Abstract
Elongation of the efferent fibers of dorsal root ganglion (DRG) neurons toward their peripheral targets occurs during development. Attractive or permissive systems may be involved in this elongation. However, the molecular mechanisms that control it are largely unknown. Here we show that class 5 semaphorin Sema5A had attractive/permissive effects on DRG axons. In mouse embryos, Sema5A was expressed in and around the path of DRG efferent fibers, and cell aggregates secreting Sema5A attracted DRG axons in vitro. We also found that ectopic Sema5A expression in the spinal cord attracted DRG axons. Together, these findings suggest that Sema5A functions as an attractant to elongate DRG fibers and contributes to the formation of the early sensory network.
Growing axons navigate along specific appropriate pathways to reach their targets. During their elongation, axons are guided by attractive and repulsive cues.Citation1 At the initial stages of dorsal root ganglion (DRG) axonal growth, both their afferent and efferent fibers respond to the same combination of repulsive and attractive cues that orient them toward their targets. Thus, DRG afferent fibers project to a restricted region of the dorsal spinal cord called the “dorsal root entry zone (DREZ)” by unknown chemoattractive cues.Citation2 Whereas DRG efferent fibers grow peripherally to the musculature and skin, guided in part by the repulsive activity of semaphorin 3A (Sema3A).Citation3-5 However, the molecular mechanism that helps DRG neurons elongate their efferent fibers to reach their peripheral targets is not known.
In vertebrates, 2 class 5 semaphorins (Sema5A and Sema5B) have been described. They are known to be membrane-bound semaphorins, although a recent report revealed that Sema5A also has a secreted form.Citation6 A previous study showed that Sema5A acts as an attractant and repellent for midbrain axons by interacting heparan sulfate proteoglycans and chondroitin sulfate proteoglycans (CSPGs), respectively.Citation7 More recently, another group revealed that Sema5B is a repellent of DRG axons innervating the spinal cord.Citation8 On the other hand, the effect of Sema5A on DRG axonal guidance is not clear.
In order to study the involvement of Sema5A in DRG axonal projections, we first examined DRG axonal trajectories in mouse embryos by immunohistochemistry with anti-class III β-tubulin antibody (Tuj1, Promega Corp.) and the mRNA expression of Sema5A by using a digoxygenin-labeled cRNA probe. At embryonic day (E) 10.5, DRG neurons extended their afferent fibers toward the DREZ (). At E12.5, these fibers entered the spinal cord and elongated rostrocaudally to form the dorsal horn (). DRG efferent fibers divided into dorsal and ventral rami, and then projected toward their peripheral targets together with motor fibers (). At E10.5, Sema5A expression was detected in the mesenchymal tissue around the DRG neurons including that along the efferent pathway of DRG fibers, but not in the mesenchyme along the afferent one (). Based on the fact that both DRG neurons and cells adjacent to the DREZ are Islet-1/2-positive,Citation9 we further examined whether Sema5A was expressed in DRG neurons and the DREZ by staining with an anti-Islet-1/2 antibody (39.4D5; Developmental Studies Hybridoma Bank). In double-stained sections, the expression patterns of Islet-1/2 and Sema5A did not overlap each other in DRG neurons or in cells around the DREZ (). The same expression pattern was observed in the E12.5 mouse embryo. Sema5A expression continued where DRG efferent fibers proceeded to elongate (). In addition, we examined the expression pattern of Sema5A mRNA in the chick embryo by using a cRNA probe for chick Sema5A. We used stage 22 and 29 chick thoracic segments because the developmental stages of E10.5 and E12.5 mouse embryos correspond to those of stage 22 and 29 chick embryos, respectively.Citation4,5,10 We confirmed the same expression pattern of Sema5A in the chick embryo (Fig. S1).
Figure 1. The expression pattern of Sema5A and Sema5B in the mouse embryo. (A and B) DRG axonal trajectories were visualized by Tuj1 staining. Transverse sections at the thoracic level of the mouse embryo at E10.5 (A) and E12.5 (B), visualizing the neural network of DRG afferent fibers (a), efferent fibers (e), and motor fibers (m). White arrowheads indicate the DREZ. (C and D) At E10.5 and E12.5, Sema5A was expressed in the mesenchymal tissues around DRG neurons (blue) where DRG efferent fibers extended. (E and F) In situ hybridization sections adjacent to C and D were labeled with anti-Islet-1/2 antibody (brown). Islet-1/2 immunohistochemistry was conducted to show DRG cells and cells adjacent to the DREZ (black arrowheads). (G) At E10.5, Sema5B was expressed broadly in the spinal cord. Sema5B signals were also detected in the dermamyotome (DM). (H) At E12.5, Sema5B was expressed in the lateral edges of the ventricular zone. The dotted lines indicate the border of the spinal cord (SC). DH, dorsal horn; dr, dorsal ramus; MN, spinal motor neurons; NC, notochord; vr, ventral ramus. (Scale bars, 200 μm).
