Abstract
While the gut epithelium represents the largest mucosal tissue, the mechanisms underlying the interaction between intestinal bacteria and the host epithelium lead to multiple outcomes that remain poorly understood at the molecular level. Deciphering such events may provide valuable information as to the mode of action of commensal and probiotic microorganisms in the gastrointestinal environment. Potential roles of such microorganisms along the privileged target represented by the intestinal immune system include maturation processes prior, during and after weaning, and the reduction of inflammatory reactions in pathogenic conditions. As commensal bacteria are naturally coated by natural and antigen-specific SIgA in the gut lumen, understanding the consequences of such an interaction may provide new clues on how the antibody contributes to homeostasis at mucosal surfaces. This review discusses several aspects of the role of SIgA in the essential communication existing between the host epithelium and members of its microbiota.
Abbreviations
| DC | = | dendritic cell |
| IEC | = | intestinal epithelial cell |
| pIgR | = | polymeric Ig receptor |
| SC | = | secretory component |
| SED | = | subepithelial dome |
| SIgA | = | secretory IgA |
Introduction
The mucosal surfaces of the body comprise an enormous interface (estimated to 400 m2 in the human adult intestine) that physically separates the interior of the body and the external environment. In contrast to the systemic compartment, which needs to remain sterile as the presence of a microorganism reflects a potentially life-threatening condition, mucosal surfaces are constantly challenged by a plethora of antigens of various nature and complexity.
A particular feature of the gastrointestinal tract is the presence of a very diverse and dense microbiota comprising as many as 1014 bacterial cells, outnumbering the amount of cells composing the human body by a factor of up to 10.Citation1 In addition to peacefully co-exist with the host, an equilibrium referred to as commensalism, bacteria residing in the gut exhibit numerous protective and metabolic features essential to the filter function of the epithelial barrier lining mucosal surface.Citation2 In contrast to enteropathogens turning on multiple pro-inflammatory circuits that will lead to their elimination, commensal bacteria populating the gastrointestinal tract are not overtly inflammatory, and this results in graded or dampened responses appropriate to ensure their symbiotic persistence.Citation3 How commensal bacteria foster the development of the mucosal immune system, and in particular the stimulation of the biosynthesis of local SIgA, has been comprehensively reviewed lately.Citation4-6
SIgA is the most abundant antibody molecule on mucosal surfaces of humans and most other mammals. Production of IgA at mucosal surfaces contributes to host defense against intestinal pathogensCitation7,8 and governs quantitative and qualitative control of commensal microbiota composition by the host.Citation9,10 Globally, the presence of SIgA at mucosal surfaces warrants that pro-inflammatory processes are kept under control, a feature that is essential to preserve the integrity and functionality of the epithelial barrier.Citation11-13 While other antibody isotypes are rapidly degraded, intact SIgA molecules can be regularly demonstrated in samples from mucosal surfaces even in the presence of large numbers of microorganisms. SIgA abundantly found in mucosal secretions results from the association during transport across epithelial cells from J chain-containing polymeric IgA with secretory component (SC), the cleavage product of the polymeric Ig receptor (pIgR).Citation14 The stability of SIgA depends largely on SC, which masks potential proteolytic cleavage sites,Citation15,16 ensuring preserved functionality in the enzymatically hostile environment that prevails on gut mucosal surfaces. In addition to its well documented role in pathogen neutralization and clearance,Citation7,8,10 SIgA is endowed with the capacity to selectively retro-transport bound antigens back into intestinal Peyer's patches across microfold (M) cellsCitation17 via the Dectin-1 receptor expressed at their surface.Citation18 In the subepithelial dome (SED) region, SIgA-based immune complexes associate with dendritic cells (DCs),Citation19 resulting in the onset of immunomodulatory types of responses.Citation20
Seminal papers published by the team of John Cebra more than 2 decades ago have demonstrated that at weaning, up to 70% of commensal bacteria are coated with natural SIgA in the mouse gastrointestinal tract, and that this association is instrumental to the maintenance of bacterial homeostasis;Citation21,22 the same holds true for pigs and calves.Citation23 Strikingly, interaction between commensals and SIgA is not only antigen-driven,Citation24 as natural polyreactive SIgA and Fab/Fc-independent, glycan-mediated binding are also likely to contribute substantially to this process.Citation25 The involvement of SIgA in the numerical control of the gut microbiota was further confirmed in germ-free or mono-associated mice.Citation26,27 In IgA-deficient mice, systemic antibody responses against commensal species are increased,Citation28,29 arguing for control of transepithelial dissemination by SIgA. The sum of these data provide evidence that mucosal SIgA is instrumental to contain and control the composition of commensal microbiota.Citation30 Provision of local SIgA appears thus to monitor the microbiota in some sort of a regulatory loop essential to ensure appropriate mucosal gut homeostasis.Citation31-32 The review covers this specific topic.
