peptide samples were then incubated overnight at 35°C for analysis of peptide conformation after 24 h. Samples from 0 and 24 h were gently agitated before transferring a 4 μl aliquot onto the grids. After 30 sec adsorption time, the excess sample was drawn off using Whatman® 541 filter paper. The grids were stained subsequently with 2% w/v potassium phosphotungstate (pH 7.2) for 10 sec. The grids were then air dried before examination. The samples were examined using a Tecnai™ 12 Transmission Electron Microscope (FEI, Eindhoven, The Netherlands) at an operating voltage of 120 kV. Images were recorded using a Megaview III CCD camera and AnalySIS camera control software (Olympus Australia).
Statistical analysis
Graphs were made using Microsoft Office Excel 2010 software and data were analyzed using PRISM® 6 (Graph Pad Software, Inc.., La Jolla, CA, USA). All data are presented as mean + SEM. Significant differences between samples were determined by comparing the mean score of samples by an unpaired Student's t-test. A P-value of <0.05 is deemed as significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work is supported in part by a grant from the Medical Advances Without Animals Trust (MAWA).
References
- Morgan C, Colombres M, Nuñez MT, Inestrosa NC. Structure and function of amyloid in Alzheimer's disease. Prog Neurobiol 2004; 74:323-49; PMID:15649580; http://dx.doi.org/10.1016/j.pneurobio.2004.10.004
- Steckmann T, Awan Z, Gerstman BS, Chapagain PP. Kinetics of peptide secondary structure conversion during amyloid β-protein fibrillogenesis. J Theor Biol 2012; 301:95-102; PMID:22586726; http://dx.doi.org/10.1016/j.jtbi.2012.02.012
- Stefani M, Dobson CM. Protein aggregation and aggregate toxicity: new insights into protein folding, misfolding diseases and biological evolution. J Molec Med (Berlin, Germany) 2003; 81:678-99
- Zagorski MG, Yang J, Shao H, Ma K, Zeng H, Hong A. Methodological and chemical factors affecting amyloid β peptide amyloidogenicity. Methods Enzymol 1999; 309:189-204; PMID:10507025; http://dx.doi.org/10.1016/S0076-6879(99)09015-1
- Chen J, Armstrong AH, Koehler AN, Hecht MH. Small molecule microarrays enable the discovery of compounds that bind the Alzheimer's Aβ peptide and reduce its cytotoxicity. J Am Chem Soc 2010; 132:17015-22; PMID:21062056; http://dx.doi.org/10.1021/ja107552s
- Macreadie I, Lotfi-Miri M, Mohotti S, Shapira D, Bennett L, Varghese J. Validation of folate in a convenient yeast assay suited for identification of inhibitors of Alzheimer's Aβ aggregation. J Alzheimers Dis 2008; 15:391-6; PMID:18997292
- Matlack KE, Tardiff DF, Narayan P, Hamamichi S, Caldwell KA, Caldwell GA, Lindquist S. Clioquinol promotes the degradation of metal-dependent amyloid-β (Aβ) oligomers to restore endocytosis and ameliorate Aβ toxicity. Proc Natl Acad Sci USA 2014; 111:4013-8; PMID:24591589; http://dx.doi.org/10.1073/pnas.1402228111
- Park SK, Pegan SD, Mesecar AD, Jungbauer LM, LaDu MJ, Liebman SW. Development and validation of a yeast high-throughput screen for inhibitors of Aβ42 oligomerization. Dis model Mech 2011; 4:822-31; PMID:21810907; http://dx.doi.org/10.1242/dmm.007963
- Roccatano D, Fioroni M, Zacharias M, Colombo G. Effect of hexafluoroisopropanol alcohol on the structure of melittin: a molecular dynamics simulation study. Protein Sci 2005; 14:2582-9; PMID:16155200; http://dx.doi.org/10.1110/ps.051426605
- Lioudyno MI, Broccio M, Sokolov Y, Rasool S, Wu J, Alkire MT, Liu V, Kozak JA, Dennison PR, Glabe CG, et al. Effect of synthetic Aβ peptide oligomers and fluorinated solvents on Kv1.3 channel properties and membrane conductance. PloS One 2012; 7:e35090; PMID:22563377; http://dx.doi.org/10.1371/journal.pone.0035090