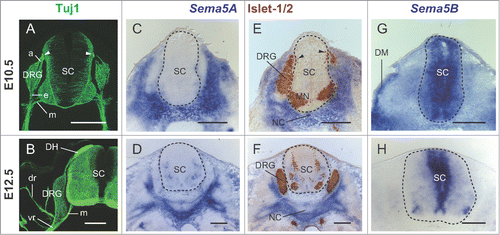
Based on the fact that Sema5B, another member of the Sema5 family in vertebrates, can repel DRG axons at early stages,Citation8 we further examined the expression pattern of Sema5B mRNA in order to compare it with that of Sema5A. Consistent with that previous report,Citation8 Sema5B was observed to be broadly distributed in the spinal cord at E10.5 (). At E12.5, Sema5B expression was restricted in the lateral edges of the ventricular zone in the spinal cord (). The finding that Sema5A and Sema5B showed complementary expression patterns in and around the developing spinal cord suggested the possibility that Sema5A and Sema5B might function differently with respect to DRG axonal guidance.
To investigate whether Sema5A acts as an attractant or repellent for DRG axons, we performed tissue culture experiments using chick DRG explants. We used stage 25 chick DRG explants whose stage corresponds to E11.5 of the mouse embryo.Citation10 Human embryonic kidney (HEK) 293-T cell aggregates were transfected with Sema5A-pSecTag2 (the pSecTag2 expression vector was designed for secretion; Invitrogen) and were co-cultured with stage 25 chick DRG explants for 24 h in a collagen gel (). For visualization, cultures were incubated with the Tuj1 antibody, followed by Alexa Fluor 488 anti-mouse IgG (Invitrogen Corp.). For quantitative analysis, we used a scale to score the degree of axon chemoattraction or chemorepulsion.Citation4 This value is a measure of axon guidance activity, with + and – value indicating attraction and repulsion, respectively. Sema5A-transfected cell aggregates attracted DRG axons (mean value: 1.03, n = 17). In addition, we observed that Sema5A-transfected cells also attracted a subset of cells in the DRG explant ().
Figure 2. Sema5A exhibited chemoattractive activities toward DRG axons. (A) Sema5A-transfected cell aggregates (HEK-Sema5A) attracted stage 25 chick DRG axons in a collagen gel. The dotted lines indicate the borders of the DRG explant and cell aggregates. The arrowhead shows cell migrating from the DRG explant. (B) The ectopic expression of Sema5A in the spinal cord (SC) caused aberrant projections of DRG axons. Transverse sections of a stage 25 chick embryo electroporated with GFP-pAPtag-5 (green) and Sema5A-pSecTag2 were labeled with the Tuj1 antibody (magenta). The inset shows the ectopically expressed Sema5A (arrows) in an adjacent section as visualized by in situ hybridization. The dotted line indicates the border of the spinal cord. (C) High magnification of the boxed area shown in B. Arrowheads indicate misrouted DRG axons. (Scale bars, 100 μm).
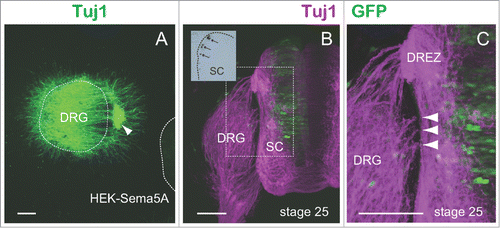
To further define the role of Sema5A in DRG axonal guidance, we tested the in vivo behavior of DRG axons by gain-of-function experiments using chick embryos. We introduced a chick Sema5A expression vector into chick spinal cords by electroporation. A mixture of the Sema5A-expressing vector (Sema5A-pSecTag2) and green fluorescent protein (GFP) vector (GFP-pAPtag-5; GenHunter Corp.) or GFP-pAPtag-5 alone was electroporated into stage 14 chick spinal cords (this stage corresponds to E8 in the mouse embryo), and the afferent trajectories of DRG axons were examined at stage 25 (). For detection of DRG axons, embryos were fixed and processed for immunohistochemistry by using the Tuj1 antibody and Alexa Fluor 594 anti-mouse IgG (Invitrogen Corp.). In the embryos that ectopically expressed Sema5A in their dorsal and intermediate spinal cord (n = 4), we observed that a subset of DRG axons became oriented aberrantly toward regions other than the DREZ (). Of the sections examined, 40% of them showed aberrant projections toward the spinal cord. In contrast, no aberrant projections toward the spinal cord were observed in the control embryos that received GFP alone (n = 3). These results indicated a high possibility that Sema5A could alter the direction of DRG axons in vivo.