SIgA-Driven Binding of Commensal Bacteria to Epithelial Cells is Mediated by the Transferrin Receptor (CD71)
The importance of commensal bacteria in the maintenance of the integrity of intestinal epithelial cell (IEC) barrier and the control of the underlying immune mechanisms ensuring homeostasis is a fundamental process, whose multiple and complex features are not yet fully understood.Citation33-35 When considering the epithelial barrier function, in vitro exposure of reconstituted polarized Caco-2 epithelial cell monolayers to commensal strains either alone or coated with SIgA leads to a completely different pattern of a) cytokine/chemokine secretion, b) pIgR expression, and c) adhesion, indicating that the nature of the bacterium and the association with the antibody results in different sensing by the epithelial cells in the absence of any other partners.Citation36 While we speculated that commensal bacteria associated with SIgA would have a reduced effect on the reconstituted epithelial layer through mechanisms similar to immune exclusion, it happened that the presence of SIgA increased the bacterial anchoring at the apical surface of IECs. This event was accompanied by an increased phosphorylation of tight junction proteins sustaining cell-to-cell interaction. Increased production of thymic stromal lymphopoietin, a chemokine involved in maintaining a noninflammatory environment at mucosal surfaces,Citation37 was measured in the presence of SIgA. Furthermore, association of commensal bacteria with SIgA promoted pIgR production by IECs, leading to more receptor available for active SIgA transcytosis.Citation34 This phenomenon could account for the sustained SIgA secretion resulting from commensal colonization.Citation38 These characteristics argue for a contribution of SIgA in maintaining commensal bacteria at bay through a delicate balance combining appropriate neutralization and proper sensing by the IECs.
Several receptors for IgA have been identified on various cell populations including myeloid cells, DCs, T and B cells, hepatocytes and epithelial cells.Citation39 The presence of an IgA receptor different of pIgR and CD89 (FcalphaR1) on the human IEC line HT29 was first reported by Kitamura et al.,Citation40 yet its precise identity was not elucidated. Studies on the transport of gliadin peptides from the lumen to the lamina propria led to the conclusion that CD71 (transferrin receptor) expressed by epithelial cells is also able to bind human polymeric and SIgA.Citation41 The possible role of apically exposed CD71 in the binding of SIgA was addressed in our laboratory by incubating fluorescently labeled mouse SIgA monoclonal antibody in the apical compartment of polarized Caco-2 epithelial cell monolayers mimicking the intestinal barrier. Interaction of the antibody (green) with CD71 (red) detected by a specific antiserum resulted in the appearance of yellow spots reflecting colocalization (, top panels). Under identical conditions, no interaction between CD71 and monomeric IgA could be observed, consistent with the lower affinity of this molecular form of the antibody.Citation42 The presence of functional CD71 at the apical surface of the Caco-2 cell monolayer was confirmed in colocalization experiments carried out upon addition of transferrin, the “natural” ligand of the receptor expressed by IECsCitation43 (, bottom panels). In addition, transcytosis via CD71 of human SIgA loaded with gliadin peptides was observed when using polarized Caco-2 cell monolayers.Citation44 The sum of these data is compatible with a role of CD71 in endocytosis/internalization into intracellular vesicles of SIgA-antigen complexes from the apical surface, with concomitant induction of specific immune responses, as this takes place for the transport of IgE-allergen complexes through CD23.Citation45 Further investigations are needed to unravel whether SIgA following this pathway can target lamina propria DCs, as this occurs for DCs in the SED region following retro-transport of SIgA across M cells in Peyer's patches.Citation17
