Abstract
The role of environmental reservoirs in H. pylori transmission remains uncertain due to technical difficulties in detecting living organisms in sources outside the stomach. Residents of some Canadian Arctic communities worry that contamination of the natural environment is responsible for the high prevalence of H. pylori infection in the region. This analysis aims to estimate associations between exposure to potential environmental sources of biological contamination and prevalence of H. pylori infection in Arctic Canada.
Using data from 3 community-driven H. pylori projects in the Northwest and Yukon Territories, we estimated effects of environmental exposures on H. pylori prevalence, using odds ratios (OR) and 95% confidence intervals (CI) from multilevel logistic regression models to adjust for household and community effects. Investigated exposures include: untreated drinking water; livestock; dogs; cats; mice or mouse droppings in the home; cleaning fish or game.
Our analysis did not identify environmental exposures associated clearly with increased H. pylori prevalence, except any exposure to mice or mouse droppings (OR = 4.6, CI = 1.2–18), reported by 11% of participants. Our multilevel models showed H. pylori clustering within households, but environmental exposures accounted for little of this clustering; instead, much of it was accounted for by household composition (especially: having infected household members; number of children).
Like the scientific literature on this topic, our results do not clearly implicate or rule out environmental reservoirs of H. pylori; thus, the topic remains a priority for future research. Meanwhile, H. pylori prevention research should seek strategies for reducing direct transmission from person to person.
Introduction
Helicobacter pylori (H. pylori) are helical, flagellar gram-negative bacteria that inhabit the lining of the human stomach and/or duodenum.1 Chronic H. pylori infection is involved in the pathogenesis of chronic gastritis, peptic ulcer disease and gastric cancer, digestive diseases responsible for a large global disease burden.1,2 Believed to have once infected the majority of humans worldwide, a decline in prevalence has been observed in areas with greater modern infrastructural development.Citation3-5 Conversely, the impact of this bacterium is still prominent in less developed regions.Citation3-6 This contrast is visible within Canada, where evidence has highlighted a disproportionately high prevalence in Indigenous Arctic communities, relative to multi-ethnic populations in the southern part of the country.Citation7-14 This inequity underlies emerging concern about H. pylori infection in these communities, as the frequency and severity of related digestive diseases is also higher relative to southern Canada. Evidence on which to base H. pylori infection control strategies for northern communities is relatively limited.
In prevalence studies of adults aged 18 to 86 y in various locations across southern Canada, prevalence of H. pylori infection ranged from 30–38%.Citation15 Some studies have reported increasing prevalence with age; for example, in a 1997 study of healthy individuals from Manitoba, including 469 aged 20 to 34 y and 265 aged 35 to 64 years, the prevalence of H. pylori infection was 35% and 46%, respectively.Citation8 Very low prevalence in southern Canadian children of 5% was shown in a 2005 study of 246 pediatric endoscopy patients aged 5 to 18 y from 4 academic centers.Citation7 Because the acquisition of chronic H. pylori infection is known to occur most frequently in childhood,2,16,17 the trend of increasing prevalence with age suggests that transmission levels have decreased over several decades.
Conversely, the literature has shown that Indigenous communities in the circumpolar region have a disproportionately high prevalence of H. pylori infection and associated health consequences. H. pylori prevalence estimates from community-based studies of Indigenous populations in Canada, Alaska, Greenland and Russia range from 51–95%.Citation9-14,18 In a study of a 306 adults from a Wasagamack Cree community in Northern Manitoba, 95% were found to be H. pylori-positive.Citation11 A study investigating H. pylori infection in 163 children aged 0 to 12 y from the same community revealed that 56% were positive.Citation13 An investigation of H. pylori infection in the Inuit communities of Chesterfield Inlet and Repulse Bay, Nunavut found that of 256 individuals of all ages, 51% in this age group were positive for H. pylori infection.Citation18
In response to questions raised by community leaders and health care providers, the Canadian North Helicobacter pylori (CANHelp) Working Group (http://canhelpworkinggroup.ca) formed to link Arctic communities, territorial health agencies and investigators from a variety of disciplines based at the University of Alberta. In the conduct of collaborative research aimed to: obtain representative data from diverse settings in northern Canada for informing regional public health strategies for reducing risks from H. pylori; conduct policy analysis to identify cost-effective H. pylori management strategies that are ethically, economically and culturally appropriate for northern communities; and develop knowledge exchange strategies that help community members understand H. pylori health risks as well as available solutions and unsolved challenges for reducing these risks.
The science surrounding the transmission of H. pylori remains unclear. Transmission has only been documented in 3 circumstances: patients undergoing endoscopic procedures; accidental infection through gastric pH electrodes; and voluntary oral ingestion of the bacteria.Citation2 Because it typically colonizes the stomach, the predominantly hypothesized routes involve the mouth as the portal of entry to the stomach. Abundant evidence suggests that the infection is usually transmitted directly from person to person and investigated pathways include fecal-oral, oral-oral, and gastro-oral (ingestion of another person's regurgitated stomach contents)Citation16; however the relative frequency of transmission through each route is unknown. The human stomach is the only known source of H. pylori and evidence from extra-gastric sources has been inconclusive,Citation19 leaving the question of how the natural environment impacts transmission unanswered to date. Using data from CANHelp Working Group community projects, this analysis investigates the hypothesis that environmental sources of biological contamination are involved in transmission of H. pylori infection.
Results
Participant characteristics
The combined number of participants from the 3 communities was 670: 564 participants provided health history data; 580 individuals provided data on their own socio-environmental exposures; 279 households provided information on socio-environmental household exposures for 650 individual household members; 652 participants were screened for H. pylori infection by 13C-urea breath test (UBT) and 645 had classifiable results; 265 participants consented to endoscopy and biopsies were obtained and analyzed from 257 (194 from Aklavik and 63 from Old Crow). The total number of participants with complete data on all environmental exposures and H. pylori status was 368 (227 from Aklavik, 89 from Old Crow and 52 from Tuktoyaktuk).
