Abstract
Epithelial cells are tightly coupled together through specialized intercellular junctions, including adherens junctions, desmosomes, tight junctions, and gap junctions. A growing body of evidence suggests epithelial cells also directly exchange information at cell-cell contacts via the Eph family of receptor tyrosine kinases and their membrane-associated ephrin ligands. Ligand-dependent and -independent signaling via Eph receptors as well as reverse signaling through ephrins impact epithelial tissue homeostasis by organizing stem cell compartments and regulating cell proliferation, migration, adhesion, differentiation, and survival. This review focuses on breast, gut, and skin epithelia as representative examples for how Eph receptors and ephrins modulate diverse epithelial cell responses in a context-dependent manner. Abnormal Eph receptor and ephrin signaling is implicated in a variety of epithelial diseases raising the intriguing possibility that this cell-cell communication pathway can be therapeutically harnessed to normalize epithelial function in pathological settings like cancer or chronic inflammation.
Abbreviations
| ADAM | = | a disintegrin and metalloprotease |
| Apc | = | adenomatous polyposis coli |
| Eph | = | erythropoietin-producing hepatocellular |
| ER | = | estrogen receptor |
| Erk | = | extracellular signal-regulated kinase |
| GEF | = | guanine nucleotide exchange factor |
| GPI | = | glycosylphosphatidylinositol |
| HER2 | = | human epidermal growth factor receptor 2 |
| HGF | = | hepatocyte growth factor |
| IBD | = | inflammatory bowel disease |
| KLF | = | Krüppel-like factor |
| MAPK | = | mitogen-activated protein kinase |
| MMTV-LTR | = | mouse mammary tumor virus-long terminal repeat |
| MT1-MMP | = | membrane-type 1 matrix metalloproteinase |
| PDZ | = | postsynaptic density protein 95, discs large 1, and zonula occludens-1 |
| PTP | = | protein tyrosine phosphatase |
| RTK | = | receptor tyrosine kinase |
| SH2 | = | Src homology 2 |
| SHIP2 | = | SH2 inositol phosphatase 2 |
| SLAP | = | Src-like adaptor protein |
| TCF | = | T-cell specific transcription factor |
| TEB | = | terminal end bud |
| TNFα | = | tumor necrosis factor α. |
Introduction
Erythropoietin-producing hepatocellular (Eph) receptor tyrosine kinases (RTKs) are activated upon binding to their membrane-associated ephrin ligands. This large family of RTKs is subdivided into 2 subclasses, EphA (1–8, 10) and EphB (1–4, 6) receptors based on amino acid sequence homology and relative binding affinities to glycosylphosphatidylinositol (GPI)-linked ephrin-A (1–5) or transmembrane ephrin-B (1–3) ligands. There is extensive promiscuity for ligand binding within receptor subclasses and more limited interaction between receptors and ligands in different subclasses.Citation1–3 While EphA and EphB receptors generally share conserved extracellular and cytoplasmic subdomain structure, the ephrin-A and -B ligand subfamilies differ substantially in the C-terminal region and mechanism of membrane-anchorage (). These molecular and biochemical features confer a considerable degree of complexity in the organization and function of Eph receptor and ephrin signaling complexes.
Figure 1. Basic molecular domain structure of Eph receptors and ephrin ligands. Eph receptors have conserved structures and domains. TheN-terminal extracellular region consists of a ligand binding domain (LBD), an epidermal growth factor-like motif within a cysteine-rich domain (CRD) and two fibronectin-type III repeats (FN III1 and FN III2). The receptors pass through the membrane via a single transmembrane domain (TM). The intracellular C-terminus starts with a juxtamembrane region (JM), followed by a tyrosine kinase domain (TKD), sterile α motif (SAM) and a PDZ (postsynaptic density protein 95, discs large 1, and zonula occludens-1) binding motif. The ephrin ligands share a conserved extracellular, N-terminal receptor binding domain (RBD). Ephrin-A ligands are attached to the cell membrane with a glycosylphosphatidylinositol (GPI) anchor. In contrast, ephrin-B ligands have a C-terminal tail that extends into the cytoplasm of the ligand-bearing cell through a TM domain. The C-termini of ephrin-B ligands contain a cytoplasmic tail with a PDZ binding motif.
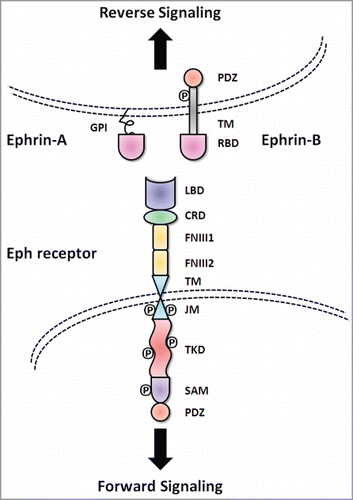
As both receptors and ligands are membrane-bound, the interaction of Eph receptors and ephrins is commonly thought to occur at cell-cell contacts. However, this is not an absolute requirement for Eph receptor signaling as ligand-independent functions for these RTKs have been described and there is evidence that EphA receptors can be activated by soluble ephrin-A ligands present in the microenvironment.Citation4,5 Ligand binding to Eph receptors at cell-cell contacts rapidly leads to the formation of higher order signaling clusters required for robust kinase activation, effector molecule recruitment, and transmission of a variety of downstream signaling cascades.Citation6,7 Contact-dependent signaling is evident where there is complementary expression of Eph receptor and ephrin in distinct cell types but there also appears to be a major role for Eph receptor signaling complexes in cell types where there is overlapping expression of receptor and ligand, particularly in epithelial tissues.
The signaling cascades induced by Eph receptors and ephrins are important for a wide range of cellular processes that govern embryonic development and tissue patterning. Frequently, this involves altered cell-cell adhesion resulting in either cell attraction or repulsion that occurs in a context-dependent manner.Citation8 Arguably, less is known about the precise Eph receptor-mediated transmission of membrane signals from the cytoplasm into the nucleus to impact gene expression compared to other RTKs. Another unique property of Eph receptor signaling is that it is bi-directional in character, with ligand-mediated activation of Eph receptor leading to forward signaling while reverse signaling is elicited in ephrin-expressing cells. Eph receptor and ephrin signaling has been shown to play a prominent role during embryogenesis, but a growing body of evidence indicates that adult epithelial tissues are another major site for Eph receptor and ephrin ligand action.Citation9-14
Epithelial tissues line body surfaces and cavities. They are composed of single (simple) or multiple (stratified) layers of epithelial cells that are tightly anchored to the underlying basement membrane and one another via specialized junctional complexes; these include integrin-based adhesion complexes for cell-extracellular matrix anchorage points while adherens junctions, desmosomes, tight junctions, and gap junctions are found at cell-cell contacts.Citation8 Eph receptors are not simply activated at cell-cell contact sites but also negatively or positively regulate these junctional complexes in a manner that depends on the character of the receptor involved, the relative abundance of ligand within the tissue, and the cell type in question.Citation15,16 As such, Eph receptor signaling can modulate epithelial tissue remodeling, integrity, and barrier function.
Homeostasis maintains cellular stability and tissue function. In actively regenerating epithelial tissues, homeostasis is maintained, in part, by the balance of cell proliferation and cell death. This balance relies on the presence of epithelial stem cells, which are found in distinct compartments within the tissue termed niches. Eph receptor and ephrin signaling has emerged as a major regulator of epithelial tissue homeostasis by regulating the organization of stem cell compartments as well as modulating the ability of progenitor cells to proliferate or differentiate.Citation17-19 Moreover, aberrant expression or activation of Eph receptors and ephrins is believed to contribute to malignant or pathologic states in epithelial tissues.Citation9-12,14,20 This review focuses on the role of Eph receptor and ephrin signaling in epithelial tissues that undergo extensive regeneration and remodeling in adults, including those present in the breast, gastrointestinal tract, and skin.
Organization of Eph Receptors and Ephrins in Epithelial Cells
Activation of Eph receptor and ephrin signaling complexes
Eph receptor signaling is distinct from that of other RTKs as receptor-ligand interactions rely on proximity between adjacent cell membranes and ephrin binding induces bi-directional signaling within the receptor-and ligand-bearing cell. The apposition of plasma membranes between adjacent epithelial cells facilitates Eph receptor and ephrin signaling but the precise molecular organization of these cell surface signaling complexes remains to be clearly elucidated in most epithelial tissues, particularly when receptor and ligand are co-expressed.
In the case of forward signaling, extracellular interactions between ephrins and Eph receptors initiate a conformational change in the receptor cytoplasmic domain that releases autoinhibitory interactions, triggering kinase activity and recruitment of Src homology 2 (SH2) domain- and phosphotyrosine binding domain-containing proteins to the cell surface.Citation21-27 Eph receptors also undergo clustering within the plasma membrane upon ligand binding in a manner that is stabilized by additional interactions with other ectodomain and cytodomain regions present on the receptor.Citation6,28-30 The size of the receptor signaling clusters can vary depending on the mobility of ephrins on adjacent membranes and the cell type in question.Citation31 Eph receptor can also form hetero-oligomer clusters with members of a different receptor subclass.Citation32,33 Taken together, this allows for a considerable degree of complexity in the molecular organization of Eph receptor signaling complexes in epithelial tissues where multiple family members are expressed.
Coincident with Eph receptor binding, reverse signaling is initiated in ephrin-expressing cells. In particular, ephrin-B ligands can cluster in the plasma membrane and are phosphorylated by Src family kinases in their cytoplasmic domain after binding Eph receptors via their ectodomains.Citation34-36 This phosphorylation of tyrosine residues facilitates interaction with SH2 domain-containing proteins but the ephrin-B cytoplasmic tail also interacts with effector proteins via serine phosphorylation-dependent events and its PDZ (postsynaptic density protein 95, discs large 1, and zonula occludens-1) binding motif.Citation37-40 Although lacking a cytoplasmic domain, GPI-linked ephrin-A ligands modulate intercellular signaling, particularly in the Src family kinase pathway, likely through clustering within lipid raft membrane domains.Citation41 As outlined below, there is evidence for forward and reverse signaling via both subclasses of Eph receptors and ephrins in epithelial tissues.
Attenuation of Eph receptor signaling complexes
Eph receptors are stabilized and become activated at cell-cell contacts but the fate of these ligand-bound receptors is not fully understood in epithelial cells. In general, attenuation of Eph receptor signaling occurs through mechanisms common to many RTKs including phosphatase-dependent dephosphorylation, proteolytic cleavage, or endocytosis.
Eph receptors can be negatively regulated by multiple protein tyrosine phosphatases (PTP) in epithelial cells. For example, PTP1B limited EphA3 kinase activity leading to the attenuation of ephrin-induced contraction of the actin cytoskeleton in human kidney epithelial cells.Citation42 Similarly, low molecular weight-PTP negatively regulated EphA2 to prevent subsequent dampening of mitogen-activated protein kinase (MAPK) signaling in prostate epithelial carcinoma cells.Citation43 Elevated PTP activity in epithelial cells may also stabilize Eph receptors at the epithelial cell surface via mechanisms that have yet to be resolved.