Figure 1. Interactions between (A) SIgA or (B) transferrin (Tf) and transferrin receptor (CD71), expressed by polarized Caco-2 epithelial cell monolayers. (A) Laser scanning confocal microscope analysis showing apical colocalization of fluorescently labeled SIgA (green) and CD71 (detected in red) in polarized Caco-2 cells as revealed by the appearance of yellow spots. (B) Physical interaction revealed by yellow dots at the apical surface between Tf and CD71. For both sets of images, cell nuclei are counterstained using 4′,6′-diamidino-2-phenylindole (DAPI, gray). SIgA or Tf were incubated for 1 hour at 37°C. The top view of 3D reconstructions from Z-stack acquisitions are depicted. Results are representative of 3 experiments performed in triplicate Transwell filters carrying polarized Caco-2 cell monolayers. White bar, 20 μm.

SIgA-Driven Entry, and Subsequent Targeting to DCs, of Commensal Bacteria in Peyer's Patches
In addition to their interaction with enterocytes, commensal bacteria are known to be sensed by immune cells localized beyond the epithelial barrier, such as DCs residing in the lamina propria of the gut mucosa or, even more importantly, in organized lymphoid immune inductive sites such as Peyer's patches.Citation46 The fact that the majority of intestinal bacteria are naturally coated by SIgA molecules (see Introduction) together with the observation that SIgA per se or bound to antigens is targeted after transport via M cells to DCs in the SED region of Peyer's patches suggests a potential implication of the antibody in the process of sensing of commensal bacteria by this tissue. Such an hypothesis has been recently demonstrated in our laboratory by following via laser scanning confocal microscopy the dynamics of entry in Peyer's patches of fluorescently labeled bacteria injected into a mouse intestinal ligated loop. Association of the strains Lactobacillus Rhamnosus CGMCC 1.3724Citation36 (referred to as LPR in the text) or Bifidobacterium lactis CNCM 1.2618Citation36 (referred to as BL in the text) with non-specific mouse SIgA prior to injection into the intestinal loop allowed accelerated internalization of the bacteria within Peyer's patches, in particular in the SED region underlying the follicle-associated epithelium (Ref. 47 and ). Moreover, once in the tissue, the antibody-coated bacteria were found in close interaction with the CD11c+CD11b+ subset of DCs prone to initiate local induction of IL-10 and TGF-β producing T cells involved in immune regulatory mechanisms.Citation48 Thus, SIgA appears as a vehicle facilitating the targeting and sensing of commensals by SED DCs ideally located to fulfill this function. This conclusion was further reinforced by the observation that bacteria injected into intestinal loops without initial association with SIgA were rapidly coated with endogenous luminal SIgA, resulting in subsequent transport across M cells followed by interaction with DCs in the SED region of Peyer's patches as well (Ref. 47 and ). Detection of SIgA-bacteria complexes with antibodies directed against the SC indicated that that bacteria were targeted to DCs in this particular form, in agreement with the capacity of SIgA to specifically interact with these cells. The time required for endogenous association to occur most likely explains the delay observed between bacteria delivered alone and those bound to SIgA prior to injection. In support of this, bacteria injected into intestinal loops of germ-free mice producing extremely reduced endogenous SIgA exhibit further limited entry into Peyer's patches as a function of time.Citation47
Figure 2. Preassociation with SIgA monoclonal antibody promotes entry of commensal bacteria into Peyer's patches (PP) and subsequent uptake by DCs in the SED region. (A) Representative picture of the tracking of FITC-labeled BL (green rods), administered alone or in complex with non-specific SIgA, 2 hours after injection in a ligated ileal loop comprising a PP. More elevated number of bacteria could be detected in the subepithelial dome (SED) region of PPs when administered in association with SIgA (right panel), with most of them observed in close contact with red-labeled DCs, resulting in the appearance of yellow spots (arrows). Cell nuclei are counterstained with 4′,6′-diamidino-2-phenylindole (DAPI, blue). L, lumen; FAE, follicule associated epithelium. (B) Quantification of FITC-labeled BL in the FAE and SED region of 6 individual sections obtained from 3 independent experiments. Numbers are median ± SE (error bars) and statistical differences calcuated with the Student's t test comparing the 2 experimental conditions at the same time-point are indicated by their respective p value.