Both our in vivo and in vitro results strongly suggest that Sema5A may be an attractive cue for early DRG axons. So far no molecule other than neurotrophins has been reported to attract DRG axons.Citation2 Therefore, this is a novel finding regarding the mechanism underlying axonal guidance events. Because Sema5A is both membrane associated and secreted, it is possible that Sema5A may not only constitute a permissive substrate for DRG axons along their route, but also function as a diffusible molecule to attract DRG axons. Based on our result showing that no Sema5A expression was detectable along the path of DRG afferent fibers and the DREZ (see ), Sema5A may not function as DREZ-derived attractants for DRG afferent fibers in vivo. Based on our in-vivo result that ectopic Sema5A could not decrease the DRG projection toward the DREZ, we suppose that unknown DREZ-derived cues may show stronger activities to attract DRG fibers than Sema5A. On the other hand, Sema5A was distributed along the path of DRG efferent fibers (dorsal and ventral rami). It is thus possible that Sema5A may attract DRG efferent fibers as substrate-bound and/or secreted forms in vivo.
A recent study revealed that Sema5-induced inhibition of retinal axon growth is mediated by PlexinA1 and PlexinA3 receptors.Citation11 In higher vertebrates, PlexinA1 is expressed in DRG neurons during development (data not shown).Citation12 Contrary to our expectation, no counterpart for PlexinA3 was found in chick databases.Citation12 Further studies will determine whether PlexinA1 alone can mediate Sem5A-induced attractive signaling.
Our findings raise an important question regarding the expression pattern of Sema5A. Although it is well known that DRG efferent fibers never orient toward the perinotochordal mesenchyme, i.e., the mesenchymal cells around the notochord, our present study found that Sema5A was distributed in the perinotochordal mesenchyme (see ). How can this discrepancy be explained? One possible explanation is based on the evidence that CSPGs were abundant in the perinotochordal mesenchyme (Fig. S2).Citation13–16 The study cited earlier showed that CSPGs can switch Sema5A from being an attractant to a repellent for midbrain axons.Citation7 Along the same line, we suppose that CSPGs might switch Sema5A from being an attractive cue to a repulsive one for DRG axons in the perinotochordal mesenchyme. Thus, it is highly possible that Sema5A along the path of DRG fibers may function as a permissive/attractive cue and that Sema5A around the notochord may inhibit DRG axonal growth by its interaction with CSPGs there.
In conclusion, we have provided evidence that Sema5A plays a key role in sensory axonal guidance and contributes to the formation of the early sensory network.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
972770_Supplemental_Figure_legends.pdf
Download PDF (39.2 KB)KCAM_A_972770_Supplemental_Figure_2.psd
Download (4.3 MB)KCAM_A_972770_Supplemental_Figure_1.psd
Download (4.5 MB)Funding
This work was supported by grant no. 24590256 from the program Grants-in-Aid for Scientific Research of the MEXT, Japan to T.M., and by a grant from the Naito Foundation, Japan to T.M.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
References
- Dickson BJ. Molecular mechanisms of axon guidance. Science 2002; 298:1959-64; PMID:12471249; http://dx.doi.org/10.1126/science.1072165
- Masuda T, Sakuma C, Taniguchi M, Kobayashi K, Kobayashi K, Shiga T, Yaginuma H. Guidance cues from the embryonic dorsal spinal cord chemoattract dorsal root ganglion axons. Neuroreport 2007; 18:1645-9; PMID:17921861; http://dx.doi.org/10.1097/WNR.0b013e3282f0b6fa