- Selkoe DJ. Alzheimer's Disease is a synaptic failure. Science 2002; 298:789-91; PMID:12399581; http://dx.doi.org/10.1126/science.1074069
- Yoshiike Y, Minai R, Matsuo Y, Chen YR, Kimura T, Takashima A. Amyloid oligomer conformation in a group of natively folded proteins. PloS One 2008; 3:e3235; PMID:18800165; http://dx.doi.org/10.1371/journal.pone.0003235
- Kumar S, Walter J. Phosphorylation of amyloid β (Aβ) peptides - a trigger for formation of toxic aggregates in Alzheimer's disease. Aging 2011; 3:803-12; PMID:21869458
- Ladiwala AR, Litt J, Kane RS, Aucoin DS, Smith SO, Ranjan S, Davis J, Van Nostrand WE, Tessier PM. Conformational differences between two amyloid β oligomers of similar size and dissimilar toxicity. J Biol Chem 2012; 287:24765-73; PMID:22547072; http://dx.doi.org/10.1074/jbc.M111.329763
- Evangelisti E, Wright D, Zampagni M, Cascella R, Fiorillo C, Bagnoli S, Relini A, Nichino D, Scartabelli T, Nacmias B, et al. Lipid rafts mediate amyloid-induced calcium dyshomeostasis and oxidative stress in Alzheimer's disease. Curr Alzheimer Res 2013; 10:143-53; PMID:22950913; http://dx.doi.org/10.2174/1567205011310020004
- Gella A, Durany N. Oxidative stress in Alzheimer disease. Cell Adh Migr 2009; 3:88-93; PMID:19372765; http://dx.doi.org/10.4161/cam.3.1.7402
- Poojari C, Kukol A, Strodel B. How the amyloid-β peptide and membranes affect each other: An extensive simulation study. Biochim Biophys Acta 2013; 1828:327-39; PMID:22975281; http://dx.doi.org/10.1016/j.bbamem.2012.09.001
- Mattson MP, Goodman Y. Different amyloidogenic peptides share a similar mechanism of neurotoxicity involving reactive oxygen species and calcium. Brain Res 1995; 676:219-24; PMID:7796173; http://dx.doi.org/10.1016/0006-8993(95)00148-J
- Nakamura M, Shishido N, Nunomura A, Smith MA, Perry G, Hayashi Y, Nakayama K, Hayashi T. Three histidine residues of amyloid-β peptide control the redox activity of copper and iron. Biochemistry 2007; 46:12737-43; PMID:17929832; http://dx.doi.org/10.1021/bi701079z
- Cali T, Ottolini D, Brini M. Mitochondria, calcium, and endoplasmic reticulum stress in Parkinson's disease. BioFactors (Oxford, England) 2011; 37:228-40; PMID:21674642; http://dx.doi.org/10.1002/biof.159
- Ferreiro E, Baldeiras I, Ferreira IL, Costa RO, Rego AC, Pereira CF, Oliveira CR. Mitochondrial- and Endoplasmic Reticulum-Associated Oxidative Stress in Alzheimer's Disease: From Pathogenesis to Biomarkers. Int J Cell Biol 2012; 2012:23; PMID:22701485; http://dx.doi.org/10.1155/2012/735206
- Selfridge JE, E L, Lu J, Swerdlow RH. Role of mitochondrial homeostasis and dynamics in Alzheimer's disease. Neurobiol Dis 2013; 51:3-12; PMID:22266017; 10; http://dx.doi.org/10.16/j.nbd.2011.12.057
- Huang CJ, Haataja L, Gurlo T, Butler AE, Wu X, Soeller WC, Butler PC. Induction of endoplasmic reticulum stress-induced β-cell apoptosis and accumulation of polyubiquitinated proteins by human islet amyloid polypeptide. Am J Physiol Endocrinol Metab 2007; 293:E1656-62; PMID:17911343; http://dx.doi.org/10.1152/ajpendo.00318.2007
- Umeda T, Tomiyama T, Sakama N, Tanaka S, Lambert MP, Klein WL, Mori H. Intraneuronal amyloid β oligomers cause cell death via endoplasmic reticulum stress, endosomal/lysosomal leakage, and mitochondrial dysfunction in vivo. J Neurosci Res 2011; 89:1031-42; PMID:21488093; http://dx.doi.org/10.1002/jnr.22640
- Porzoor A, Macreadie IG. Application of yeast to study the tau and Amyloid-β abnormalities of Alzheimer's disease. J Alzheimers Dis 2013; 35; PMID:23396350
- Xie L, Helmerhorst E, Taddei K, Plewright B, Van Bronswijk W, Martins R. Alzheimer's β-amyloid peptides compete for insulin binding to the insulin receptor. J Neurosci 2002; 22:Rc221; PMID:12006603