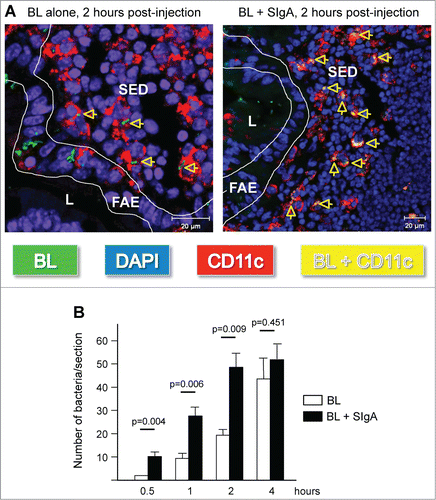
Figure 3. In vivo coating of BL by engodenous intestinal SIgA and subsequent entry into a Peyer's patch. (A) Representative image of the visualization of FITC-labeled BL (green rods) administered as such in a mouse ligated ileal loop containing a Peyer's patch. Cell nuclei are stained in blue (DAPI). (B) Three-dimensional reconstructed image from the magnified area (yellow square) depicted in panel (A). Magnification of the subepithelial dome (SED) region demonstrates surface (yellow) and internal (green) co-localization with red-labeled DCs. Bacteria interacting with DCs are associated with endogenous SIgA (arrows in magnified insets 1 and 2). In the insets, the red channel was not activated to allow visualization of coating SIgA on the bacterial surface (pink). L, lumen; V, villus.
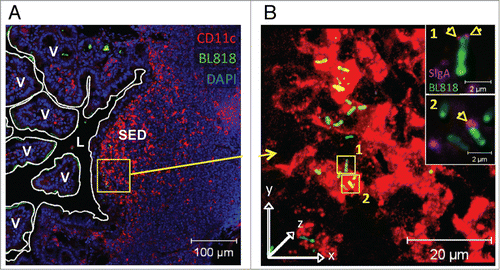
Interestingly, the dynamics of entry appeared to be different between the 2 investigated strains; while internalized BL administered into a ligated loop as such could be detected in close association with DCs in the SED region within 2 hours, LPR necessitated as much as 6 hours. Whether this is related to intrinsic bacterial strain properties, including capacity to bind to SIgA or luminal localization closer to the sampling site, or to host-related features as for instance the endogenous microbiota, is in need of further investigations. It can be postulated that the presence of more SIgA specific for Bifidobacteria than for Lactobacilli, although mice are more colonized by bacteria from the later genus, may impact on the way by which bacteria-SIgA complexes are formed and subsequently perceived by the host mucosal immune system. While the above-reported experiments support an intrinsic effect of the association of bacteria with non-specific SIgA via interactions with carbohydrate moieties,Citation49 we cannot exclude that antigen-specific recognition may be of an added value for the sensing process. In this respect, association between an antigen and its cognate specific SIgA triggers structural changes in the antibody, which in turn confers enhanced interaction with cognate receptors,Citation50 a feature which may explain the favored interaction of SIgA-coated commensals to M cells or DCs. Moreover, experiments done with mutant mice displaying limited diversity of the Ig repertoire due to a severe defect in somatic hypermutation, but having wild-type equivalent intestinal total IgA production, demonstrated that the incapacity to generate antigen-specific antibodies affected immune responsiveness to mucosal challenges as expected, but altered also the microbiota balance.Citation51
In conclusion, all these recent in vivo results strongly support the concept that association with SIgA not only facilitates selective entry of commensal bacteria across the gut epithelium, but may additionally be an important driver toward the optimal sensing of commensal bacteria in mucosal inductive sites such as PPs. These data provide partial clues as to the still puzzling issue of how SIgA contributes to the complex mechanisms of actively regulating mucosal immune homeostasis, in particular discrimination between noxious and resident microorganisms.Citation52 The question of the fine tuning of such SIgA-driven process in controlling commensal bacteria sensing by, and priming of, DCs remains to be further investigated.