Upon ligand binding, Eph receptors can undergo a specialized form of internalization termed transendocytosis; this involves collective internalization of Eph receptor and ephrin complexes into the same cell. A clathrin-dependent internalization mechanism that relied on Rac signaling and Rho-guanine nucleotide exchange factor (GEF) Tiam1 binding to the EphA receptor juxtamembrane domain has been described in pancreatic carcinoma and kidney epithelial cells.Citation44,45 Conversely, EphA2 was stabilized at the cell surface in invasive breast cancer cells by SH2-containing 5’-inositol phosphatase 2 (SHIP2) through sterile α motif domain interactions between the respective proteins.Citation46 EphA2 stabilization was dependent on SHIP2-mediated dephosphorylation of PIP3, which inhibits Rac1-GTPase-dependent endocytosis. Thus, Eph receptor recruitment of signaling effectors that differentially regulate Rac1 can determine receptor fate at the epithelial cell surface.
While receptor endocytosis eliminates Eph signaling at cell-cell contacts, internalized Eph receptor complexes continue to signal inside the cell. For example, EphA2 in early endosomes exhibited high tyrosine phosphorylation content and a fraction of internalized EphA2 recycled back to the plasma membrane where it presumably engaged new ligand.Citation44 Investigation into the regulation of Eph receptor endosomal trafficking events will likely reveal factors important for receptor recycling or degradation pathways that influence signaling persistence in epithelial cells.
Eph receptor signaling complexes can also be internalized following protease-mediated cleavage of ephrins or the receptor. For example, EphA3 bound by ephrin-A5 was internalized upon proteolytic cleavage of the ligand by a disintegrin and metalloprotease 10 (ADAM10) in kidney epithelial cells and EphA2 was cleaved by membrane-type 1 matrix metalloproteinase (MT1-MMP) in breast carcinoma cell lines.Citation47,48 Ubiquitin-mediated degradation provides another pathway to attenuate Eph receptor signaling in epithelial cells. In particular, ligand-dependent degradation of EphA receptors is mediated through interaction with c-Cbl resulting in receptor ubiquitination.Citation44,49,50 While c-Cbl-mediated ubiquitination led to EphA receptor degradation, this process can be inhibited by binding to Odin, a member of the Anks family of ankyrin-rich repeat proteins, resulting in prolonged receptor signaling.Citation51,52 Another route of EphA2 degradation has been shown to be dependent on the Src-like adaptor protein (SLAP). SLAP negatively regulated EphA2 signaling through SH2-mediated binding to a Src-phosphorylated tyrosine site in the EphA2 tail.Citation53 The subsequent degradation of EphA2 was dependent on the ubiquitination protein UBE4A. Overall, SLAP exerted tumor suppressive properties in colorectal cancer cells by attenuating EphA2 signaling through receptor destabilization, ubiquitin-dependent proteasomal degradation, and downstream attenuation of Akt signaling.Citation53 Collectively, these findings indicate multiple modes of action converge to limit Eph receptor signaling events at epithelial cell-cell contacts.
Eph Receptors and Ephrins in Breast Epithelium
Eph receptor and ephrin expression in the mammary gland
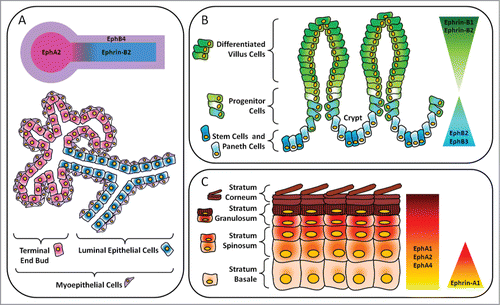
The simple, cuboidal epithelium lining the ducts of the mammary gland undergoes repeated cycles of expansion and regression in response to changes in hormone levels. These epithelial remodeling events rely on stem cells located throughout the epithelial ductal tree giving rise to myoepithelial and luminal cell lineages that populate the mammary gland.Citation54 Multiple Eph receptors and ephrins are expressed in various breast epithelial cell compartments. For example, EphB4 and ephrin-B2 are found in the myoepithelium and luminal epithelium, respectively, while EphA2 is concentrated in terminal end buds (TEBs) ().Citation55-57 The spatiotemporal expression pattern of Eph receptors and ephrins is important for breast epithelial tissue morphogenesis and function.
Figure 2. Layout of major Eph receptors and ephrin regulators in epithelial compartments. (A) Mammary gland. In the luminal epithelial cells lining the ducts, there is expression of ephrin-B2. Adjacent myoepithelial cells express EphB4. EphA2 is expressed in the terminal end bud. (B) Gastrointestinal tract. In the intestines, there is an inverse gradient of EphB and ephrin-B expression along the crypt-villous axis. EphB2 and EphB3 are important for maintenance of stem, progenitor and Paneth cells in the crypts. In the villi, ephrin-B1 and ephrin-B2 maintain the segregation of differentiated cells in the upper regions from precursor cells in the lower regions. (C) Epidermis. Ephrin-A1 is expressed in the progenitor layer, or stratum basale, of human epidermis while EphA1, EphA2 and EphA4 are present throughout all layers and are especially concentrated in the more differentiated suprabasal layers.
Eph receptor gene expression in breast epithelial cell compartments is partially dependent on hormone levels. An inverse correlation between estrogen receptor (ER) and EphA2 levels was found in nontransformed and breast carcinoma cells with estrogen treatment leading to a reduction in EphA2.Citation58,59 In contrast, EphB4 expression was lost in ovariectomized mice and could be restored by exogenous administration of estrogen. This is consistent with the finding that EphB4 expression in myoepithelial cells is tightly regulated during the estrous cycle of mice.Citation57,60 In turn, EphB4 was shown to modulate ER expression and activity in breast cancer cell lines, suggesting a feedback loop between Eph receptor activity and hormonal signaling pathways.Citation61 The hormone-dependent action on Eph receptors is particularly relevant for mammary epithelium remodeling but may also impact non-reproductive tissues where gonadal steroid receptors are expressed, including the skin and gut.Citation62,63
Eph receptor and ephrin function in mammary gland development and homeostasis
Mammary gland expansion and regression are hormone-dependent processes mediated by a variety of signaling factors, including Eph receptors and ephrins. The most dynamic structures in the mammary gland are TEBs as they undergo branching morphogenesis to form extensive ductal networks in response to hormones in preparation for lactation. EphA2 expression is enriched in TEBs compared to ducts.Citation55,56 EphA2 was required for proliferation and hepatocyte growth factor (HGF)-induced branching morphogenesis as post-pubertal mice lacking this receptor exhibited reduced ductal penetration into the fat pad, which is a requirement for TEB formation. These studies suggest that EphA2 is a positive regulator of branching morphogenesis in mammary gland development. However, ligand-dependent EphA2 signaling inhibited HGF-induced branching morphogenesis in a 3-dimensional in vitro model of kidney morphogenesis suggesting EphA2 action in this epithelial remodeling process may be tissue-specific.Citation64
EphB receptors and ephrin-B ligands also play a major role in mammary epithelial tissue homeostasis. In particular, ephrin-B2 has been implicated in the maintenance of mammary epithelial stem cell compartments through control of differentiation.Citation65 Conditional deletion of ephrin-B2 in the mammary epithelium of lactating mice led to a loss of glandular architecture and tissue integrity. Moreover, ephrin-B2-deficient alveolar epithelial cells exhibited impaired cell-cell adhesion with reduced levels of E-cadherin and increased β-catenin in the cytoplasm and nucleus.Citation66 Ephrin-B2 reverse signaling may account for this phenotype as a signaling-deficient mutant of ephrin-B2 lacking its cytoplasmic domain disrupted mammary epithelial cell polarity apparent by mislocalization of the apical junction complex proteins, Par-3 and zonula occludens-1. Ultimately, the loss of ephrin-B2-dependent polarity disrupted the breast epithelial stem cell niche and TEB organization by increasing progenitor cell proliferation and altering differentiation.Citation65 EphB4 is predominantly expressed in the myoepithelial cells lining the outside of the ductal luminal epithelium.Citation57 Ectopic EphB4 expression in breast luminal epithelial cells interfered with differentiation in the mammary epithelial tree by increasing luminal and progenitor cell populations.Citation67 Collectively, these data suggest that spatial regulation of Eph receptor and ephrin expression is critical for signaling events that control the organization of mammary gland epithelial tissue compartments.
Eph receptor and ephrin signaling in breast cancer
Given the key role of Eph receptor and ephrin signaling in proper mammary gland morphogenesis, it is not surprising that these proteins have been associated with malignant breast phenotypes. For example, increased EphA2 expression has been found in aggressive breast cancers and was significantly correlated with decreased overall survival and invasive ductal carcinoma.Citation68 Although breast ductal carcinomas express EphA2 and ephrin-A1, ligand expression is reduced in corresponding lymph node metastases. Breast carcinoma cell line invasiveness also correlated with a loss of ephrin-A1 and mislocalization of EphA2 in the cytoplasm.Citation48,69,70 Furthermore, ectopic expression of EphA2 was sufficient to transform normal breast epithelial cells in a manner that could be reverted by exogenous delivery of ephrin-A1.Citation71 Collectively, these data indicate that EphA2 functions in a ligand-independent manner to promote breast cancer progression and metastasis but this RTK can subsequently normalize breast cancer phenotypes once re-engaged by ephrin-A ligand.
Perhaps underlying its association with aggressive breast cancers, EphA2 induced an estrogen-independent and tamoxifen-resistant growth phenotype in breast cancer cell lines that maintain expression of ER.Citation59,72 In addition, EphA2 conferred resistance to therapy targeting oncogenic human epidermal growth factor receptor 2 (HER2) in breast cancer cells and xenograft tumors.Citation73 Thus, EphA2 exhibits extensive cross-talk with other receptor-mediated signaling pathways to impact breast epithelial tumorigenesis.
The contribution of EphA2 in breast cancer may also be related to its role in modulating cell-cell adhesion complexes. For example, EphA2 was concentrated in the cytoplasm of invasive breast cancer cell lines in association with reduced E-cadherin levels. Silencing of EphA2 normalized E-cadherin-mediated adhesion in these breast cancer cells.Citation48,70 Similarly, EphA2 overexpression was capable of disrupting adherens junctions through a RhoA-mediated mechanism in breast epithelial cells but, in this case, without altering adherens junction protein or phosphorylation levels.Citation74 Since a cytoplasmic truncation EphA2 mutant lacking the C-terminal tail region did not disrupt cell-cell adhesion, this study suggested a direct role for EphA2 downstream signaling events that lead to adherens junction dissolution.