Classification of H. pylori status
Of the 368 individuals included in the analysis, 4% (n = 16) did not have a UBT result and 51% (n = 188) did not have histopathology or culture results. Among those with results from all 3 tests used to classify H. pylori infection status, 81% (n = 145) were concordant on all tests. Of those with results on just 2 tests, 93% (14/15) were concordant on the 2 tests. Of 35 participants with 3 test results that were not concordant, 40% were positive on 2 of the 3 tests and 60% were negative on 2 of the 3 tests. These participants were classified based on the results of the 2 tests that agreed (40% positive, 60% negative). Of these 35 participants, 74% (n = 26) had a culture result that disagreed with the other 2 tests. A high level of agreement was observed between the UBT and histopathology; of those with results on both tests (n = 165), 95% were concordant.
Prevalence of H. pylori infection
H. pylori prevalence was 63% (408/645) in the total study population: 61% (217/354) in Aklavik; 69% (132/190) in Old Crow; 58% (59/101) in Tuktoyaktuk. In those with complete data on all variables, H. pylori prevalence was 62% (227/368).
Socio-demographic effects
Results of purposeful selection procedures for regression modeling indicated the most important adjustment variables were age, sex, household income, highest educational attainment of each individual, ethnicity and community. Considerable variation across households was observed (standard deviation (SD): 1.3; 95%CI: 0.69, 2.6). The distribution of socio-demographic characteristics and the estimated ORs and 95% CIs for their effects on the prevalence odds of H. pylori infection in individuals with complete data on all variables (n = 368) are presented in . In order to more accurately adjust for the non-linear effect of age, a cubic spline with 4 knots was fitted; for this reason, an OR for age is not reported.
Table 1 Effects of socio-demographic characteristics on H. pylori prevalence odds among 368 community H. pylori project participants, Northwest and Yukon Territories, 2008–2011.
Table 2 Pathways for zoonotic transmission: Exposure prevalence and exposure-specific H. pylori prevalence by community among 368 community H. pylori project participants, Northwest and Yukon Territories, 2008–2011
Pathways for zoonotic transmission
The distribution of zoonotic exposures and exposure-specific prevalence of H. pylori infection is shown by community in . The most common zoonotic exposure was contact with animal innards (76%; 279/368), followed by caring for animals (75%; 277/368) and, more specifically, caring for dogs (73%; 269/368). The prevalence of H. pylori infection ranged widely from 42–75% across categories of zoonotic exposures. Observed prevalence was lowest in individuals who reported caring for cats (42%; 15/36) and highest in those who reported evidence of mice in their homes (78%; 31/40).
Table 3 Pathways for zoonotic transmission: Results of multivariable logistic regression analysis of effects on H. pylori prevalence odds among 368 community H. pylori project participants, Northwest and Yukon Territories, 2008–2011
Results of logistic regression analysis for zoonotic exposures appear in , with unadjusted ORs presented along with ORs from 2 multivariable models: the first including age as a cubic spline, sex, ethnicity, income, education, community as a fixed effect, and household as a random effect; and the second adding to that set of variables all water and zoonotic exposures. The largest effect observed was for the comparison of individuals who reported having seen mice or mouse droppings in their homes compared to those who reported that they had not. The most fully adjusted OR for the effect of exposure to mice compared to no exposure to mice on prevalent H. pylori infection was 4.6 (95%CI: 1.2, 18). It should be noted, however, that only 14.4% of participants reported exposure to mice. Remaining effects were modest, with the exception of the crude OR for the effect of cat ownership compared to not owning cats. However, this effect became weak and highly imprecise following adjustment for socio-demographic and other environmental variables (OR: 1.4; 95%CI: 0.34, 5.4).
Table 4 Pathways for waterborne transmission: Exposure prevalence and exposure-specific H. pylori prevalence by community among 368 community H. pylori project participants, Northwest and Yukon Territories, 2008–2011
Pathways for waterborne transmission
The distribution of exposure to pathways for waterborne transmission and associated prevalence of H. pylori infection in each community is shown in . Having ever consumed untreated water was the most commonly reported exposure to water potentially contaminated with human pathogens (77%; 282/368). Prevalence of H. pylori infection in different exposure categories ranged from 59–61%. The combined prevalence among those included in the analysis fell just outside of this range (62%). Further, this range is much narrower than that of the community-specific estimates of prevalence. H. pylori-positivity was highest in those who reported ever consuming untreated water (61%; 172/282) and doing so in the past year (61%; 75/123). It should be noted that H. pylori prevalence among participants reporting consumption of untreated water was much lower in Tuktoyaktuk than the other 2 communities.
Table 5: Pathways for waterborne transmission: Results of multivariable logistic regression analysis of effects on H. pylori prevalence odds among 368 community H. pylori project participants, Northwest and Yukon Territories, 2008–2011
Results of logistic regression analysis for exposure to sources of water potentially contaminated with human pathogens are presented in . The largest effect was for the comparison of individuals who had consumed untreated water at some point in their life compared to those who had not. The OR estimated a strong inverse association following adjustment for socio-demographic variables (OR: 0.44; 95%CI: 0.20, 0.96) and other environmental exposures (OR: 0.36; 95%CI: 0.14, 0.94), consistent with a protective effect of having ever consumed untreated water on H. pylori infection prevalence odds. Given the large difference between Tuktoyaktuk and the other communities, this effect was also estimated among participants from Aklavik and Old Crow alone (OR: 0.62; 95%CI: 0.29, 1.4)
Table 6: Effects of household composition variables on H. pylori prevalence odds among 368 community H. pylori project participants, Northwest and Yukon Territories, 2008–2011
Household effect
A random effects parameter for household was required for the multivariable logistic regression models because the odds of H. pylori infection among participants residing in the same household cannot be assumed to be independent. This parameter captures the degree to which H. pylori infection clusters by household among participants and yields information about any residual effect of household membership on H. pylori prevalence odds that cannot be explained by variables included in the model. The size of the standard deviation (SD) of the random effects parameter reflects the degree of residual household effect, with the SD shrinking as the random effect diminishes. shows the random household effect relative to the effect of independent variables from logistic regression models with different subsets of independent variables. Comparison of these models shows a strong effect of household membership on H. pylori prevalence beyond that captured by the investigated environmental exposures. Adding cohabitation with another research participant who was H. pylori –positive considerably reduced the residual household effect (SD: 0.62; 95%CI: 0.96, 4.04), as did the number of children in the home (SD: 43; 95%CI: 0.016, 11.3).