Effect of SIgA on Commensal-Bacteria-Driven Early Maturation of the Gut Immune System
While SIgA-driven commensal bacteria sampling and sensing appears as a constant ongoing process ensuring gut homeostasis, there are situations where its consequence is of outstanding importance. This is for example the case in the early life period; although neonates are exposed to a unique microbiome prior to birth, as mostly analyzed via 16S rRNA,Citation53,54 progressive colonization by a developing microbiota ecosystem shortly after vaginal delivery requires control mechanisms to ensure appropriate symbiosis.Citation55 This colonization process and its interplay with the host is now commonly seen as a key factor to ensure proper post-natal maturation of the gut epithelium barrier and early programming of physiological processes, 2 features which positively influence health throughout life.Citation56-58 It is now generally accepted that induction/completion of immune maturation relies on the presence of newly established commensal intestinal microbes, and that such a process will lead to production of endogenous SIgA, which in turn shapes the composition of the microbiota.Citation59 This is illustrated by the virtually complete absence of IgA in neonates, as well as by its drastic reduction in germ-free animals, a status that returns to normality following colonization with a commensal microbiota.Citation60 The momentarily absence of endogenous SIgA production in newborns is compensated in mammals by provision of such antibodies through breast milk, whose concentration in humans ranges between 0.4 to 1.0 g/L. Breast milk SIgA may thus play an important role in early-life bacterial sensing by ensuring optimal microbiota-driven maturation and programming of the mucosal or systemic immune system.
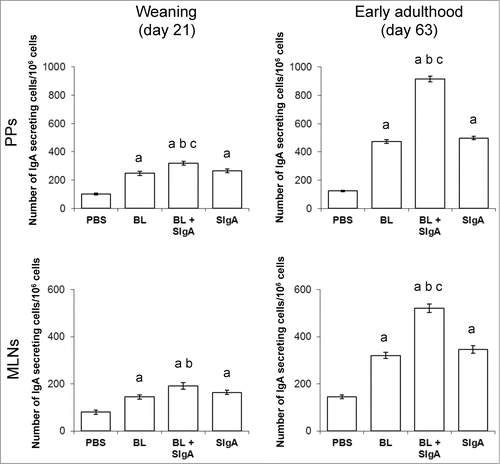
To address this hypothesis, we investigated the impact of neonatal SIgA supplementation in an immune maturation's mouse model in which germ-free dams, whose breast milk is thus almost deprived of SIgA, were used to feed their pups exposed to mouse fecal bacteria to permit natural microbial colonization. Pups were daily supplemented during the pre-weaning period with either a saline solution (control for absence of SIgA), or the commensal bacteria BL known to promote immune maturation in humans,Citation61 or BL associated with non-specific murine SIgA, or the equivalent amount of the same SIgA alone. Monitoring of gut mucosal immune maturation through measurement of endogenous IgA production by ELISPOT measuring the number of IgA secreting cells in PPs and mesenteric lymph nodes revealed that association of BL with SIgA prior to administration was able to further potentiate the effect of BL on IgA production (), possibly through driving of the access of the commensal to mucosal immune inductive sites, as discussed in the previous section. Interestingly, administration of SIgA alone also promoted immune maturation. This may result from the association of exogenous SIgA with the endogenous growing microbiota in the pups, resulting in enhanced sampling and sensing in Peyer's patches in comparison to the SIgA-deprived control protocol. Noteworthy, the fact that supplementation of only very small amounts of SIgA, such as 500 pg/day, provided an measurable effect on local IgA-producing cells further supports a key role of the antibody in the microbiota-host interaction taking place during the very particular and crucial early-life period. Noteworthy, the effect of the presence of SIgA were not only visible at weaning, i.e. at the end of the supplementation period (, left panels), but was maintained for at least 6 weeks post-weaning (early adulthood, , right panels). This supports the concept of a long-lasting impact on immune programming when priming is enhanced via the presence of SIgA during the very early life period. The recent observation that immunoregulation of DCs by SIgA is potent in inhibiting autoimmune responses in mouse models suggest that the antibody is important to establish healthy homeostasis and possibly to serve in disease prevention.Citation62