EphA2 can also regulate signaling pathways that alter the organization of the actin cytoskeleton to impact cell motility. For example, ephexin4 is a GEF that activated RhoG-induced chemotaxis of breast cancer cells downstream of EphA2.Citation75 Activated RhoG recruited its effectors ELMO2 and Dock4 into a complex with EphA2 at the leading edges of migrating breast cancer cells. In a separate mechanism for promoting motility in breast cancer cells, EphA2 internalization by MT1-MMP-dependent cleavage enhanced RhoA signaling leading to collapse of cytoskeletal architecture, cell rounding, and single-cell migration.Citation48 This migratory phenotype also depended on a serine 897 residue present on the EphA2 cytoplasmic tail, a site directly phosphorylated by Akt in the absence of ephrin binding.Citation76,77 EphA2 and Akt signaling may also intersect in normal mammary epithelial development since loss of Akt1 and Akt2 in mice disrupted expansion of the epithelial mammary tree during pregnancy and lactation similar to EphA2 loss.Citation55,78-80
The deregulation of EphB and ephrin-B signaling also contributes to breast cancer phenotypes. Specifically, lack of ephrin-B2 reverse signaling was associated with breast cancer progression by interfering with proper development of the mammary gland. Generation of transgenic mice expressing a C-terminal truncation mutant of ephrin-B2 driven by the mouse mammary tumor virus-long-terminal repeat (MMTV-LTR) promoter, which induces expression of mutant ephrin-B2 specifically in the mammary gland, disrupted development and correlated with increased primary tumor formation and lung or liver metastasis.Citation81,82 In contrast, expression of full-length ephrin-B2 did not alter tumor latency and only modestly induced lung metastasis. Similar to the ephrin-B2-null phenotype, mice bearing ephrin-B2 cytoplasmic tail mutations showed marked reduction in the expression of E-cadherin and an increase in nuclear β-catenin in breast epithelial-derived tumors. Furthermore, ectopic expression of EphB4 disrupted proper mammary gland involution following lactation and resulted in enhancement of breast cancer stem cells, decreased tumor latency, and the presence of lung metastases in MMTV-LTR-neuT/ephB4 double transgenic mice but not in MMTV-LTR-ephB4 transgenic mice.Citation82,83 These results indicate that overexpression of EphB4 potentiates the action of oncogenes such as HER2 (neu) during breast cancer progression. Additionally, EphB4 was shown to be a key regulator of oncogenic phenotypes in breast cancer cells since its loss or inhibition reduced survival, anti-apoptotic proteins, migration, invasion, tumor size, and vascularity.Citation84,85 In the breast carcinoma cell lines tested, robust expression of EphB4 correlated with little to no detection of its cognate ligand, ephrin-B2. When ligand-induced receptor activation was restored with recombinant ephrin-B2, there was a significant decrease in cell survival.Citation84,85 Cumulatively, these results suggest that gain of ligand independent EphB4 signaling combined with loss of ephrin-B2 reverse signaling facilitates breast cancer progression.
Additional Eph receptors have also been shown to be altered in breast cancer, including EphA4, EphA7, EphB4 and EphB6, which were significantly correlated with decreased overall survival and invasive ductal carcinoma.Citation68 Epigenetic repression of EphA5 was associated with predictors of poor prognoses including high tumor grade, lymph node metastasis, and progesterone receptor negative status indicative of resistance to endocrine therapy.Citation86 A genome-wide association study of more than 2,000 invasive breast cancer samples and matched controls revealed changes in EphB1, EphA3, and EphA7.Citation87 This study proposed that Eph receptors serve as “driver kinases” that are somatically mutated in breast cancer.
Overall, Eph receptor and ephrin signaling contributes to normal mammary gland development but deregulation of this signaling axis can promote tumorigenesis in breast epithelial cells. Specifically, ligand-independent Eph receptor signaling and reverse signaling through ephrins appears to play a key role in mediating aggressive breast cancer phenotypes with poor clinical prognoses.
Eph Receptors and Ephrins in Gut Epithelium
Eph receptor and ephrin expression in the gastrointestinal tract
The single-layered, mucosal epithelium lining the gut has a high turnover rate that depends on the activity of stem cells located within specific compartments of its crypt-villous structure. In particular, stem cells are present at the bottom of crypts and divide asymmetrically to maintain self-renewal and produce a population of rapidly proliferating progeny. These transient amplifying cells ultimately give rise to differentiated cells along the crypt-villous axis as well as terminally differentiated Paneth cells in the base of the crypt. Interaction between intestinal epithelial stem cells and their daughter cells are important for the maintenance of this stem cell niche.Citation88,89
Human intestinal epithelia contain mRNA transcripts for the majority of Eph receptor and ephrin family members and a few of these have been directly implicated in intestinal epithelial homeostasis.Citation90,91 The general expression pattern of Eph receptors and ephrins is relatively similar in the mucosal epithelium of the small and large intestines.Citation90 In particular, EphB2 and EphB3 expression is concentrated in the cells that populate the crypt nadirs and decreases toward the top of the villi.Citation92-94 Ephrin-B1 and ephrin-B2 are expressed in an inverse manner along villi with highest expression at the apices. This reciprocal gradient of receptor and ligand expression reflects a functional transition zone between proliferative and differentiated epithelial cell compartments within the intestine (). EphA receptors and ephrin-A ligands are also differentially expressed along the crypt-villous axis. For example, EphA1 is found in crypts while EphA2 and ephrin-A1 are present at the top of villi.Citation91 However, a role for EphA and ephrin-A signaling in intestinal epithelial development or architecture has yet to be reported.
Regulation of Eph receptor and ephrin expression in the gut is dependent on several key signaling pathways. In particular, Wnt/β-catenin signaling is required for the maintenance of intestinal epithelial stem cells in crypts and targets EphB receptor gene expression by T-cell specific transcription factor (TCF)/β-catenin complexes.Citation93,95,96 Accordingly, inhibition of TCF/β-catenin-mediated transcription by a dominant-negative TCF factor induced a loss of EphB2 and EphB3 mRNA transcripts while ephrin-B1 mRNA was up-regulated in colorectal cancer cells.Citation97 Krüppel-like factor 5 (KLF5) has also been implicated in the positive regulation of EphB3 expression in the intestinal epithelium.Citation98 Intestinal-specific loss of KLF5 led to reduction in EphB3 and ephrin-B1 expression coincident with severely compromised intestinal crypt architecture and barrier function. This intestinal epithelial defect may stem from aberrant β-catenin signaling as intestine-specific deletion of KLF5 further led to the loss of β-catenin nuclear localization. Transforming growth factor β signaling plays a critical role in wound healing responses of the intestinal mucosal epithelium in a manner that depends somewhat on Smad3-mediated induction of EphB2 and EphB3.Citation99,100 EphB expression in colonic crypts by Smad3 accelerated re-epithelialization by regulating proliferation of intestinal epithelial cells. Hence, loss of EphB expression may account for some of the wound healing defects reported in the intestinal epithelium of Smad3 knockout mice.Citation101 While EphA receptors and ephrin-A ligands are present in the gut, very little is known about how they are regulated in this tissue.
Eph receptor and ephrin function in gut epithelial homeostasis
EphB receptors and ephrin-B ligands have emerged as major regulators of intestinal crypt architecture.Citation92,102 In particular, EphB and ephrin-B signaling is required for positioning and differentiation of intestinal epithelial cells as they transit along the crypt-villous axis. Ablation of EphB signaling in EphB2 and EphB3 double knockout mice decreased progenitor cell proliferation by limiting cell cycle reentry in intestinal crypts. This modification of the progenitor proliferative zone led to altered distribution of cells along intestinal crypts. These findings suggest that differential expression of EphB receptors and ephrin-B ligands provides a contextual cue that maintains the organization of progenitor and differentiated cell compartments in intestinal epithelium.Citation92
Sorting of EphB and ephrin-B expressing cells in the intestinal epithelium was shown to be driven by cell-cell repulsion events through destabilization of E-cadherin.Citation103 EphB-expressing cells in apposition with ephrin-B-expressing cells recruit ADAM10 to cell-cell borders where it cleaves E-cadherin and disrupts adherens junctions. Destabilization of cell-cell adhesion complexes limits intermingling of EphB- and ephrin-B-expressing cell populations, providing a putative molecular mechanism for maintaining epithelial cell compartments in the intestine. In support of this model, loss of ADAM10 in Paneth cells resulted in their improper positioning along the crypt axis.Citation103 This phenotype was also seen following loss of EphB2.Citation104 Interestingly, EphB2 appears to function in a kinase-dependent or -independent manner with distinct biological outcomes in the gut. Kinase-dependent signaling of EphB2 promoted cell cycle entry and proliferation in intestinal crypt progenitor cells, at least in part, through modulation of cyclin-D1 levels and Abl kinase activity.Citation104 In contrast, the ability of EphB2 to maintain stem cell compartments was kinase-independent and reliant on phosphatidylinositol 3-kinase activity.Citation104 Thus, EphB receptors and ephrin-B ligands help segregate progenitor and differentiated cell populations within the intestinal epithelium via modulation of adhesion complexes and downstream signaling pathways.
While EphA2 is not thought to be an essential regulator of intestinal morphogenesis, this RTK subtype may play a role in modulating barrier function in gut epithelium through destabilization of tight junctions.Citation105 Activation of EphA2 with ephrin-A1 promoted interaction between the receptor and claudin-4, a tight junction-associated protein, in several epithelial cell lines including HT29 colon carcinoma cells.Citation105 EphA2 led to claudin-4 phosphorylation and its mislocalization from cell-cell contacts coincident with an increase in paracellular permeability. The negative regulation of tight junctions by EphA2 was contrasted by the reinforcement of the tight junction barrier by ephrin-B1 which itself interacted with claudins-1 and -4 in HT29 cells.Citation106 Thus, different Eph receptor and ephrin subclasses appear to have opposing effects on epithelial tight junction integrity and function in the gut.
As in the intestine, EphB receptors are concentrated in the crypt epithelium while ephrin-B ligands are prominently expressed in the upper villous regions of the stomach. Cells in the isthmus, the region connecting the gastric pits and gastric glands, proliferate and differentiate giving rise to distinct cell lineages that populate the gastric mucosal epithelium. The exact role of Eph receptors and ephrins in the upper regions of the human gastrointestinal tract remains largely unknown. However, EphB and ephrin-B signaling may be important in the transition between stratified squamous and simple columnar epithelium in the murine stomach. In particular, EphB2 is restricted to the junctional zone between these two epithelial compartments while ephrin-B1 occupies the stratified epithelial layers in the stomach.Citation107,108 These findings support the notion that EphB and ephrin-B signaling provide cues in the cellular microenvironment that help orchestrate the organization of structurally and functionally distinct epithelial cell compartments within the gastrointestinal tract.
Eph receptor and ephrin signaling in gastrointestinal disease
As key regulators of intestinal homeostasis, abnormal EphB and ephrin-B expression is linked to malignant and diseased states in the gut.Citation109,110 In the intestinal epithelium of patients with inflammatory bowel disease (IBD), there was a significant up-regulation of ephrin-B2 in lesions with little change in EphB expression.Citation90 Treatment of rat intestinal epithelium cells with a recombinant EphB1 ectodomain to stimulate ephrin-B reverse signaling enhanced scratch wound closure in vitro.Citation90 These findings suggest that ephrin-B2 responds to EphB receptors in order to enhance intestinal epithelial repair and barrier function but it is also possible that ephrin-B2 functions in the absence of EphB receptor stimulation in the gut epithelium of IBD patients.