Figure 1. Random effect of household (SD) relative to the effect of independent variables in the model. Model 1. Household Random Effect (SD): 1.26; 95%CI: 0.70, 2.3 Model 2. Household Random Effect (SD): 1.16; 95%CI: 0.59, 2.3) Model 3. Household Random Effect (SD): 1.11; 95%CI: 0.54, 2.3 Model 4. Household Random Effect (SD): 0.62; 95%CI: 0.96, 4.04 Model 5. Household Random Effect (SD): 0.43; 95%CI: 0.016, 11.3.
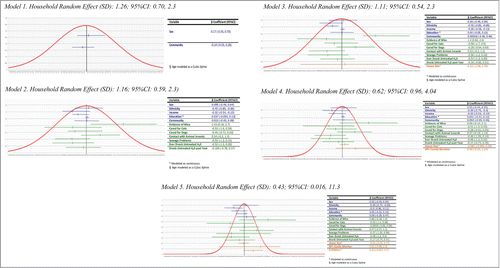
shows estimated ORs for of the effects of the household composition variables on prevalent H. pylori infection. Though estimated somewhat imprecisely, the estimated ORs show a strong positive association with increasing household size and an even stronger one with an increasing number of children in the home. The weak effect estimated for living with an H. pylori-positive household member may be due to many households including members who did not participate in the community projects.
Table 7: Sensitivity analysis showing estimates based on different methods for classifying H. pylori infection status of participants with discordant results (n = 368)
Sensitivity analysis
shows results from models that repeat the analysis using different approaches to classifying participants with discordant results on H. pylori tests: classifying them in one model based on the culture result, in another model based on the histopathology result and in another model based on the UBT result. While the largest changes are noted for the model that bases the classification on culture, the degree of change across the models does not substantially alter the interpretations of the estimated effects. Of note, none of the estimates in any of the 3 models fall outside of the originally estimated 95%CIs.
Table 8 Variable definitions and response options for environmental exposures
Discussion
The prevalence of H. pylori infection observed in the CANHelp Working Group community projects in the Northwest and Yukon Territories of 62% falls within the expected range for Indigenous communities in the circumpolar north, based on reports from the eastern Canadian Arctic and other Arctic countries. In contrast to evidence from major urban centers across Canada that suggests decreasing H. pylori transmission over time and an average prevalence of approximately 20–30%,Citation7,8 the much higher H. pylori prevalence observed in these western Canadian Arctic communities shows it to be a health inequity and justifies the concerns expressed by leaders of these communities and their health care providers. Among the major research goals of interest in these community-driven projects is finding out if there are local environmental reservoirs of H. pylori infection that can be eliminated or reduced. The present analysis does not clearly identify indicators of exposure to waterborne or zoonotic pathways as exposures of concern in the participating communities.
While this analysis showed participants who reported evidence of mice in their homes to have a relatively high prevalence of H. pylori infection compared to others, only 40 of 368 (11%) participants reported this exposure, thus it is unlikely that mice play a major role in transmission, unless the exposure generally goes unnoticed, is otherwise underreported, or occurs more frequently outside the home. A review of the literature pertaining to mice and H. pylori transmission revealed the pervasive use of mice in animal models and repeated demonstration of the ability to inoculate mice with H. pylori, supporting the plausibility of mice playing a role in transmission.Citation20–22 However, the literature lacks epidemiologic investigations of the effect of exposure to mice on the risk of H. pylori infection. Thus, conclusions about this observation cannot be drawn without further research to determine whether this association is observed in other settings, and if so, if it reflects a role in transmission or is confounded by other risk factors. The estimated effects of regular contact with dogs or cats on H. pylori prevalence were modest and imprecise. The estimate for regular care of dogs was slightly more precise, with a 95%CI indicating the data are compatible with effect estimates ranging from a large protective effect to a small detrimental effect. The imprecision around the effect estimate for cat ownership on H. pylori prevalence is likely due to the very small number of participants who reported owning a cat.
These findings were consistent with a body of literature that examined the prevalence of non-pylori Helicobacter organisms in a variety of animals, with reported prevalence of 67 to 100% in some species.Citation23-28 While the prevalence of these other Helicobacter species is quite high in some animal species, it is estimated that no more than 1% of humans are infected with these other species, indicating they are not readily transmitted between animals kept as pets or livestock and humansCitation16,29 The present analysis is inconclusive about a moderate effect of exposure to animal innards in the transmission of H. pylori in the participating communities.
The estimated effects of sources of potential exposure to contaminated water on H. pylori prevalence show inverse associations, with widely varied effects across communities. Based on the reviewed literature, inverse associations were not expected. While the scientific community has been unable to demonstrate conclusively whether H. pylori organisms are able to retain infectivity in water,Citation30-33 epidemiologic investigations of exposure to sources of untreated water suggest the potential for waterborne transmission of H. pylori.Citation34-45 However, a large proportion of the estimates reported in the literature have 95% CIs that indicate the association may actually be closer to the null.Citation34,36,40,41,43,45 While some authors have reported null associations between untreated water consumption and prevalence of H. pylori infection,Citation16,36,46 null findings were not commonly reported in the literature. This may be due, in part, to a tendency for papers presenting positive results to be favored for publication over those presenting null associations. It may be that our analysis failed to identify factors that confound the association between untreated water consumption and prevalent H. pylori infection among our community project participants, or perhaps was affected by differential recall of water consumption.
An important limitation of this analysis is misclassification of exposure and confounding variables caused by errors in questionnaire data, which likely occurred to some degree due to the respondents’ imperfect recall. Additionally, some of the exposures of interest had a low prevalence among participants, and this led to poor statistical precision for some of the estimated effects. Selection bias due to differential participation rates in relevant project components is also possible. At the same time, major strengths of this analysis include the population-based dataset and the high level of engagement of community members who seek solutions for this health problem and their health care providers.