Figure 4. Presence of SIgA during the pre-weaning period promotes early-life immune maturation. Mouse pups from germ-free dams were daily supplemented for the last 14 d of the suckling period with either a saline solution (PBS, control for absence of SIgA), 104 CFU of the commensal bacteria BL known to promote immune maturation in humans, 104 CFU of BL associated with 500 pg of non-specific murine SIgA monoclonal antibodyCitation28, or the equivalent amount of the same SIgA alone. Germ-free dams have an almost completely SIgA-deprived milk. Concomitantly to supplementation, pups were exposed to conventional mouse fecal bacteria to induce natural microbial colonization. Monitoring of mucosal immune maturation was assessed through quantification of the number IgA secreting cells in Peyer's patches (PPs, top panels) and mesenteric lymph nodes (MLNs, bottom panels), as measured by the ELISPOT technique (median ± SEM). IgA production was assessed at the end of the supplementation period (weaning, day 21 of age, left panels) and 6 weeks later (early adulthood, day 63 of age, right panels). Statistical significance (Wilcoxon test) is indicated by the letters: a = P < 0.001 vs. PBS control; b = P ≤ 0.002 vs. BL alone; c = P < 0.001 vs. SIgA alone. n = 10 to 11 pups per experimental group for each organ and time point investigated.
The importance of SIgA to ensure optimal immune maturation and to support long-term gut homeostasis was recently further supported in a totally different experimental model. Indeed, exploiting a mother foster approach with pIgR-sufficient and deficient mice, whose milk either contains or was devoid of SIgA, respectively, it was demonstrated that early exposure to maternal breast milk SIgA promoted intestinal epithelial barrier function.Citation63 In suckling neonates, this resulted in prevention of colonic damage caused by the epithelial-disrupting agent dextran sulfate sodium. More long-term effects including modulation of expression of intestinal epithelial cell genes associated with intestinal inflammatory diseases in humans and promotion of a healthy microbiota in adult mice were also observed. IgA-mediated control of the nature of microbiota partners during post-natal development has also been reported in a recent study,Citation64 suggesting that this mode of action of the antibody may find other examples in the future.
Conclusion
The above-described studies shed light on the beneficial effects of surrogate natural SIgA (nonspecific monoclonal IgA) and maternal IgA in the mouse intestine, as illustrated by epithelial cell programming, maintenance of tissue homeostasis or the regulation of immune maturation. The strict quantitative and qualitative contribution of commensal-specific affinity-matured SIgA in such a process remains to be confirmed through the use of purified antibody. It appears a sound speculation to anticipate that the key role of natural SIgA of low affinity would be to support SIgA-mediated sensing of commensals to drive optimal immune priming by the nascent microbiota. Such an early event would in turn foster initial gut homeostasis early in life, while affinity-matured antigen specific SIgA would be involved in the protection against less frequently encountered pathogens.Citation65 The dichotomy in the “aggressiveness” of the mucosal humoral response may represent a mechanism ensuring the symbiotic relationship between the host and its microbiota, as opposed to blocking and elimination required to fight invasive bacteria.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
The laboratory of Blaise Corthésy is supported by grants nos. 3100-138422 and 3100-156806 from the Swiss Science Research Foundation.