The complementary expression of EphB receptors and ephrin-B ligands is also perturbed in colorectal cancers. EphB2 and EphB3 are lost during colorectal cancer progression and the extent of down-regulation correlates with higher tumor grade.Citation111 EphB receptor activation was positively associated with E-cadherin-mediated adhesion of colorectal cancer cells, which contributed to their segregation from cells expressing ephrin-B ligand.Citation112 Interestingly, ephrin-B1 loss disrupted the organization of tumor cell compartments and accelerated growth of intestinal tumors that spontaneously develop in Apcmin/+ mice, which may be relevant to patients with hereditary human colorectal cancers with mutations in the adenomatous polyposis coli (APC) tumor suppressor gene.Citation113,114 This study provided additional support for the idea that ephrins present within the tumor microenvironment can create repulsive cues to prohibit Eph receptor-expressing cancer cell growth and invasion.
EphA receptors are also deregulated in gastrointestinal malignancies. Expression of EphA1 and EphA2 is associated with poor prognosis in gastric cancer.Citation115,116 Increased EphA1 expression significantly correlated with increased tumor grade and lymph node metastasis and decreased cumulative survival in gastric carcinomas. Ephrin-A1 mRNA transcripts independently predicted poor prognosis in colorectal cancer patients and knockdown of this ligand inhibited proliferation and migration of colon carcinoma cell lines.Citation117 As in the mammary gland, EphA expression may synergize with oncogenic pathways to enhance cancer progression. Notably, EphA2 and ephrin-A1 differentially impact tumor burden in Apcmin/+ mice: ephrin-A1 promotes tumorigenesis while loss of EphA2 reduces tumor formation.Citation118,119 It is therefore possible that an “Eph-switch” from B type to A type receptors contributes to the malignant transformation of gut epithelia.
Taken together, these data suggest an important role for Eph receptor and ephrin signaling in organizing epithelial stem cell compartments in the gastrointestinal tract through governance of proliferation in crypts and differentiation in villi. Loss of Eph receptor and ephrin signaling that disrupts intestinal epithelial stem cell compartments appears to render the gut more vulnerable to tumorigenic progression.
Eph Receptors and Ephrins in Skin
Eph receptor and ephrin expression in epidermis and hair follicles
The epidermis is composed of a stratified squamous epithelium that undergoes a specialized form of differentiation, termed keratinization. This constantly renewing epithelial tissue depends on progenitor cells in the basal layer to replace epidermal cells lost by desquamation or through injury in the more superficial layers.Citation120 Keratinocytes are not only tightly anchored to their neighbors via junctional complexes but also directly exchange information with one another in a manner that controls their differentiation state.Citation121 Recent studies indicate that Eph receptors and ephrins mediate cell-cell communication in the epidermis to regulate keratinocyte proliferation, migration, adhesion, differentiation, and survival.
While Eph receptors from both subclasses are present in the epidermis, the regulated expression EphA receptors in keratinocytes is somewhat more clear and varies with differentiation.Citation122 In particular, EphA2 is prominently expressed by primary keratinocytes in culture but is markedly down-regulated upon differentiation in vitro.Citation123 In contrast, EphA1 and EphA4 are abundantly expressed in cultured, differentiated keratinocytes and are also found in the suprabasal layers of the epidermis. Interestingly, ephrin-A1 is concentrated in the progenitor cell-containing basal layer of human epidermis ().Citation18,123 This cellular localization pattern suggests that EphA receptors and ephrin-A ligands may play an important role as keratinocytes commit to a pathway of differentiation at the transition between the basal and suprabasal layers.
Several factors have been shown to regulate EphA and ephrin-A gene expression in the epidermis. For example, p63 is a master regulator of epidermal differentiation capable of increasing EphA1 and EphA4 mRNA levels via KLF4 induction, which reflects the suprabasal expression pattern of these RTK subtypes in vivo.Citation124,125 In contrast, EphA2 levels are increased following activation of epidermal growth factor receptor in multiple cell types, including epidermal carcinoma cells; this might explain why receptor levels are kept low under homeostatic conditions in skin.Citation126 Accordingly, inflammatory signals and environmental stress impact epidermal EphA2 expression as this receptor is upregulated in keratinocytes by several pro-inflammatory cytokines and ultraviolet radiation.Citation123,127–129 Interestingly, a cluster of ephrin-A genes (EFNA1, EFNA3, EFNA4) exists on the q arm of chromosome 1 in an area proximal to the epidermal differentiation complex harboring several genes coordinately regulated during keratinization. While ephrin-A1 is a known target of tumor necrosis factor α (TNFα) signaling in many cell types, including keratinocytes, very little is known about what regulates expression of most other ephrins in the epidermis.Citation127
The hair follicle is a skin appendage that is contiguous with the epidermis and undergoes continuous cycling through the stages of anagen (growth), catagen (death), and telogen (rest). This regeneration process relies on hair follicle stem cells located in the bulge region and the hair germ.Citation120,130 Bulge stem cells also participate in the wound healing and repair of the interfollicular epidermis.Citation131 EphA and EphB receptor subtypes are found in the hair follicle with EphA4, EphB4, ephrin-A3, and ephrin-B1 concentrated in the bulge region. Ephrin-A3 and ephrin-B2 are also found in the dermal papilla, a fibroblast-enriched stromal region important for hair follicle cycling events.Citation132–135 Increased ephrin-A mRNA transcripts in mouse skin correlated with the onset of anagen with ephrin-A3 exhibiting the highest expression during this active growth phase.Citation135 The regulated expression of Eph receptors and ephrins in hair follicles and other skin appendages (i.e. sebaceous glands, sweat glands) may play a role in determining cell fate and positioning during the hair cycle or epidermal wound repair, but this remains to be rigorously investigated.
Eph receptor and ephrin function in epidermal homeostasis
To a certain degree, epidermal homeostasis reflects a balance between cell proliferation in the basal layer and cell differentiation and death in the upper layers. Eph receptor and ephrin signaling is emerging as a major pathway that controls these aspects of epidermal homeostasis. For example, the extracellular signal-regulated kinase 1/2 (Erk1/2)-MAPK pathway supports keratinocyte proliferation and must be dampened to allow for cell cycle exit and differentiation.Citation136 EphA2 acts in a ligand-dependent manner to attenuate Erk1/2 signaling in a variety of epithelial cells, including keratinocytes.Citation43,137-139 A role for Eph receptor and ephrin signaling in epidermal growth control is further supported by the observation that injection of recombinant proteins containing ectodomain fragments of Eph receptors or ephrin ligands increased keratinocyte proliferation in the epidermis and hair follicles.Citation140 While Eph receptor and ephrin signaling likely serve to limit keratinocyte proliferation, these RTKs may also play a role in cell survival pathways to eliminate keratinocytes damaged by environmental insults. In particular, EphA2 is up-regulated in keratinocytes following UV radiation and was required for apoptosis.Citation129
Ephrin-A ligands appear to be particularly important for eliciting differentiation in keratinocytes, at least in cell culture models. In particular, delivery of soluble ephrin-A ligand led to keratinocyte stratification and differentiation.Citation141 Specifically, ephrin-A1-mediated activation of EphA2 induced the expression of the desmosomal cadherin desmoglein 1, which was required for strengthening of cell-cell adhesion and robust differentiation. While desmoglein 1 induction represents a major signaling node for efficient execution of a keratinocyte differentiation program, ephrin action in the epidermis is likely to be more complex.Citation142 Accordingly, the profiling of genes regulated by ephrin-A ligands in epidermal keratinocytes revealed targets not only involved in adhesion and differentiation but also proliferation, migration, and proteolysis.Citation143 These studies uncovered subsets of genes that were similarly regulated by ephrin-A3, ephrin -4, and ephrin -5 while ephrin-A1 and ephrin-A2 gene targets were somewhat distinct, highlighting non-redundant roles for the various ephrin members in the epidermis.
Interestingly, reverse signaling via ephrin-B ligands in keratinocytes can also induce differentiation and restrict migration. Treatment of keratinocytes with EphB2-Fc fusion dimers to stimulate ephrin-B ligands led to an upregulation of genes related to differentiation and coincided with a loss of integrins and cell cycle regulators.Citation144 While ephrin-B2 appears to be dispensable in the mouse epidermis, there is a role for dermal ephrin-B2 during the perinatal period of mice through indirect control of keratinocyte proliferation in the basal layer.Citation133 Thus, Eph receptor and ephrin signaling seem to generally provide information about the cellular microenvironment that supports epidermal differentiation even though the specific signaling effectors are likely to vary among receptor and ligand subclasses.
Eph receptor and ephrin signaling in skin disease
Eph/ephrin signaling has been associated with a variety of human skin diseases, including nonmelanoma skin cancers and psoriasis. For example, the epidermis of psoriatic lesions is characterized by hyperplastic keratinocytes that poorly differentiate, leading to the formation of thick, scaly plaques. These lesions show a marked increase in EphA2 levels coincident with a reduction in ephrin-A1 and ephrin-A3 expression.Citation123 Similarly, EphA2 was increased by a variety of growth factors and pro-inflammatory cytokines, including epidermal growth factor, interleukin-1α and TNFα, in a 3-dimensional organotypic culture model of human epidermis. Interestingly, targeting of EphA2 for activation and subsequent downregulation with recombinant ephrin-A1 ligand was capable of normalizing keratinocyte differentiation in this human skin culture model of inflammation. Thus, ephrin-A1 delivery may ultimately prove effective in resolving lesions of patients with psoriasis when combined with treatments that target inflammation.Citation123
EphA receptors also serve as tumor suppressors in the skin. In particular, deletion of EphA2 in mice led to significantly enhanced susceptibility to 2-stage skin carcinogenesis resulting in increased tumor size, number, and invasiveness.Citation137 The highly related EphA1 receptor has also been implicated as a tumor suppressor in skin. Loss of EphA1 expression is associated with poorly differentiated basal and squamous cell carcinomas although the specific role of this particular RTK in normal or transformed keratinocytes remains largely unexplored.Citation122 Moreover, the consequence of ephrin-A ligand loss for skin carcinogenesis merits additional investigation. Defining specific roles for Eph receptor and ephrin signaling complexes in skin will likely reveal pathways that can normalize keratinocyte behavior in disease states such as cancer and psoriasis.
Conclusion
A growing body of work has revealed key roles for Eph receptors and ephrins in the maintenance of epithelial tissue homeostasis, particularly in the breast, gut, and skin. As mediators of a diverse set of epithelial cell responses that impact proliferation, migration, differentiation, survival, and the organization of stem cell compartments, Eph receptors and ephrins must be tightly regulated to prevent their possible contributions to epithelial-derived diseases. Understanding the genetic and epigenetic regulation of these receptors and ligands in epithelial tissues remains an area to be more fully explored and an important hurdle to clear as the expression of Eph receptors and ephrins appears to be organ- and tissue-specific with extensive post-translational control of their cellular levels.