The observations reported here likely apply more broadly to understanding H. pylori transmission in northern Canadian communities and similar populations. Clear identification of H. pylori transmission pathways is needed for the development of meaningful public health policy aimed at preventing the spread of the bacteria. While the science surrounding transmission remains unclear, evidence suggests that H pylori often spreads directly from person to person through contact with digestive fluids containing the organism. The prospect of contamination of the local environment with H. pylori is a commonly expressed concern among CANHelp Working Group community project participants. Because the available evidence does not clearly rule out waterborne or zoonotic transmission of H pylori infection, it remains important to investigate potential environmental reservoirs. Additional data from other Arctic communities will permit more precise estimation of the effects of exposure to environmental sources of biological contamination on prevalent H. pylori infection. At the same time, H. pylori control efforts should help communities focus on strategies for reducing the frequency of communicable diseases that spread directly from person to person.
Materials and Methods
Study populations
Health officials in the Northwest Territories (NT) sought this research on behalf of communities like the Hamlet of Aklavik, where leaders expressed concern about the role of H. pylori in gastric cancer, perceived as afflicting an excessive number of community members. Thus, the CANHelp Working Group selected Aklavik NT as the target community for beginning its research in 2007, as described elsewhere.Citation47 According to the 2006 census, Aklavik had 590 residents with 92% identifying with either Gwitch’in (Athabascan First Nations) or Inuvialuit (western Canadian Inuit) cultures.Citation48 In 2010, the second community project began in Old Crow, Yukon Territory (YT), at the request of community leaders. According to the 2011 census, the population of Old Crow was 245, with 86% identifying as Vuntut Gwitch’in.Citation49 The third community project began in Tuktoyaktuk, NT in 2011. According to the 2011 census, the population of Tuktoyaktuk was 854, with 85% identifying as Inuvialuit, First Nations or Métis (officially recognized by the Canadian government as an Aboriginal group with mixed European and Indigenous ancestry).Citation50 Many residents of these communities follow a traditional lifestyle of hunting, trapping and fishing, incorporating modern technologies such as computers and snowmobiles. Aklavik and Tuktoyaktuk are accessible by water or air in the summer and ice road in the winter. Old Crow is accessible only by air.Citation48-52
Study design and community projects
This analysis used data collected in cross-sectional studies of H. pylori infection as part of the CANHelp Working Group community projects in Aklavik, Old Crow and Tuktoyaktuk. The cross-sectional design is appropriate for initial investigations of the burden of disease from H. pylori in a community setting, given that the onset of this infection generally goes undetected and often persists indefinitely without symptoms. Thus, the starting point for describing the frequency of H. pylori infection in a community is screening to detect prevalent cases. Projects were established independently in each community, with the guidance of a local planning committee. Each participating community chose to follow a similar design to previous projects, to allow for comparability. Community projects included 5 components: questionnaire-based interviews to collect information on relevant personal and household characteristics, non-invasive screening for H. pylori infection, endoscopy of the stomach with gastric biopsy for endoscopic and histopathological assessment of gastro-duodenal disease and isolation of H. pylori to investigate characteristics of bacterial strains, treatment to eliminate H. pylori, and knowledge exchange. Planning committees comprised community representatives and University of Alberta project staff. The planning committees guided the design and conduct of the projects to ensure that the research addressed local priorities in a culturally appropriate manner. Community planning committees were given the opportunity to review this report and provided feedback prior to publication.
Each community project sought to enrol all consenting community members during defined enrolment periods. Recruitment occurred in Aklavik primarily from November 2007 through February 2008, in Old Crow primarily from November 2010 through February 2011 and in Tuktoyaktuk during February-March 2011 and March-May of 2012. With local guidance from planning committees in each community, recruitment activities included community gatherings, flyers, radio announcements, information tables in high traffic locations and door-to-door outreach.
The planning process highlighted the scientific importance of using similar methodological approaches across communities for the purpose of comparability and each planning committee chose to keep the data collection methods as similar as possible to those used in other communities, with only minor differences arising from variations in the local setting. Due to logistic constraints, the community projects were not all carried out simultaneously, but there is no evidence to suggest that the occurrence of H. pylori infection followed any secular trend during the brief time span of these community projects. For these reasons, this analysis combined the data collected from individual projects in order to enhance statistical power for estimating associations that appeared homogeneous across communities and to explore differences across communities to gain a better understanding of the factors that contribute to the burden of disease at the community level.
Classification of H. pylori infection status
The 13C-urea breath test (UBT) was the primary method used for to detect H. pylori infection in community project participants. Participants who had endoscopy were also classified for H. pylori status according to pathological examination of gastric biopsies and culture. The H. pylori status of each participant was classified using all available information, with a systematic algorithm used in cases with discordant results. Given uncertainty regarding the classification of discordant results, a sensitivity analysis was performed to assess the extent to which the estimated effects would change if the classification scheme for discordant results were altered and the test results of participants with discordant results not adjusted based on all available information. This analysis used 3 variations of the fully adjusted model; each classified the infection status of participants with discordant results based solely on one of the 3 tests (culture, histopathology, UBT).
The UBT is considered the most accurate and convenient noninvasive method for detecting active H. pylori infection in children and adults.Citation53–57 The sample collection protocol and interpretation of test values was adapted from the IRIS and labeled urea manufacturer (http://www.helikit.com/en/physician-information/) instructions, modified according to the conclusions of the Gisbert and Parajes (2004) systematic review of validation studies.Citation57 While providing breath samples, participants were asked about factors believed to impair UBT accuracy (recent use of specific medications, when they last ate, height/weight for children aged 5 y and younger). Most participants were screened by UBT upon enrolment while providing study data in response to interviewer-administered questionnaires. The sample bags were packaged loosely in plastic containers to avoid being put under pressure during transport while being shipped to the University of Alberta. All UBTs were analyzed using an infrared breath test analyzer (IRIS by Wagner).
For participants aged 5 y or younger, methods adapted from Klein et al. (1999)Citation58 were used to correct for the influence of anthropometric differences in C02 production believed to inflate the test values. A borderline test value was interpreted as meaning the participant might have the infection but another factor may have influenced the result, for example, a proton pump inhibiting medication, or having recently consumed food or drink with a high 13C level. Individuals with a test result classified as borderline were advised to repeat the test for a more accurate result. Individuals were also advised to repeat their test if the CO2 concentration in either sample was too low for accurate analysis or the test value was implausible. For repeat tests, the test with the best CO2 concentration was used.