References
- Savage DC. Microbial ecology of the gastrointestinal tract. Annu Rev Microbiol 1977; 31:107-33; PMID:334036; http://dx.doi.org/10.1146/annurev.mi.31.100177.000543
- O’Hara AM, Shanahan F. The gut flora as a forgotten organ. EMBO Rep 2006; 7:688-93; PMID:16819463; http://dx.doi.org/10.1038/sj.embor.7400731
- Neish AS. Microbes in gastrointestinal health and disease. Gastroenterology 2009; 136:65-80; PMID:19026645; http://dx.doi.org/10.1053/j.gastro.2008.10.080
- Macpherson AJ, Slack E, Geuking MB, McCoy KD. The mucosal firewalls against commensal intestinal microbes. Semin Immunopathol 2009; 31:145-49; PMID:19707762; http://dx.doi.org/10.1007/s00281-009-0174-3
- Gutzeit C, Magri G, Cerutti A. Intestinal IgA production and its role in host-microbe interaction. Immunol Rev 2014; 260:76-85; PMID:24942683; http://dx.doi.org/10.1111/imr.12189
- Kaetzel CS. Cooperativity among secretory IgA, the polymeric immunoglobulin receptor, and the gut microbiota promotes host-microbial mutualism. Immuno Lett 2014; in press; S0165-2478(14):00100-X; PMID:24877874; http://dx.doi.org/10.1016/j.imlet.2014.05.008
- Cerutti A, Chen K, Chorny A. Immunoglobulin responses at the mucosal interface. Annu Rev Immunol 2011; 29:273-93; PMID:21219173; http://dx.doi.org/10.1146/annurev-immunol-031210-101317
- Corthésy B. Multi-faceted functions of secretory IgA at mucosal surfaces. Front Immunol 2013; 4:185; PMID:23874333; http://dx.doi.org/10.3389fimmu.2013.00185
- Hooper LV, Macpherson AJ. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nature Rev Immunol 2010; 10:159-69; PMID:20182457; http://dx.doi.org/10.1038/nri2710
- Brandtzaeg P. Gate-keeper function of the intestinal epithelium. Benef Microbes 2013; 4:67-82; PMID:23257015; http://dx.doi.org/10.3920/BM2012.0024
- Corthésy B. Roundtrip ticket for secretory IgA: role in mucosal homeostasis? J Immunol 2007; 178:27-32; PMID:17182536; http://dx.doi.org/10.4049/jimmunol.178.1.27
- Boullier S, Tanguy M, Kadaoui KA, Caubet C, Sansonetti P, Corthésy B, Phalipon A. Secretory IgA-mediated neutralization of Shigella flexneri prevents intestinal tissue destruction by down-regulating inflammatory circuits. J Immunol 2009; 183:5879-85; PMID:19828639; http://dx.doi.org/10.4049/jimmunol.0901838
- Corthésy, B. Role of secretory IgA in infection and maintenance of homeostasis. Autoimmun Rev 2013; 12:661-5; PMID:23201924; http://dx.doi.org/10.1016/j.autrev.2012.10.012
- Kaetzel CS. The polymeric immunoglobulin receptor: bridging innate and adaptive immune responses at mucosal surfaces. Immunol Rev 2005; 206:83-99; PMID:16048543; http://dx.doi.org/10.1111/j.0105-2896.2005.00278.x
- Prydz H, Brandtzaeg P. Direct evidence for an integrating function of J chain and secretory component in epithelial transport of immunoglobulins. Nature 1984; 311:71-3; PMID:6433206; http://dx.doi.org/10.1038/311071a0
- Crottet P, Corthésy B. Secretory component delays the conversion of secretory IgA into antigen binding competent F(ab)’2: A possible implication for mucosal defense. J Immunol 1998; 161:5445-53; PMID:9820520
- Rey J, Garin N, Spertini F, Corthésy B. Targeting of secretory IgA to Peyer's patch dendritic and T cells after transport by intestinal M cells. J Immunol 2004; 172:3026-33; PMID:14978107; http://dx.doi.org/10.4049/jimmunol.172.5.3026