Eph receptors and ephrins represent a large family of signaling proteins that can operate in concert or independent of one another. Functional redundancy and combinatorial interactions between receptor and ligand increases the complexity of their signaling potential; this can complicate interpretation of studies aimed at defining specific roles for Eph receptors and ephrins in epithelial tissue homeostasis. As Eph receptor and ephrin-mediated cell-cell communication is altered in most epithelial-derived cancers, it will be important to elucidate the context-specific signal transduction pathways elicited by Eph receptors and ephrins in various epithelia. Ultimately, Eph receptors and ephrins serve as attractive pharmacological targets for disease as downstream signaling pathways impacted by these surface receptor-ligand complexes help maintain epithelial tissue homeostasis.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported in part by a grant to SG (AR062110) and a Core Center grant to Northwestern University (Skin Disease Research Center, AR057216) from the National Institutes of Health (NIH). BEPW was supported by an NIH-T32 training program fellowship (AR060710).
References
- Gale NW, Holland SJ, Valenzuela DM, Flenniken A, Pan L, Ryan TE, Henkemeyer M, Strebhardt K, Hirai H, Wilkinson DG, et al. Eph receptors and ligands comprise two major specificity subclasses and are reciprocally compartmentalized during embryogenesis. Neuron 1996; 17:9-19; PMID:8755474; http://dx.doi.org/10.1016/S0896-6273(00)80276-7
- Eph Nomenclature Committee. Unified nomenclature for Eph family receptors and their ligands, the ephrins. Cell 1997; 90:403-4; PMID:9267020; http://dx.doi.org/10.1016/S0092-8674(00)80500-0
- Himanen JP, Chumley MJ, Lackmann M, Li C, Barton WA, Jeffrey PD, Vearing C, Geleick D, Feldheim DA, Boyd AW, et al. Repelling class discrimination: ephrin-A5 binds to and activates EphB2 receptor signaling. Nat Neurosci 2004; 7:501-9; PMID:15107857; http://dx.doi.org/10.1038/nn1237
- Alford S, Watson-Hurthig A, Scott N, Carette A, Lorimer H, Bazowski J, Howard PL. Soluble ephrin a1 is necessary for the growth of HeLa and SK-BR3 cells. Cancer Cell Int 2010; 10:1475-2867; http://dx.doi.org/10.1186/1475-2867-10-41
- Beauchamp A, Lively MO, Mintz A, Gibo D, Wykosky J, Debinski W. EphrinA1 is released in three forms from cancer cells by matrix metalloproteases. Mol Cell Biol 2012; 32:3253-64; PMID:22688511; http://dx.doi.org/10.1128/MCB.06791-11
- Himanen JP, Yermekbayeva L, Janes PW, Walker JR, Xu K, Atapattu L, Rajashankar KR, Mensinga A, Lackmann M, Nikolov DB, et al. Architecture of Eph receptor clusters. Proc Natl Acad Sci U S A 2010; 107:10860-5; PMID:20505120; http://dx.doi.org/10.1073/pnas.1004148107
- Janes PW, Nievergall E, Lackmann M. Concepts and consequences of Eph receptor clustering. Semin Cell Dev Biol 2012; 23:43-50; PMID:22261642; http://dx.doi.org/10.1016/j.semcdb.2012.01.001
- Singh A, Winterbottom E, Daar IO. Ephephrin signaling in cell-cell and cell-substrate adhesion. Front Biosci 2012; 17:473-97; http://dx.doi.org/10.2741/3939
- Coulthard MG, Morgan M, Woodruff TM, Arumugam TV, Taylor SM, Carpenter TC, Lackmann M, Boyd AW. EphEphrin signaling in injury and inflammation. Am J Pathol 2012; 181:1493-503; PMID:23021982; http://dx.doi.org/10.1016/j.ajpath.2012.06.043
- Merlos-Suarez A, Batlle E. Eph-ephrin signalling in adult tissues and cancer. Curr Opin Cell Biol 2008; 20:194-200; PMID:18353626; http://dx.doi.org/10.1016/j.ceb.2008.01.011
- Nievergall E, Lackmann M, Janes PW. Eph-dependent cell-cell adhesion and segregation in development and cancer. Cell Mol Life Sci 2012; 69:1813-42; PMID:22204021; http://dx.doi.org/10.1007/s00018-011-0900-6
- Kandouz M. The EphEphrin family in cancer metastasis: communication at the service of invasion. Cancer Metastasis Rev 2012; 31:353-73; PMID:22549394; http://dx.doi.org/10.1007/s10555-012-9352-1
- Frisen J, Barbacid M. Genetic analysis of the role of Eph receptors in the development of the mammalian nervous system. Cell Tissue Res 1997; 290:209-15; PMID:9321682; http://dx.doi.org/10.1007/s004410050925
- Ivanov AI, Romanovsky AA. Putative dual role of ephrin-Eph receptor interactions in inflammation. IUBMB Life 2006; 58:389-94; PMID:16801213; http://dx.doi.org/10.1080/15216540600756004
- Arvanitis D, Davy A. Ephephrin signaling: networks. Genes Dev 2008; 22:416-29; PMID:18281458; http://dx.doi.org/10.1101/gad.1630408
- Miao H, Wang B. Ephephrin signaling in epithelial development and homeostasis. Int J Biochem Cell Biol 2009; 41:762-70; PMID:18761422; http://dx.doi.org/10.1016/j.biocel.2008.07.019
- Gucciardo E, Sugiyama N, Lehti K. Eph- and ephrin-dependent mechanisms in tumor and stem cell dynamics. Cell Mol Life Sci 2014; 4:4.
- Lin S, Wang B, Getsios S. Ephephrin signaling in epidermal differentiation and disease. Semin Cell Dev Biol 2012; 23:92-101; PMID:22040910; http://dx.doi.org/10.1016/j.semcdb.2011.10.017
- Genander M. Eph and ephrins in epithelial stem cell niches and cancer. Cell Adh Migr 2012; 6:126-30; PMID:22568955; http://dx.doi.org/10.4161/cam.18932
- Genander M, Frisen J. Ephrins and Eph receptors in stem cells and cancer. Curr Opin Cell Biol 2010; 22:611-6; PMID:20810264; http://dx.doi.org/10.1016/j.ceb.2010.08.005
- Kalo MS, Pasquale EB. Multiple in vivo tyrosine phosphorylation sites in EphB receptors. Biochemistry 1999; 38:14396-408; PMID:10572014; http://dx.doi.org/10.1021/bi991628t
- Binns KL, Taylor PP, Sicheri F, Pawson T, Holland SJ. Phosphorylation of tyrosine residues in the kinase domain and juxtamembrane region regulates the biological and catalytic activities of Eph receptors. Mol Cell Biol 2000; 20:4791-805; PMID:10848605; http://dx.doi.org/10.1128/MCB.20.13.4791-4805.2000
- Wybenga-Groot LE, Baskin B, Ong SH, Tong J, Pawson T, Sicheri F. Structural basis for autoinhibition of the Ephb2 receptor tyrosine kinase by the unphosphorylated juxtamembrane region. Cell 2001; 106:745-57; PMID:11572780; http://dx.doi.org/10.1016/S0092-8674(01)00496-2
- Bartley TD, Hunt RW, Welcher AA, Boyle WJ, Parker VP, Lindberg RA, Lu HS, Colombero AM, Elliott RL, Guthrie BA, et al. B61 is a ligand for the ECK receptor protein-tyrosine kinase. Nature 1994; 368:558-60; PMID:8139691; http://dx.doi.org/10.1038/368558a0
- Fang WB, Brantley-Sieders DM, Hwang Y, Ham AJ, Chen J. Identification and functional analysis of phosphorylated tyrosine residues within EphA2 receptor tyrosine kinase. J Biol Chem 2008; 283:16017-26; PMID:18387945; http://dx.doi.org/10.1074/jbc.M709934200
- Hock B, Bohme B, Karn T, Feller S, Rubsamen-Waigmann H, Strebhardt K. Tyrosine-614, the major autophosphorylation site of the receptor tyrosine kinase HEK2, functions as multi-docking site for SH2-domain mediated interactions. Oncogene 1998; 17:255-60; PMID:9674711; http://dx.doi.org/10.1038/sj.onc.1201907
- Zisch AH, Kalo MS, Chong LD, Pasquale EB. Complex formation between EphB2 and Src requires phosphorylation of tyrosine 611 in the EphB2 juxtamembrane region. Oncogene 1998; 16:2657-70; PMID:9632142; http://dx.doi.org/10.1038/sj.onc.1201823
- Thanos CD, Goodwill KE, Bowie JU. Oligomeric structure of the human EphB2 receptor SAM domain. Science 1999; 283:833-6; PMID:9933164; http://dx.doi.org/10.1126/science.283.5403.833
- Wimmer-Kleikamp SH, Janes PW, Squire A, Bastiaens PI, Lackmann M. Recruitment of Eph receptors into signaling clusters does not require ephrin contact. J Cell Biol 2004; 164:661-6; PMID:14993233; http://dx.doi.org/10.1083/jcb.200312001
- Sharonov GV, Bocharov EV, Kolosov PM, Astapova MV, Arseniev AS, Feofanov AV. Point mutations in dimerization motifs of transmembrane domain stabilize active or inactive state of the EphA2 receptor tyrosine kinase. J Biol Chem 2014; 14:14.