Endoscopies were offered to individuals aged 15 y or older in Aklavik in February of 2008 and Old Crow in January of 2012, irrespective of infection status. (The endoscopy phase of the project had not yet taken place in Tuktoyaktuk at the time of this analysis.) In each community, an endoscopy unit was set up in the health center and a medical team led by project gastroenterologists performed unsedated upper gastrointestinal endoscopies using thin gastroscopes. For consenting participants, endoscopists examined the stomach for gastric lesions and took 7 biopsies of the gastric mucosa, 2 for microbiological examination and 5 for histopathological examination, from pre-specified locations in the stomach. The biopsy sampling protocol adhered to the updated Sydney protocol.Citation59 If an endoscopically visible lesion was present, the endoscopist took an extra biopsy of the lesion for pathological examination. Of the biopsies collected for microbiological examination, one was taken from the antrum and one from the body of the stomach. Upon culture of Helicobacter organisms, project microbiologists confirmed that the organisms were H. pylori. A single pathologist examined all biopsies to identify H. pylori, measure its density in the gastric mucosa, and characterize histopathological abnormalities.
Exposure ascertainment
Selection of environmental exposures of interest was based on the scientific literature and relevance to the communities. Environmental exposures were grouped based on their relevance to known modes of transmission of infectious agents: pathways for zoonotic transmission (evidence of mice in the home; caring for animals; caring for dogs; caring for cats; and contact with animal innards) and pathways for waterborne transmission (ever consumed untreated water; consumed untreated water in the past year; exposure to sewage (contaminated water)).
Structured interviews conducted by trained interviewers were used to collect data on health history, demographic characteristics and exposure to relevant socio-environmental factors. The questionnaire instruments included items pertaining to individuals and households as appropriate. Questionnaires that ascertained characteristics and exposures of individuals were administered to each participant. Parents decided if participating children were mature enough to respond for themselves. Additionally, a household questionnaire was administered to one adult member of each household. Questionnaires were adapted from previous research conducted by the principal investigator and were informed by relevant scientific literature.Citation16,60 Members on each community planning committee reviewed the questionnaires to assist in tailoring their content to the cultural context of each community. Environmental exposure variables were taken from responses provided in structured interviews (). The questionnaire data included other variables of interest as potential confounding factors: family size and structure, educational attainment, occupation, residential crowding and hygienic practices.
Ethics approval
This research was approved by the University of Alberta Health Research Ethics Board, as well as the Aurora Research Institute, which issues licenses for the conduct of research in the Northwest Territories, and the Heritage Resource Unit of the Yukon Department of Tourism and Culture, which issues licenses of the conduct of research in the Yukon Territory.
Statistical analysis
The goal of this analysis was to estimate the effect of specified environmental exposures on the prevalence of H. pylori infection in the combined population of the 3 participating communities. To examine the underlying relationships of relevant variables, H. pylori prevalence was compared across categories of exposure variables by community. Prevalence odds ratios (OR) and 95% confidence intervals (CI) were used to estimate effects, as recommended for prevalence studies by Pearce (2004).Citation61 In order to account for lack of independence of response probabilities given a contagious outcome and participants clustered in households and communities, a mixed logistic regression model was used, adjusting for clustering in communities as a fixed effect and in households as a random effect. The statistical software package STATA version 10 was used for statistical analyses.
Purposeful selection, as proposed by Hosmer and Lemeshow (2000),Citation62 was used to select variables to control confounding in multivariable models. Given the large number of factors to consider, each potential confounder was assessed in a logistic regression model that estimated the crude OR for its association with the dependent variable. Variables with unadjusted ORs yielding a P-value ≤0 .25 were subsequently included in a multivariable logistic regression model. Variables included in the multivariable model were then removed one at a time; if removal changed the coefficient of any independent variable by ≥10 %, the removed variable was included as a confounder in the final model. Exposures of interest and scientifically important variables were included regardless of statistical significance.
Lowess plots were used to visually assess whether continuous variables had a linear relationship with the respective outcome variable. If the relationship did not appear linear, appropriate transformations were tested. In order to faithfully adjust for the shape of the continuous data, cubic splines were fitted to the variable. The mathematical function used to create the cubic spline included terms which allowed the line to move up or down with the data, minimizing residual confounding resulting from fitting a straight-line relationship to non-linear data. The number of knots was chosen based on the visual assessment of the data and locations of the knots generated by STATA were checked by visual assessment of the lowess plot to ensure adequate placement. The LR test was used to statistically assess the fit of a model containing the continuous variable modeled as a cubic spline, relative to a model with the continuous variable modeled as having a linear relationship with the outcome. If the resulting P-value was ≤0 .05, the model containing the cubic spline was deemed a better fit for the data.
To identify factors that accounted for household clustering of the infection, the coefficient for the household random effect was compared across models that included subsets of the study variables. The household random effects parameter coefficient is the standard deviation (SD), which can be interpreted as an estimate of the change in the log-odds of prevalent H. pylori infection by household. To compare the relative contributions to the household effect of environmental exposures and composition of household membership, independent variables measuring aspects of household composition (household size in one-person increments, cohabitation with one or more research participants who tested H. pylori-positive, and number of children ≤ 12 y of age living in the home in one-person increments) were added one at a time to the model including the selected socio-demographic and environmental variables, to observe their influence on the residual household effect. Because random effects follow a normal distribution with a mean of 0, the standard deviations for the random effects parameter from each model were plotted as normal distributions using Excel. The β coefficients for each independent variable and their 95% confidence intervals were plotted along the x-axis of each graph, to show the amount of variation explained by the independent variables relative to the residual household effect.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
At the time this research was conducted, the CANHelp Working Group research program was supported by grants from the Canadian Institute of Health Research (FRN: 115031) and ArcticNet Network of Centers of Excellence. Emily V. Hastings was supported by a graduate studentship from the Nasivvik Center for Inuit Health and Changing Environments. Karen J. Goodman is a Health Senior Scholar supported by Alberta Innovates Health Solutions. The authors acknowledge the support of the Aklavik and Old Crow Project Planning Committees and the Inuvialuit Regional Corporation.