- Wang HY, Lee DH, Davis CB, Shank RP. Amyloid peptide Abeta(1-42) binds selectively and with picomolar affinity to alpha7 nicotinic acetylcholine receptors. J Neurochem 2000; 75:1155-61; PMID:10936198; http://dx.doi.org/10.1046/j.1471-4159.2000.0751155.x
- Renner M, Lacor PN, Velasco PT, Xu J, Contractor A, Klein WL, Triller A. Deleterious effects of amyloid β oligomers acting as an extracellular scaffold for mGluR5. Neuron 2010; 66:739-54; PMID:20547131; http://dx.doi.org/10.1016/j.neuron.2010.04.029
- Lauren J, Gimbel DA, Nygaard HB, Gilbert JW, Strittmatter SM. Cellular prion protein mediates impairment of synaptic plasticity by amyloid-β oligomers. Nature 2009; 457:1128-32; PMID:19242475; http://dx.doi.org/10.1038/nature07761
- Chacińska A, Woźny W, Boguta M, Misicka A, Brzyska M, Elbaum D. Effects of β-amyloid on proliferation and morphology of yeast Saccharomyces cerevisiae. Lett Pept Sci 2002; 9:197-201
- Bharadwaj P, Waddington L, Varghese J, Macreadie IG. A new method to measure cellular toxicity of non-fibrillar and fibrillar Alzheimer's Aβ using yeast. J Alzheimers Dis 2008; 13:147-50; PMID:18376056
- Ryan TM, Caine J, Mertens HD, Kirby N, Nigro J, Breheney K, Waddington LJ, Streltsov VA, Curtain C, Masters CL, et al. Ammonium hydroxide treatment of Abeta produces an aggregate free solution suitable for biophysical and cell culture characterization. PeerJ 2013; 1:e73; PMID:23678397; http://dx.doi.org/10.7717/peerj.73
- Bharadwaj, P. Ph. D. Thesis. Yeast as a model for studying Aβ aggregation, toxicity and clearance. (2011); http://ro.ecu.edu.au/cgi/viewcontent.cgi?article=1403&context=theses
- Bezsonov EE, Groenning M, Galzitskaya OV, Gorkovskii AA, Semisotnov GV, Selyakh IO, Ziganshin RH, Rekstina VV, Kudryashova IB, Kuznetsov SA, et al. Amyloidogenic peptides of yeast cell wall glucantransferase Bgl2p as a model for the investigation of its pH-dependent fibril formation. Prion 2013; 7:175-84; PMID:23208381; http://dx.doi.org/10.4161/pri.22992
- Gorkovskii AA, Bezsonov EE, Plotnikova TA, Kalebina TS, Kulaev IS. Revealing of Saccharomyces cerevisiae yeast cell wall proteins capable of binding thioflavin T, a fluorescent dye specifically interacting with amyloid fibrils. Biochem (Mosc) 2009; 74:1219-24; PMID:19916936; http://dx.doi.org/10.1134/S0006297909110066
- Kalebina TS, Plotnikova TA, Gorkovskii AA, Selyakh IO, Galzitskaya OV, Bezsonov EE, Gellissen G, Kulaev IS. Amyloid-like properties of Saccharomyces cerevisiae cell wall glucantransferase Bgl2p: prediction and experimental evidences. Prion 2008; 2:91-6; PMID:19098439; http://dx.doi.org/10.4161/pri.2.2.6645
- Ramsook CB, Tan C, Garcia MC, Fung R, Soybelman G, Henry R, Litewka A, O'Meally S, Otoo HN, Khalaf RA, et al. Yeast cell adhesion molecules have functional amyloid-forming sequences. Eukaryotic cell 2010; 9:393-404; PMID:20038605; http://dx.doi.org/10.1128/EC.00068-09
- Allen C, Buttner S, Aragon AD, Thomas JA, Meirelles O, Jaetao JE, Benn D, Ruby SW, Veenhuis M, Madeo F, et al. Isolation of quiescent and nonquiescent cells from yeast stationary-phase cultures. J Cell Biol 2006; 174:89-100; PMID:16818721; http://dx.doi.org/10.1083/jcb.200604072
- Crescenzi O, Tomaselli S, Guerrini R, Salvadori S, D'Ursi AM, Temussi PA, Picone D. Solution structure of the Alzheimer amyloid β-peptide (1–42) in an apolar microenvironment. Eur J Biochem 2002; 269:5642-8; PMID:12423364; http://dx.doi.org/10.1046/j.1432-1033.2002.03271.x
- LeVine H, 3rd. Thioflavine T interaction with synthetic Alzheimer's disease β-amyloid peptides: detection of amyloid aggregation in solution. Protein Sci 1993; 2:404-10; PMID:8453378; http://dx.doi.org/10.1002/pro.5560020312