- Salaita K, Nair PM, Petit RS, Neve RM, Das D, Gray JW, Groves JT. Restriction of receptor movement alters cellular response: physical force sensing by EphA2. Science 2010; 327:1380-5; PMID:20223987; http://dx.doi.org/10.1126/science.1181729
- Janes PW, Griesshaber B, Atapattu L, Nievergall E, Hii LL, Mensinga A, Chheang C, Day BW, Boyd AW, Bastiaens PI, et al. Eph receptor function is modulated by heterooligomerization of A and B type Eph receptors. J Cell Biol 2011; 195:1033-45; PMID:22144690; http://dx.doi.org/10.1083/jcb.201104037
- Fox BP, Kandpal RP. A paradigm shift in EPH receptor interaction: biological relevance of EPHB6 interaction with EPHA2 and EPHB2 in breast carcinoma cell lines. Cancer Genomics Proteomics 2011; 8:185-93; PMID:21737611
- Bruckner K, Pasquale EB, Klein R. Tyrosine phosphorylation of transmembrane ligands for Eph receptors. Science 1997; 275:1640-3; PMID:9054357; http://dx.doi.org/10.1126/science.275.5306.1640
- Palmer A, Zimmer M, Erdmann KS, Eulenburg V, Porthin A, Heumann R, Deutsch U, Klein R. EphrinB phosphorylation and reverse signaling: regulation by Src kinases and PTP-BL phosphatase. Mol Cell 2002; 9:725-37; PMID:11983165; http://dx.doi.org/10.1016/S1097-2765(02)00488-4
- Parker M, Roberts R, Enriquez M, Zhao X, Takahashi T, Pat Cerretti D, Daniel T, Chen J. Reverse endocytosis of transmembrane ephrin-B ligands via a clathrin-mediated pathway. Biochem Biophys Res Commun 2004; 323:17-23; PMID:15351694; http://dx.doi.org/10.1016/j.bbrc.2004.07.209
- Cowan CA, Henkemeyer M. The SH2SH3 adaptor Grb4 transduces B-ephrin reverse signals. Nature 2001; 413:174-9; PMID:11557983; http://dx.doi.org/10.1038/35093123
- Qiu R, Wang J, Tsark W, Lu Q. Essential role of PDZ-RGS3 in the maintenance of neural progenitor cells. Stem Cells 2010; 28:1602-10; PMID:20629178; http://dx.doi.org/10.1002/stem.478
- Lin D, Gish GD, Songyang Z, Pawson T. The carboxyl terminus of B class ephrins constitutes a PDZ domain binding motif. J Biol Chem 1999; 274:3726-33; PMID:9920925; http://dx.doi.org/10.1074/jbc.274.6.3726
- Essmann CL, Martinez E, Geiger JC, Zimmer M, Traut MH, Stein V, Klein R, Acker-Palmer A. Serine phosphorylation of ephrinB2 regulates trafficking of synaptic AMPA receptors. Nat Neurosci 2008; 11:1035-43; PMID:19160501; http://dx.doi.org/10.1038/nn.2171
- Gauthier LR, Robbins SM. Ephrin signaling: One raft to rule them all? One raft to sort them? One raft to spread their call and in signaling bind them? Life Sci 2003; 74:207-16; PMID:14607248; http://dx.doi.org/10.1016/j.lfs.2003.09.029
- Nievergall E, Janes PW, Stegmayer C, Vail ME, Haj FG, Teng SW, Neel BG, Bastiaens PI, Lackmann M. PTP1B regulates Eph receptor function and trafficking. J Cell Biol 2010; 191:1189-203; PMID:21135139; http://dx.doi.org/10.1083/jcb.201005035
- Parri M, Buricchi F, Taddei ML, Giannoni E, Raugei G, Ramponi G, Chiarugi P. EphrinA1 repulsive response is regulated by an EphA2 tyrosine phosphatase. J Biol Chem 2005; 280:34008-18; PMID:16051609; http://dx.doi.org/10.1074/jbc.M502879200
- Boissier P, Chen J, Huynh-Do U. EphA2 signaling following endocytosis: role of Tiam1. Traffic 2013; 14:1255-71; PMID:24112471; http://dx.doi.org/10.1111/tra.12123
- Yoo S, Shin J, Park S. EphA8-ephrinA5 signaling and clathrin-mediated endocytosis is regulated by Tiam-1, a Rac-specific guanine nucleotide exchange factor. Mol Cells 2010; 29:603-9; PMID:20496116; http://dx.doi.org/10.1007/s10059-010-0075-2
- Zhuang G, Hunter S, Hwang Y, Chen J. Regulation of EphA2 receptor endocytosis by SHIP2 lipid phosphatase via phosphatidylinositol 3-Kinase-dependent Rac1 activation. J Biol Chem 2007; 282:2683-94; PMID:17135240; http://dx.doi.org/10.1074/jbc.M608509200
- Janes PW, Wimmer-Kleikamp SH, Frangakis AS, Treble K, Griesshaber B, Sabet O, Grabenbauer M, Ting AY, Saftig P, Bastiaens PI, et al. Cytoplasmic relaxation of active Eph controls ephrin shedding by ADAM10. PLoS Biol 2009; 7:13; http://dx.doi.org/10.1371/journal.pbio.1000215
- Sugiyama N, Gucciardo E, Tatti O, Varjosalo M, Hyytiainen M, Gstaiger M, Lehti K. EphA2 cleavage by MT1-MMP triggers single cancer cell invasion via homotypic cell repulsion. J Cell Biol 2013; 201:467-84; PMID:23629968; http://dx.doi.org/10.1083/jcb.201205176
- Walker-Daniels J, Riese DJ 2nd, Kinch MS. c-Cbl-dependent EphA2 protein degradation is induced by ligand binding. Mol Cancer Res 2002; 1:79-87; PMID:12496371
- Wang Y, Ota S, Kataoka H, Kanamori M, Li Z, Band H, Tanaka M, Sugimura H. Negative regulation of EphA2 receptor by Cbl. Biochem Biophys Res Commun 2002; 296:214-20; PMID:12147253; http://dx.doi.org/10.1016/S0006-291X(02)00806-9
- Kim J, Lee H, Kim Y, Yoo S, Park E, Park S. The SAM domains of Anks family proteins are critically involved in modulating the degradation of EphA receptors. Mol Cell Biol 2010; 30:1582-92; PMID:20100865; http://dx.doi.org/10.1128/MCB.01605-09
- Shin J, Gu C, Park E, Park S. Identification of phosphotyrosine binding domain-containing proteins as novel downstream targets of the EphA8 signaling function. Mol Cell Biol 2007; 27:8113-26; PMID:17875921; http://dx.doi.org/10.1128/MCB.00794-07
- Naudin C, Sirvent A, Leroy C, Larive R, Simon V, Pannequin J, Bourgaux JF, Pierre J, Robert B, Hollande F, et al. SLAP displays tumour suppressor functions in colorectal cancer via destabilization of the SRC substrate EPHA2. Nat Commun 2014; 5:3159; PMID:24457997; http://dx.doi.org/10.1038/ncomms4159
- Smith GH, Chepko G. Mammary epithelial stem cells. Microsc Res Tech 2001; 52:190-203; PMID:11169867; http://dx.doi.org/10.1002/1097-0029(20010115)52:2%3c190::AID-JEMT1005%3e3.0.CO;2-O
- Vaught D, Chen J, Brantley-Sieders DM. Regulation of mammary gland branching morphogenesis by EphA2 receptor tyrosine kinase. Mol Biol Cell 2009; 20:2572-81; PMID:19321667; http://dx.doi.org/10.1091/mbc.E08-04-0378
- Kouros-Mehr H, Werb Z. Candidate regulators of mammary branching morphogenesis identified by genome-wide transcript analysis. Dev Dyn 2006; 235:3404-12; PMID:17039550; http://dx.doi.org/10.1002/dvdy.20978
- Nikolova Z, Djonov V, Zuercher G, Andres AC, Ziemiecki A. Cell-type specific and estrogen dependent expression of the receptor tyrosine kinase EphB4 and its ligand ephrin-B2 during mammary gland morphogenesis. J Cell Sci 1998; 111:2741-51; PMID:9718367
- Zelinski DP, Zantek ND, Walker-Daniels J, Peters MA, Taparowsky EJ, Kinch MS. Estrogen and Myc negatively regulate expression of the EphA2 tyrosine kinase. J Cell Biochem 2002; 85:714-20; PMID:11968011; http://dx.doi.org/10.1002/jcb.10186
- Gokmen-Polar Y, Toroni RA, Hocevar BA, Badve S, Zhao Q, Shen C, Bruckheimer E, Kinch MS, Miller KD. Dual targeting of EphA2 and ER restores tamoxifen sensitivity in EREphA2-positive breast cancer. Breast Cancer Res Treat 2011; 127:375-84; PMID:20602165; http://dx.doi.org/10.1007/s10549-010-1004-y
- Andres AC, Reid HH, Zurcher G, Blaschke RJ, Albrecht D, Ziemiecki A. Expression of two novel eph-related receptor protein tyrosine kinases in mammary gland development and carcinogenesis. Oncogene 1994; 9:1461-7; PMID:8152808
- Schmitt F, Nguyen PH, Gupta N, Mayer D. Eph receptor B4 is a regulator of estrogen receptor alpha in breast cancer cells. J Recept Signal Transduct Res 2013; 33:244-8; PMID:23725356; http://dx.doi.org/10.3109/10799893.2013.795971
- Papaxoinis K, Triantafyllou K, Sasco AJ, Nicolopoulou-Stamati P, Ladas SD. Subsite-specific differences of estrogen receptor beta expression in the normal colonic epithelium: implications for carcinogenesis and colorectal cancer epidemiology. Eur J Gastroenterol Hepatol 2010; 22:614-9; PMID:20173645; http://dx.doi.org/10.1097/MEG.0b013e328335ef50
- Thornton MJ. The biological actions of estrogens on skin. Exp Dermatol 2002; 11:487-502; PMID:12473056; http://dx.doi.org/10.1034/j.1600-0625.2002.110601.x
- Miao H, Nickel CH, Cantley LG, Bruggeman LA, Bennardo LN, Wang B. EphA kinase activation regulates HGF-induced epithelial branching morphogenesis. J Cell Biol 2003; 162:1281-92; PMID:14517207; http://dx.doi.org/10.1083/jcb.200304018
- Kaenel P, Antonijevic M, Richter S, Kuchler S, Sutter N, Wotzkow C, Strange R, Andres AC. Deregulated ephrin-B2 signaling in mammary epithelial cells alters the stem cell compartment and interferes with the epithelial differentiation pathway. Int J Oncol 2012; 40:357-69; PMID:22020958
- Weiler S, Rohrbach V, Pulvirenti T, Adams R, Ziemiecki A, Andres AC. Mammary epithelial-specific knockout of the ephrin-B2 gene leads to precocious epithelial cell death at lactation. Dev Growth Differ 2009; 51:809-19; PMID:19843150; http://dx.doi.org/10.1111/j.1440-169X.2009.01140.x
- Kaenel P, Hahnewald S, Wotzkow C, Strange R, Andres AC. Overexpression of EphB4 in the mammary epithelium shifts the differentiation pathway of progenitor cells and promotes branching activity and vascularization. Dev Growth Differ 2014; 56:255-75; PMID:24635767; http://dx.doi.org/10.1111/dgd.12126
- Brantley-Sieders DM, Jiang A, Sarma K, Badu-Nkansah A, Walter DL, Shyr Y, Chen J. Ephephrin profiling in human breast cancer reveals significant associations between expression level and clinical outcome. PLoS One 2011; 6:15; http://dx.doi.org/10.1371/journal.pone.0024426