References
- Velázquez M, Feirtag JM. Helicobacter pylori: characteristics, pathogenicity, detection methods and mode of transmission implicating foods and water. Int J Food Microbiol 1999 53:95–104; http://dx.doi.org/10.1016/S0168-1605(99)00160-9
- Goodman KJ, Correa P. The transmission of helicobacter pylori. A critical review of the evidence. Int J Epidemiol 1995; 24:875-87; PMID:8557443; http://dx.doi.org/10.1093/ije/24.5.875
- Gessner BD, Bruce MG, Parkinson AJ, Gold BD, Muth PT, Dunaway E, Baggett HC. A Randomized trial of triple therapy for pediatric helicobacter pylori infection and risk factors for treatment failure in a population with a high prevalence of infection. Clin Infect Dis 2005; 41:1261-8; PMID:16206100; http://dx.doi.org/10.1086/496925
- Fischbach LA, Goodman KJ, Feldman M, Aragaki C. Sources of variation of Helicobacter pylori treatment success in adults worldwide: a meta-analysis. Int J Epidemiol 2002; 31:128-39; PMID:11914309; http://dx.doi.org/10.1093/ije/31.1.128
- Khurana R, Fischbach L, Chiba N, VAN Zanten SV, Sherman PM, George BA, Goodman KJ, Gold BD. Meta-analysis: Helicobacter pylori eradication treatment efficacy in children. Aliment Pharmacol Ther 2007; 25:523-6; PMID:17305754; http://dx.doi.org/10.1111/j.1365-2036.2006.03236.x
- Hartgrink HH, Jansen EP, van Grieken NC, van de Velde CJ Gastric cancer. Lancet 2009; 374:477-90; http://dx.doi.org/10.1016/S0140-6736(09)60617-6
- Jacobson K. The changing prevalence of Helicobacter pylori infection in Canadian children: should screening be performed in high-risk children? Can J Gastroenterol 2005; 19:412-4
- Pérez-Pérez GI, Bhat N, Gaensbauer J, Fraser A, Taylor DN, Kuipers EJ, Zhang L, You WC, Blaser MJ. Country-specific constancy by age in cagA+ proportion of Helicobacter pylori infections. Int J Cancer 1997; 72:453-6; PMID:9247289; http://dx.doi.org/10.1002/(SICI)1097-0215(19970729)72 :3%3c453::AID-IJC13%3e3.0.CO;2-D
- Koch A, Krause TG, Krogfelt K, Olsen OR, Fischer TK, Melbye M. Seroprevalence and risk factors for helicobacter pylori infection in greenlanders. Helicobacter 2005; 10:433-42; PMID:16181354; http://dx.doi.org/10.1111/j.1523-5378.2005.00351.x
- Milman N, Byg KE, Andersen LP, Mulvad G, Pedersen HS, Bjerregaard P. Indigenous Greenlanders have a higher sero-prevalence of IgG antibodies to Helicobacter pylori than Danes. Int J Circumpolar Health 2003; 62:54-60; PMID:12725341; http://dx.doi.org/10.3402/ijch.v62i1.17528
- Bernstein CN, McKeown I, Embil JM, Blanchard JF, Dawood M, Kabani A, Kliewer E, Smart G, Coghlan G, MacDonald S, et al. Seroprevalence of Helicobacter pylori, incidence of gastric cancer, and peptic ulcer-associated hospitalizations in a Canadian Indian population. Dig Dis Sci 1999; 44:668-74; PMID:10219820; http://dx.doi.org/10.1023/A:1026689103952
- Sinha SK, Martin B, Sargent M, McConnell JP, Bernstein CN. Age at acquisition of Helicobacter pylori in a pediatric Canadian First Nations population. Helicobacter 2002; 7:76-85; PMID:11966865; http://dx.doi.org/10.1046/j.1083-4389.2002.00063.x
- Zhu J, Davidson M, Leinonen M, Saikku P, Gaydos CA, Canos DA, Gutman KA, Howard BV, Epstein SE; GOCADAN Study Investigators. Prevalence and persistence of antibodies to herpes viruses, Chlamydia pneumoniae and Helicobacter pylori in Alaskan Eskimos: the GOCADAN Study. Clin Microbiol Infect 2006; 12:118-22; PMID:16441448; http://dx.doi.org/10.1111/j.1469-0691.2005.01319.x
- Reshetnikov OV, Nikitin YP, Kholmogortsev MV, Kurilovich SA, Pycllik OA. Helicobacter pylori in a Chukotka Native male population. Int J Circumpolar Health 1998; 57 Suppl 1:293-5; PMID: 10093292
- Thomson ABR, Barkun AN, Armstrong D, Chiba N, White RJ, Daniels S, Escobedo S, Chakraborty B, Sinclair P, Van Zanten SJ. The prevalence of clinically significant endoscopic findings in primary care patients with uninvestigated dyspepsia: the Canadian Adult Dyspepsia Empiric Treatment – Prompt Endoscopy (CADET–PE) study. Aliment Pharmacol Ther 2003; 17:1481-91; PMID:12823150; http://dx.doi.org/10.1046/j.1365-2036.2003.01646.x
- Goodman KJ, Correa P, Tenganá Aux HJ, Ramírez H, DeLany JP, Guerrero Pepinosa O, López Quiñones M, Collazos Parra T. Helicobacter pylori Infection in the Colombian Andes: a population-based study of transmission pathways. Am J Epidemiol 1996; 144:290-9; PMID:8686698; http://dx.doi.org/10.1093/oxfordjournals.aje.a008924
- Brown LM. Helicobacter pylori: epidemiology and routes of transmission. Epidemiol Rev 2000; 22:283-97; PMID:11218379; http://dx.doi.org/10.1093/oxfordjournals.epirev.a018040
- McKeown I, Orr P, Macdonald S, Kabani A, Brown R, Coghlan G, Dawood M, Embil J, Sargent M, Smart G. Helicobacter pylori in the Canadian arctic: seroprevalence and detection in community water samples. Am J Gastroenterol 1999; 94:1823-29; PMID:10406242; http://dx.doi.org/10.1111/j.1572-0241.1999.01212.x