- Masuda T, Tsuji H, Taniguchi M, Yagi T, Tessier-Lavigne M, Fujisawa H, Okado N, Shiga T. Differential non-target-derived repulsive signals play a critical role in shaping initial axonal growth of dorsal root ganglion neurons. Dev Biol 2003; 254:289-302; PMID:12591248; http://dx.doi.org/10.1016/S0012-1606(02)00087-8
- Masuda T, Sakuma C, Taniguchi M, Kanemoto A, Yoshizawa M, Satomi K, Tanaka H, Takeuchi K, Ueda S, Yaginuma H, et al. Development of the dorsal ramus of the spinal nerve in the chick embryo: A close relationship between development and expression of guidance cues. Brain Res 2012; 1480:30-40; PMID:22981415; http://dx.doi.org/10.1016/j.brainres.2012.08.055
- Masuda T, Taniguchi M, Sakuma C, Yamagishi T, Ueda S, Kawaguchi M, Yaginuma H. Development of the dorsal ramus of the spinal nerve in the mouse embryo: Involvement of semaphorin 3A in dorsal muscle innervation. Congenit Anom 2013; 53:122-6; PMID:23998265; http://dx.doi.org/10.1111/cga.12019
- Sadanandam A, Sidhu SS, Wullschleger S, Singh S, Varney ML, Yang CS, Ashour AE, Batra SK, Singh RK. Secreted semaphorin 5A suppressed pancreatic tumour burden but increased metastasis and endothelial cell proliferation. Br J Cancer 2012; 107:501-7; PMID:22782341; http://dx.doi.org/10.1038/bjc.2012.298
- Kantor DB, Chivatakarn O, Peer KL, Oster SF, Inatani M, Hansen MJ, Flanagan JG, Yamaguchi Y, Sretavan DW, Giger RJ, et al. Semaphorin 5A is a bifunctional axon guidance cue regulated by heparan and chondroitin sulfate proteoglycans. Neuron 2004; 44:961-75; PMID:15603739; http://dx.doi.org/10.1016/j.neuron.2004.12.002
- Liu RQ, Wang W, Legg A, Abramyan J, O’Connor TP. Semaphorin 5B is a repellent cue for sensory afferents projecting into the developing spinal cord. Development 2014; 141:1940-9; PMID:24718987; http://dx.doi.org/10.1242/dev.103630
- Masuda T, Kai N, Sakuma C, Kobayashi K, Koga H, Yaginuma H. Laser capture microdissection and cDNA array analysis for identification of mouse KIAA/FLJ genes differentially expressed in the embryonic dorsal spinal cord. Brain Res 2009; 1249:61-7; PMID:19026994; http://dx.doi.org/10.1016/j.brainres.2008.10.028
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. Dev Dyn 1992; 195:231-72; PMID:1304821; http://dx.doi.org/10.1002/aja.1001950404
- Matsuoka RL, Chivatakarn O, Badea TC, Samuels IS, Cahill H, Katayama K, Kumar SR, Suto F, Chédotal A, Peachey NS, et al. Class 5 transmembrane semaphorins control selective mammalian retinal lamination and function. Neuron 2011; 71:460-73; PMID:21835343; http://dx.doi.org/10.1016/j.neuron.2011.06.009
- Mauti O, Sadhu R, Gemayel J, Gesemann M, Stoeckli ET. Expression patterns of plexins and neuropilins are consistent with cooperative and separate functions during neural development. BMC Dev Biol 2006; 6:32; PMID:16846494; http://dx.doi.org/10.1186/1471-213X-6-32
- Bundy J, Rogers R, Hoffman S, Conway SJ. Segmental expression of aggrecan in the non-segmented perinotochordal sheath underlies normal segmentation of the vertebral column. Mech Dev 1998; 79:213-7; PMID:10349634; http://dx.doi.org/10.1016/S0925-4773(98)00179-8
- Domowicz M, Li H, Hennig A, Henry J, Vertel BM, Schwartz NB. The biochemically and immunologically distinct CSPG of notochord is a product of the aggrecan gene. Dev Biol 1995; 171:655-64; PMID:7556944; http://dx.doi.org/10.1006/dbio.1995.1312
- Masuda T, Fukamauchi F, Takeda Y, Fujisawa H, Watanabe K, Okado N, Shiga T. Developmental regulation of notochord-derived repulsion for dorsal root ganglion axons. Mol Cell Neurosci 2004; 25:217-27; PMID:15019939; http://dx.doi.org/10.1016/j.mcn.2003.10.005
- Perissinotto D, Iacopetti P, Bellina I, Doliana R, Colombatti A, Pettway Z, Bronner-Fraser M, Shinomura T, Kimata K, Mörgelin M, et al. Avian neural crest cell migration is diversely regulated by the two major hyaluronan-binding proteoglycans PG-M/versican and aggrecan. Development 2000; 127:2823-42; PMID:10851128