- Fox BP, Kandpal RP. Invasiveness of breast carcinoma cells and transcript profile: Eph receptors and ephrin ligands as molecular markers of potential diagnostic and prognostic application. Biochem Biophys Res Commun 2004; 318:882-92; PMID:15147954; http://dx.doi.org/10.1016/j.bbrc.2004.04.102
- Zantek ND, Azimi M, Fedor-Chaiken M, Wang B, Brackenbury R, Kinch MS. E-cadherin regulates the function of the EphA2 receptor tyrosine kinase. Cell Growth Differ 1999; 10:629-38; PMID:10511313
- Zelinski DP, Zantek ND, Stewart JC, Irizarry AR, Kinch MS. EphA2 overexpression causes tumorigenesis of mammary epithelial cells. Cancer Res 2001; 61:2301-6; PMID:11280802
- Lu M, Miller KD, Gokmen-Polar Y, Jeng MH, Kinch MS. EphA2 overexpression decreases estrogen dependence and tamoxifen sensitivity. Cancer Res 2003; 63:3425-9; PMID:12810680
- Zhuang G, Brantley-Sieders DM, Vaught D, Yu J, Xie L, Wells S, Jackson D, Muraoka-Cook R, Arteaga C, Chen J. Elevation of receptor tyrosine kinase EphA2 mediates resistance to trastuzumab therapy. Cancer Res 2010; 70:299-308; PMID:20028874; http://dx.doi.org/10.1158/0008-5472.CAN-09-1845
- Fang WB, Ireton RC, Zhuang G, Takahashi T, Reynolds A, Chen J. Overexpression of EPHA2 receptor destabilizes adherens junctions via a RhoA-dependent mechanism. J Cell Sci 2008; 121:358-68; PMID:18198190; http://dx.doi.org/10.1242/jcs.017145
- Hiramoto-Yamaki N, Takeuchi S, Ueda S, Harada K, Fujimoto S, Negishi M, Katoh H. Ephexin4 and EphA2 mediate cell migration through a RhoG-dependent mechanism. J Cell Biol 2010; 190:461-77; PMID:20679435; http://dx.doi.org/10.1083/jcb.201005141
- Miao H, Li DQ, Mukherjee A, Guo H, Petty A, Cutter J, Basilion JP, Sedor J, Wu J, Danielpour D, et al. EphA2 mediates ligand-dependent inhibition and ligand-independent promotion of cell migration and invasion via a reciprocal regulatory loop with Akt. Cancer Cell 2009; 16:9-20; PMID:19573808; http://dx.doi.org/10.1016/j.ccr.2009.04.009
- Kawai H, Kobayashi M, Hiramoto-Yamaki N, Harada K, Negishi M, Katoh H. Ephexin4-mediated promotion of cell migration and anoikis resistance is regulated by serine 897 phosphorylation of EphA2. FEBS Open Bio 2013; 3:78-82; PMID:23772378; http://dx.doi.org/10.1016/j.fob.2013.01.002
- Maroulakou IG, Oemler W, Naber SP, Klebba I, Kuperwasser C, Tsichlis PN. Distinct roles of the three Akt isoforms in lactogenic differentiation and involution. J Cell Physiol 2008; 217:468-77; PMID:18561256; http://dx.doi.org/10.1002/jcp.21518
- Boxer RB, Stairs DB, Dugan KD, Notarfrancesco KL, Portocarrero CP, Keister BA, Belka GK, Cho H, Rathmell JC, Thompson CB, et al. Isoform-specific requirement for Akt1 in the developmental regulation of cellular metabolism during lactation. Cell Metab 2006; 4:475-90; PMID:17141631; http://dx.doi.org/10.1016/j.cmet.2006.10.011
- Chen CC, Boxer RB, Stairs DB, Portocarrero CP, Horton RH, Alvarez JV, Birnbaum MJ, Chodosh LA. Akt is required for Stat5 activation and mammary differentiation. Breast Cancer Res 2010; 12:17; http://dx.doi.org/10.1186/bcr2781
- Haldimann M, Custer D, Munarini N, Stirnimann C, Zurcher G, Rohrbach V, Djonov V, Ziemiecki A, Andres AC. Deregulated ephrin-B2 expression in the mammary gland interferes with the development of both the glandular epithelium and vasculature and promotes metastasis formation. Int J Oncol 2009; 35:525-36; PMID:19639173
- Kaenel P, Schwab C, Mulchi K, Wotzkow C, Andres AC. Preponderance of cells with stem cell characteristics in metastasising mouse mammary tumours induced by deregulated EphB4 and ephrin-B2 expression. Int J Oncol 2011; 38:151-60; PMID:21109936
- Munarini N, Jager R, Abderhalden S, Zuercher G, Rohrbach V, Loercher S, Pfanner-Meyer B, Andres AC, Ziemiecki A. Altered mammary epithelial development, pattern formation and involution in transgenic mice expressing the EphB4 receptor tyrosine kinase. J Cell Sci 2002; 115:25-37; PMID:11801721
- Kumar SR, Singh J, Xia G, Krasnoperov V, Hassanieh L, Ley EJ, Scehnet J, Kumar NG, Hawes D, Press MF, et al. Receptor tyrosine kinase EphB4 is a survival factor in breast cancer. Am J Pathol 2006; 169:279-93; PMID:16816380; http://dx.doi.org/10.2353/ajpath.2006.050889
- Rutkowski R, Mertens-Walker I, Lisle JE, Herington AC, Stephenson SA. Evidence for a dual function of EphB4 as tumor promoter and suppressor regulated by the absence or presence of the ephrin-B2 ligand. Int J Cancer 2012; 131:11; http://dx.doi.org/10.1002/ijc.27392
- Fu DY, Wang ZM, Wang BL, Chen L, Yang WT, Shen ZZ, Huang W, Shao ZM. Frequent epigenetic inactivation of the receptor tyrosine kinase EphA5 by promoter methylation in human breast cancer. Hum Pathol 2010; 41:48-58; PMID:19733895; http://dx.doi.org/10.1016/j.humpath.2009.06.007
- Bonifaci N, Gorski B, Masojc B, Wokolorczyk D, Jakubowska A, Debniak T, Berenguer A, Serra Musach J, Brunet J, Dopazo J, et al. Exploring the link between germline and somatic genetic alterations in breast carcinogenesis. PLoS One 2010; 5:0014078; http://dx.doi.org/10.1371/journal.pone.0014078
- Clevers H, Batlle E. SnapShot: the intestinal crypt. Cell 2013; 152:1198; PMID:23452862; http://dx.doi.org/10.1016/j.cell.2013.02.030
- Clevers H. The intestinal crypt, a prototype stem cell compartment. Cell 2013; 154:274-84; PMID:23870119
- Hafner C, Meyer S, Langmann T, Schmitz G, Bataille F, Hagen I, Becker B, Roesch A, Rogler G, Landthaler M, et al. Ephrin-B2 is differentially expressed in the intestinal epithelium in Crohn's disease and contributes to accelerated epithelial wound healing in vitro. World J Gastroenterol 2005; 11:4024-31; PMID:15996027
- Kosinski C, Li VS, Chan AS, Zhang J, Ho C, Tsui WY, Chan TL, Mifflin RC, Powell DW, Yuen ST, et al. Gene expression patterns of human colon tops and basal crypts and BMP antagonists as intestinal stem cell niche factors. Proc Natl Acad Sci U S A 2007; 104:15418-23; PMID:17881565; http://dx.doi.org/10.1073/pnas.0707210104
- Holmberg J, Genander M, Halford MM, Anneren C, Sondell M, Chumley MJ, Silvany RE, Henkemeyer M, Frisen J. EphB receptors coordinate migration and proliferation in the intestinal stem cell niche. Cell 2006; 125:1151-63; PMID:16777604; http://dx.doi.org/10.1016/j.cell.2006.04.030
- Batlle E, Henderson JT, Beghtel H, van den Born MM, Sancho E, Huls G, Meeldijk J, Robertson J, van de Wetering M, Pawson T, et al. Beta-catenin and TCF mediate cell positioning in the intestinal epithelium by controlling the expression of EphBephrinB. Cell 2002; 111:251-63; PMID:12408869; http://dx.doi.org/10.1016/S0092-8674(02)01015-2
- George MD, Wehkamp J, Kays RJ, Leutenegger CM, Sabir S, Grishina I, Dandekar S, Bevins CL. In vivo gene expression profiling of human intestinal epithelial cells: analysis by laser microdissection of formalin fixed tissues. BMC Genomics 2008; 9:209.: 10.1186471-2164-9-209; PMID:18457593; http://dx.doi.org/10.1186/1471-2164-9-209
- Kuhl SJ, Kuhl M. On the role of Wntbeta-catenin signaling in stem cells. Biochim Biophys Acta 2013; 2:16
- Sancho E, Batlle E, Clevers H. Live and let die in the intestinal epithelium. Curr Opin Cell Biol 2003; 15:763-70; PMID:14644203; http://dx.doi.org/10.1016/j.ceb.2003.10.012
- van de Wetering M, Sancho E, Verweij C, de Lau W, Oving I, Hurlstone A, van der Horn K, Batlle E, Coudreuse D, Haramis AP, et al. The β-cateninTCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell 2002; 111:241-50; PMID:12408868; http://dx.doi.org/10.1016/S0092-8674(02)01014-0
- McConnell BB, Kim SS, Yu K, Ghaleb AM, Takeda N, Manabe I, Nusrat A, Nagai R, Yang VW. Kruppel-like factor 5 is important for maintenance of crypt architecture and barrier function in mouse intestine. Gastroenterology 2011; 141:1302-13; PMID:21763241; http://dx.doi.org/10.1053/j.gastro.2011.06.086
- Beck PL, Rosenberg IM, Xavier RJ, Koh T, Wong JF, Podolsky DK. Transforming growth factor-beta mediates intestinal healing and susceptibility to injury in vitro and in vivo through epithelial cells. Am J Pathol 2003; 162:597-608; PMID:12547717; http://dx.doi.org/10.1016/S0002-9440(10)63853-9
- Furukawa K, Sato T, Katsuno T, Nakagawa T, Noguchi Y, Tokumasa A, Yokote K, Yokosuka O, Saito Y. Smad3 contributes to positioning of proliferating cells in colonic crypts by inducing EphB receptor protein expression. Biochem Biophys Res Commun 2011; 405:521-6; PMID:21276420; http://dx.doi.org/10.1016/j.bbrc.2011.01.045
- Owen CR, Yuan L, Basson MD. Smad3 knockout mice exhibit impaired intestinal mucosal healing. Lab Invest 2008; 88:1101-9; PMID:18711354; http://dx.doi.org/10.1038/labinvest.2008.77
- Richmond CA, Breault DT. Regulation of gene expression in the intestinal epithelium. Prog Mol Biol Transl Sci 2010; 96:207-29; PMID:21075346; http://dx.doi.org/10.1016/B978-0-12-381280-3.00009-9
- Solanas G, Cortina C, Sevillano M, Batlle E. Cleavage of E-cadherin by ADAM10 mediates epithelial cell sorting downstream of EphB signalling. Nat Cell Biol 2011; 13:1100-7; PMID:21804545; http://dx.doi.org/10.1038/ncb2298
- Genander M, Halford MM, Xu NJ, Eriksson M, Yu Z, Qiu Z, Martling A, Greicius G, Thakar S, Catchpole T, et al. Dissociation of EphB2 signaling pathways mediating progenitor cell proliferation and tumor suppression. j 2009; 139:679-92
- Tanaka M, Kamata R, Sakai R. EphA2 phosphorylates the cytoplasmic tail of Claudin-4 and mediates paracellular permeability. J Biol Chem 2005; 280:42375-82; PMID:16236711; http://dx.doi.org/10.1074/jbc.M503786200