- Travis PB, Goodman KJ, O'Rourke KM, Groves FD, Sinha D, Nicholas JS, VanDerslice J, Lackland D, Mena KD. The association of drinking water quality and sewage disposal with Helicobacter pylori incidence in infants: the potential role of water-borne transmission. J Water Health 2010; 8:192-203; PMID:20009261; http://dx.doi.org/10.2166/wh.2009.040
- Lee A, O'Rourke J, De Ungria MC, Robertson B, Daskalopoulos G, Dixon MF. A standardized mouse model of Helicobacter pylori infection: introducing the Sydney strain. Gastroenterology 1997; 112:1386-97; PMID:9098027; http://dx.doi.org/10.1016/S0016-5085(97)70155-0
- Mohammadi M, Redline R, Nedrud J, Czinn S. Role of the host in pathogenesis of Helicobacter-associated gastritis: H. felis infection of inbred and congenic mouse strains. Infect Immun 1996; 64:238-45; PMID:8557346
- Ghiara P, Marchetti M, Blaser MJ, Tummuru MK, Cover TL, Segal ED, Tompkins LS, Rappuoli R. Role of the Helicobacter pylori virulence factors vacuolating cytotoxin, CagA, and urease in a mouse model of disease. Infect Immun 1995; 63:4154-60; PMID: 7558333
- Jalava K, On SL, Vandamme PA, Happonen I, Sukura A, Hänninen ML. Isolation and Identification ofHelicobacter spp. from canine and feline gastric mucosa. Appl Environ Microbiol 1998; 64:3998-4006; PMID:9758832
- Neiger R, Simpson KW. Helicobacter infection in dogs and cats: facts and fiction. J Vet Intern Med 2000; 14:125-33; PMID:10772482; http://dx.doi.org/10.1892/0891-6640(2000)014%3c0125:IIDACF%3e2.3.CO;2
- Eaton KA, Dewhirst FE, Paster BJ, Tzellas N, Coleman BE, Paola J, Sherding R. Prevalence and varieties of Helicobacter species in dogs from random sources and pet dogs: animal and public health implications. J Clin Microbiol 1996; 34:3165-70; PMID:8940465
- Neiger R, Dieterich C, Burnens A, Waldvogel A, Corthésy-Theulaz I, Halter F, Lauterburg B, Schmassmann A. Detection and prevalence of helicobacter infection in pet cats. J Clin Microbiol 1998; 36:634-7; PMID:9508286
- Happonen I, Linden J, Saari S, Karjalainen M, Hänninen ML, Jalava K, Westermarck E. Detection and effects of helicobacters in healthy dogs and dogs with signs of gastritis. J Am Vet Med Assoc 1998; 213:1767-74; PMID:9861972
- Yamasaki K, Suematsu H, Takahashi T. Comparison of gastric lesions in dogs and cats with and without gastric spiral organisms. J Am Vet Med Assoc 1998; 212:529-33; PMID:9491160
- Dubois A, Berg DE, Incecik ET, Fiala N, Heman-Ackah LM, Perez-Perez GI, Blaser MJ. Transient and persistent experimental infection of nonhuman primates with Helicobacter pylori: implications for human disease. Infect Immun 1996; 64:2885-91; PMID:8757808
- Bode G, Mauch F, Malfertheiner P. The coccoid forms of helicobacter pylori. Criteria for their viability. Epidemiol Infect 1993; 111:483-90; PMID:8270008; http://dx.doi.org/10.1017/S0950268800057216
- M Shahamat, U Mai, C Paszko-Kolva, M Kessel & RR Colwell. Use of autoradiography to assess viability of Helicobacter pylori in water. applied and environmental microbiology. Appl Env Microbiol 1993; 59:1231-5
- Bellack NR, Koehoorn MW, MacNab YC, Morshed MG. A conceptual model of water's role as a reservoir in Helicobacter pylori transmission: a review of the evidence. Epidemiol Infect 2006; 134:439-49; PMID:16512966; http://dx.doi.org/10.1017/S0950268806006005
- Degnan AJ, Sonzogni WC, Standridge JH. Development of a plating medium for selection of helicobacter pylori from water samples. Appl Environ Microbiol 2003; 69:2914-8; PMID:12732566; http://dx.doi.org/10.1128/AEM.69.5.2914-2918.2003
- Herbarth O, Krumbiegel P, Fritz GJ, Richter M, Schlink U, Müller DM, Richter T. Helicobacter pylori prevalences and risk factors among school beginners in a German urban center and its rural county. Environ Health Perspect 2001; 109:573-7; PMID:11445510; http://dx.doi.org/10.1289/ehp.01109573
- Iso N, Matsuhisa T, Shimizu K. Helicobacter pylori Infection among patients visiting a clinic in Kasama City, Ibaraki Prefecture. J Nippon Med Sch 2005; 72:341-54; PMID:16415514; http://dx.doi.org/10.1272/jnms.72.341
- Redlinger T, O’Rourke K, Goodman KJ. Age distribution of helicobactor pulori seroprevalence among young children in a United SatesMexicoBorder Community: evidence for transitory infection. Am J Epidemiol 1999; 150:225-30; PMID:10430225; http://dx.doi.org/10.1093/oxfordjournals.aje.a009991
- Lyra AC, Santana G, Santana N, Silvany-Neto A, Magalhães E, Pereira EM, Mascarenhas R, Lyra MC, Veiga A, Ferreira K. Seroprevalence and risk factors associated with helicobacter pylori infection in blood donors in Salvador, Northeast-Brazil. Braz J Infect Dis 2003; 7:339-45; PMID:14552744; http://dx.doi.org/10.1590/S1413-86702003000500009
- O’Rourke K, Goodman KJ, Grazioplene M, Redlinger T, Day RS. Determinants of geographic variation in helicobacter pylori infection among children on the US-Mexico border. Am J Epidemiol 2003; 158:816-24; http://dx.doi.org/10.1093/aje/kwg219
- Klein PD, Graham DY. Water source as risk factor for Helicobacter pylori infection in Peruvian children. Lancet 1991; 337:1503; PMID:1675369; http://dx.doi.org/10.1016/0140-6736(91)93196-G