- Tanaka M, Kamata R, Sakai R. Phosphorylation of ephrin-B1 via the interaction with claudin following cell-cell contact formation. Embo J 2005; 24:3700-11; PMID:16211011; http://dx.doi.org/10.1038/sj.emboj.7600831
- Ogawa K, Takemoto N, Ishii M, Pasquale EB, Nakajima T. Complementary expression and repulsive signaling suggest that EphB receptors and ephrin-B ligands control cell positioning in the gastric epithelium. Histochem Cell Biol 2011; 136:617-36; PMID:21959989; http://dx.doi.org/10.1007/s00418-011-0867-2
- Ogawa K, Saeki N, Igura Y, Hayashi Y. Complementary expression and repulsive signaling suggest that EphB2 and ephrin-B1 are possibly involved in epithelial boundary formation at the squamocolumnar junction in the rodent stomach. Histochem Cell Biol 2013; 140:659-75; PMID:23881165; http://dx.doi.org/10.1007/s00418-013-1129-2
- Liu W, Ahmad SA, Jung YD, Reinmuth N, Fan F, Bucana CD, Ellis LM. Coexpression of ephrin-Bs and their receptors in colon carcinoma. Cancer 2002; 94:934-9; PMID:11920461; http://dx.doi.org/10.1002/cncr.10122
- Herath NI, Boyd AW. The role of Eph receptors and ephrin ligands in colorectal cancer. Int J Cancer 2010; 126:2003-11; PMID:20039322; http://dx.doi.org/10.1002ijc.25147
- Batlle E, Bacani J, Begthel H, Jonkheer S, Gregorieff A, van de Born M, Malats N, Sancho E, Boon E, Pawson T, et al. EphB receptor activity suppresses colorectal cancer progression. Nature 2005; 435:1126-30; PMID:15973414; http://dx.doi.org/10.1038/nature03626
- Cortina C, Palomo-Ponce S, Iglesias M, Fernandez-Masip JL, Vivancos A, Whissell G, Huma M, Peiro N, Gallego L, Jonkheer S, et al. EphB-ephrin-B interactions suppress colorectal cancer progression by compartmentalizing tumor cells. Nat Genet 2007; 39:1376-83; PMID:17906625; http://dx.doi.org/10.1038/ng.2007.11
- Moser AR, Pitot HC, Dove WF. A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science 1990; 247:322-4; PMID:2296722; http://dx.doi.org/10.1126/science.2296722
- Kinzler KW, Vogelstein B. Lessons from hereditary colorectal cancer. Cell 1996; 87:159-70; PMID:8861899; http://dx.doi.org/10.1016/S0092-8674(00)81333-1
- Huang J, Xiao D, Li G, Ma J, Chen P, Yuan W, Hou F, Ge J, Zhong M, Tang Y, et al. EphA2 promotes epithelial-mesenchymal transition through the Wntbeta-catenin pathway in gastric cancer cells. Oncogene 2014; 33:2737-47. Epub Jun 10; PMID:23752181; http://dx.doi.org/10.1038/onc.2013.238
- Wang J, Dong Y, Wang X, Ma H, Sheng Z, Li G, Lu G, Sugimura H, Zhou X. Expression of EphA1 in gastric carcinomas is associated with metastasis and survival. Oncol Rep 2010; 24:1577-84; PMID:21042754
- Yamamoto H, Tei M, Uemura M, Takemasa I, Uemura Y, Murata K, Fukunaga M, Ohue M, Ohnishi T, Ikeda K, et al. Ephrin-A1 mRNA is associated with poor prognosis of colorectal cancer. Int J Oncol 2013; 42:549-55; PMID:23258614
- Shi L, Itoh F, Itoh S, Takahashi S, Yamamoto M, Kato M. Ephrin-A1 promotes the malignant progression of intestinal tumors in Apc(min+) mice. Oncogene 2008; 27:3265-73. Epub 2008 Feb 4; PMID:18246128; http://dx.doi.org/10.1038/sj.onc.1210992
- Bogan C, Chen J, O’Sullivan MG, Cormier RT. Loss of EphA2 receptor tyrosine kinase reduces ApcMin +tumorigenesis. Int J Cancer 2009; 124:1366-71; PMID:19089910; http://dx.doi.org/10.1002/ijc.24083
- Cotsarelis G, Sun T, Lavker R. Label-retaining cells reside in the bulge area of pilosebaceous unit: Implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell 1990; 61:1328-37; http://dx.doi.org/10.1016/0092-8674(90)90696-C
- Simpson CL, Patel DM, Green KJ. Deconstructing the skin: cytoarchitectural determinants of epidermal morphogenesis. Nat Rev Mol Cell Biol 2011; 12:565-80; PMID:21860392; http://dx.doi.org/10.1038/nrm3175
- Hafner C, Becker B, Landthaler M, Vogt T. Expression profile of Eph receptors and ephrin ligands in human skin and downregulation of EphA1 in nonmelanoma skin cancer. Mod Pathol 2006; 19:1369-77; PMID:16862074; http://dx.doi.org/10.1038/modpathol.3800660
- Gordon K, Kochkodan JJ, Blatt H, Lin SY, Kaplan N, Johnston A, Swindell WR, Hoover P, Schlosser BJ, Elder JT, et al. Alteration of the EphA2Ephrin-A signaling axis in psoriatic epidermis. J Invest Dermatol 2013; 133:712-22; PMID:23190894; http://dx.doi.org/10.1038/jid.2012.391
- Truong AB, Kretz M, Ridky TW, Kimmel R, Khavari PA. p63 regulates proliferation and differentiation of developmentally mature keratinocytes. Genes Dev 2006; 20:3185-97; PMID:17114587; http://dx.doi.org/10.1101/gad.1463206
- Sen GL, Boxer LD, Webster DE, Bussat RT, Qu K, Zarnegar BJ, Johnston D, Siprashvili Z, Khavari PA. ZNF750 is a p63 target gene that induces KLF4 to drive terminal epidermal differentiation. Dev Cell 2012; 22:669-77; PMID:22364861; http://dx.doi.org/10.1016/j.devcel.2011.12.001
- Larsen AB, Pedersen MW, Stockhausen MT, Grandal MV, van Deurs B, Poulsen HS. Activation of the EGFR gene target EphA2 inhibits epidermal growth factor-induced cancer cell motility. Mol Cancer Res 2007; 5:283-93; PMID:17374733; http://dx.doi.org/10.1158/1541-7786.MCR-06-0321
- Banno T, Gazel A, Blumenberg M. Effects of tumor necrosis factor-alpha (TNF alpha) in epidermal keratinocytes revealed using global transcriptional profiling. J Biol Chem 2004; 279:32633-42; PMID:15145954; http://dx.doi.org/10.1074/jbc.M400642200
- Banno T, Gazel A, Blumenberg M. Pathway-specific profiling identifies the NF-kappa B-dependent tumor necrosis factor alpha-regulated genes in epidermal keratinocytes. J Biol Chem 2005; 280:18973-80. Epub 2005 Feb 18; PMID:15722350; http://dx.doi.org/10.1074/jbc.M411758200
- Zhang G, Njauw CN, Park JM, Naruse C, Asano M, Tsao H. EphA2 is an essential mediator of UV radiation-induced apoptosis. Cancer Res 2008; 68:1691-6; PMID:18339848; http://dx.doi.org/10.1158/0008-5472.CAN-07-2372
- Lavker RM, Sun TT, Oshima H, Barrandon Y, Akiyama M, Ferraris C, Chevalier G, Favier B, Jahoda CA, Dhouailly D, et al. Hair follicle stem cells. J Investig Dermatol Symp Proc 2003; 8:28-38; PMID:12894992; http://dx.doi.org/10.1046/j.1523-1747.2003.12169.x
- Blanpain C, Fuchs E. Epidermal homeostasis: a balancing act of stem cells in the skin. Nat Rev Mol Cell Biol 2009; 10:207-17; PMID:19209183; http://dx.doi.org/10.1038/nrm2636
- Midorikawa T, Chikazawa T, Yoshino T, Takada K, Arase S. Different gene expression profile observed in dermal papilla cells related to androgenic alopecia by DNA macroarray analysis. J Dermatol Sci 2004; 36:25-32; PMID:15488702; http://dx.doi.org/10.1016/j.jdermsci.2004.05.001
- Egawa G, Osawa M, Uemura A, Miyachi Y, Nishikawa S. Transient expression of ephrin b2 in perinatal skin is required for maintenance of keratinocyte homeostasis. J Invest Dermatol 2009; 129:2386-95; PMID:19571816; http://dx.doi.org/10.1038/jid.2009.105
- Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, Fuchs E. Defining the epithelial stem cell niche in skin. Science 2004; 303:359-63; PMID:14671312; http://dx.doi.org/10.1126/science.1092436
- Yamada Y, Midorikawa T, Oura H, Yoshino T, Ohdera M, Kubo Y, Arase S. Ephrin-A3 not only increases the density of hair follicles but also accelerates anagen development in neonatal mice. J Dermatol Sci 2008; 52:178-85; PMID:18640011; http://dx.doi.org/10.1016/j.jdermsci.2008.05.007
- Dumesic PA, Scholl FA, Barragan DI, Khavari PA. Erk12 MAP kinases are required for epidermal G2M progression. J Cell Biol 2009; 185:409-22; PMID:19414607; http://dx.doi.org/10.1083/jcb.200804038
- Guo H, Miao H, Gerber L, Singh J, Denning MF, Gilliam AC, Wang B. Disruption of EphA2 receptor tyrosine kinase leads to increased susceptibility to carcinogenesis in mouse skin. Cancer Res 2006; 66:7050-8; PMID:16849550; http://dx.doi.org/10.1158/0008-5472.CAN-06-0004
- Petty A, Myshkin E, Qin H, Guo H, Miao H, Tochtrop GP, Hsieh JT, Page P, Liu L, Lindner DJ, et al. A small molecule agonist of EphA2 receptor tyrosine kinase inhibits tumor cell migration in vitro and prostate cancer metastasis in vivo. PLoS One 2012; 7:15; http://dx.doi.org/10.1371/journal.pone.0042120
- Lema Tome CM, Palma E, Ferluga S, Lowther WT, Hantgan R, Wykosky J, Debinski W. Structural and functional characterization of monomeric EphrinA1 binding site to EphA2 receptor. J Biol Chem 2012; 287:14012-22; PMID:22362770; http://dx.doi.org/10.1074/jbc.M111.311670
- Genander M, Holmberg J, Frisen J. Ephrins negatively regulate cell proliferation in the epidermis and hair follicle. Stem Cells 2010; 28:1196-205; PMID:20506314
- Lin S, Gordon K, Kaplan N, Getsios S. Ligand targeting of EphA2 enhances keratinocyte adhesion and differentiation via desmoglein 1. Mol Biol Cell 2010; 21:3902-14; PMID:20861311; http://dx.doi.org/10.1091/mbc.E10-03-0242
- Getsios S, Simpson CL, Kojima S, Harmon R, Sheu LJ, Dusek RL, Cornwell M, Green KJ. Desmoglein 1-dependent suppression of EGFR signaling promotes epidermal differentiation and morphogenesis. J Cell Biol 2009; 185:1243-58; PMID:19546243; http://dx.doi.org/10.1083/jcb.200809044
- Walsh R, Blumenberg M. Specific and shared targets of ephrin A signaling in epidermal keratinocytes. J Biol Chem 2011; 286:9419-28; PMID:21193391; http://dx.doi.org/10.1074/jbc.M110.197087
- Walsh R, Blumenberg M. Eph-2B, acting as an extracellular ligand, induces differentiation markers in epidermal keratinocytes. J Cell Physiol 2012; 227:2330-40; PMID:21809346; http://dx.doi.org/10.1002/jcp.22968