- Elitsur Y, Short JP, Neace C. Prevalence of Helicobacter pylori infection in children from urban and rural West Virginia. Dig Dis Sci 1998; 43:773-8; PMID:9558033; http://dx.doi.org/10.1023/A:1018866030977
- Nabwera HM, Nguyen-Van-Tam JS, Logan RF, Logan RP. Prevalence of Helicobacter pylori infection in Kenyan schoolchildren aged 3-15 years and risk factors for infection. Eur J Gastroenterol Hepatol 2000; 12:483-7; PMID:10833089; http://dx.doi.org/10.1097/00042737-200012050-00002
- Olmos JA, Ríos H, Higa R. Prevalence of Helicobacter pylori infection in Argentina: results of a nationwide epidemiologic study. Argentinean Hp Epidemiologic Study Group. J Clin Gastroenterol 2000; 31:33-7; PMID:10914773; http://dx.doi.org/10.1097/00004836-200007000-00008
- Yilmaz E, Doğan Y, Gürgöze MK, Ünal S. Seroprevalence of Helicobacter pylori infection among children and their parents in eastern Turkey. J Paediatr Child Health 2002; 38:183-6; PMID:12031003
- Rolle-Kampczyk UE, Fritz GJ, Diez U, Lehmann I, Richter M, Herbarth O. Well water – one source of Helicobacter pylori colonization. Int J Hyg Environ Health 2004; 207:363-8; PMID:15471100; http://dx.doi.org/10.1078/1438-4639-00301
- Lindkvist P, Enquselassie F, Asrat D, Nilsson I, Muhe L, Giesecke J. Helicobacter pylori infection in Ethiopian children: a cohort study. Scand J Infect Dis 1999; 31:475-80; PMID:10576126; http://dx.doi.org/10.1080/00365549950163996
- Naficy AB, Frenck RW, Abu-Elyazeed R, Kim Y, Rao MR, Savarino SJ, Wierzba TF, Hall E, Clemens JD. Seroepidemiology of Helicobacter pylori infection in a population of Egyptian children Int J Epidemiol 2000; 29:928-32; PMID:11034980; http://dx.doi.org/10.1093/ije/29.5.928
- Cheung J, Goodman K, Munday R, Heavner K, Huntington J, Morse J, Veldhuyzen van Zanten S, Fedorak RN, Corriveau A, Bailey RJ; CANHelp work. Helicobacter pylori infection in Canada's arctic: searching for the solutions. Can J Gastroenterol 2008; 22:912-6; PMID:19018336
- Gwich’in Social and Cultural Institute. Aklavik: The Gwich’in. 2006. at http:www.gwichin.caTheGwichinaklavik.html
- Statistics Canada. Old Crow, Yukon (Code 6001043) and Yukon, Yukon (Code 6001) (table). Census Profile. 2011 Census. Statistics Canada Catalogue no. 98-316-XWE 2012.
- Statistics Canada. Tuktoyaktuk, Northwest Territories (Code 6101036) and Region 1, Northwest Territories (Code 6101) (table). Census Profile. 2011 Census. Statistics Canada Catalogue no. 98-316-XWE. 2012. Available from http:www12.statcan.gc.cacensus-recensement2011dp-pdprofdetailspage.cfm?Lang=E&Geo1=CSD&Code1=6101036&Geo2=CD&Code2=6101&Data=Count&SearchText=Tuktoyaktuk&SearchType=Begins&SearchPR=61&B1=All&Custom=&TABID=1
- Council of Yukon First Nations. Gwich’in Tribal Council. at http://www.cyfn.caourhistory
- Bureau of Statistics. Aklavik Profile. 2004
- Hunt R, Thomson AB. Canadian Helicobacter pylori consensus conference. Canadian Association of Gastroenterology. Can J Gastroenterol 1998; 12:31-41
- Bourke B, Ceponis P, Chiba N, Czinn S, Ferraro R, Fischbach L, Gold B, Hyunh H, Jacobson K, Jones NL, et al. Canadian Helicobacter Study Group Consensus Conference: Update on the approach to Helicobacter pylori infection in children and adolescents–an evidence-based evaluation. Can J Gastroenterol 2005; 19:399-408; PMID:16010300
- Malfertheiner P, Megraud F, O'Morain C, Bazzoli F, El-Omar E, Graham D, Hunt R, Rokkas T, Vakil N, Kuipers EJ. Current concepts in the management of Helicobacter pylori infection: the Maastricht III consensus report. Gut 2007; 56:772-81; PMID:17170018; http://dx.doi.org/10.1136/gut.2006.101634
- Graham DY Klein PD, Evans DJ Jr, Evans DG, Alpert LC, Opekun AR, Boutton TW. Campylobacter pylori detected noninvasively by the 13C-urea breath test. Lancet 1987; 329:1174-7; PMID:2883491; http://dx.doi.org/10.1016/S0140-6736(87)92145-3
- Gisbert JP, Pajares JM. Review article: 13C-urea breath test in the diagnosis of Helicobacter pylori infection – a critical review. Aliment Pharmacol Ther 2004; 20:1001-17; PMID:15569102; http://dx.doi.org/10.1111/j.1365-2036.2004.02203.x
- Klein PD, Malaty HM, Czinn SJ, Emmons SC, Martin RF, Graham DY. Normalizing results of 13C-urea breath testing for CO2 production rates in children. J Pediatr Gastroenterol Nutr 1999; 29:297-01; PMID:10467995; http://dx.doi.org/10.1097/00005176-199909000-00011
- Stolte M, Meining A. The updated Sydney system: classification and grading of gastritis as the basis of diagnosis and treatment. Can J Gastroenterol 2001; 15:591-8
- Goodman KJ, Correa P, Tenganá Aux HJ, DeLany JP, Collazos T. Nutritional factors and Helicobacter pylori infection in Colombian children. J Pediatr Gastroenterol Nutr 1997; 25:507-15; PMID:9360204; http://dx.doi.org/10.1097/00005176-199711000-00004
- Pearce N. Effect Measures in Prevalence Studies. Environ Health Perspect 2004; 112:1047-50; PMID:15238274; http://dx.doi.org/10.1289/ehp.6927
- Hosmer DW, Lemeshow S. Applied Logistic Regression. New York: John Wiley & Sons, 2000.