- Rochereau N, Drocourt D, Perouzel E, Pavot V, Redelinghuys P, Brown GD, Tibary G, Roblin X, Verrier B, Genin C. Dectin-1 is essential for reverse transcytosis of glycosylated SIgA-antigen complexes by intestinal M cells. PLoS Biol 2013; 11(9):e1001658; PMID:24068891; http://dx.doi.org/10.1371/journal.pbio.1001658
- Kadaoui KA, Corthésy B. Secretory IgA mediates bacterial translocation to dendritic cells in mouse Peyer's patches with restriction to mucosal compartment. J Immunol 2007; 179:7751-7; PMID:18025221; http://dx.doi.org/10.4049/jimmunol.179.11.7751
- Favre L, Spertini F, Corthésy B. Secretory IgA possesses intrinsic modulatory properties stimulating mucosal and systemic immune responses. J Immunol 2005; 175:2793-800; PMID:16116164; http://dx.doi.org/10.4049/jimmunol.175.5.2793
- van der Waaij LA, Limburg PC, Mesander G, van der Waaij D. In vivo coating of anaerobic bacteria in human feces. Gut 1996; 38:348-54; PMID:8675085; http://dx.doi.org/10.1136/gut.38.3.348
- Cebra JJ, Periwal SB, Lee G, Lee F, Shroff KE. Development and maintenance of the gut-associated lymphoid tissue (GALT): the roles of enteric bacteria and viruses. Dev Immunol 1998; 6:13-8; PMID:9716901; http://dx.doi.org/10.1155/1998/68382
- Tsuruta T, Inoue R, Tsukahara T, Nakamoto M, Hara H, Ushida K, Yajima T. Commensal bacteria coated by secretory immunoglobulin A and immunoglobulin G in the gastrointestinal tract of pigs and calves. Anim Sci J 2012; 83:799-804; PMID:23216546; http://dx.doi.org/10.1111/j.1740-0929.2012.01026.x
- Lindner C, Wahl B, Fohse L, Suerbaum S, Macpherson AJ, Prinz I, Pabst O. Age, microbiota, and T cells shape diverse individual IgA repertoires in the intestine. J Exp Med 2012; 209:365-77; PMID:22249449; http://dx.doi.org/10.1084/jem.20111980
- Mathias A, Corthésy B. Recognition of gram-positive intestinal bacteria by hybridoma- and colostrum-derived secretory immunoglobulin A is mediated by carbohydrates. J Biol Chem 2011; 286:17239-47; PMID:21454510; http://dx.doi.org/10.1074/jbc.M110.209015
- Suzuki K, Meek B, Doi Y, Muramatsu M, Chiba T, Honjo T, Fagarasan S. Aberrant expansion of segmented filamentous bacteria in IgA-deficient gut. Proc Natl Acad Sci USA 2004; 101:1981-6; PMID:14766966; http://dx.doi.org/10.1073/pnas.0307317101
- Macpherson AJ, Uhr T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science 2004; 303:1662-65; PMID:15016999; http://dx.doi.org/10.1126/science.1091334
- Sait LC, Galic M, Price JD, Simpfendorfer KR, Diavatopoulos DA, Uren TK, Janssen PH, Wijburg OL, Strugnell RA. Secretory antibodies reduce systemic antibody responses against the gastrointestinal commensal flora. Int Immunol 2007; 19:257-65; PMID:17255112; http://dx.doi.org/10.1093/intimm/dxl142
- Peterson DA, McNulty NP, Guruge JL, Gordon JI. IgA response to symbiotic bacteria as a mediator of gut homeostasis. Cell Host Microbe 2007; 2:328-39; PMID:18005754; http://dx.doi.org/10.1016/j.chom.2007.09.013
- Kawamoto S, Tran TH, Maruya M, Suzuki K, Doi Y, Tsutsui Y, Kato LM, Fagarasan S. The inhibitory receptor PD-1 regulates IgA selection and bacterial composition in the gut. Science 2012; 336:485-9; PMID:22539724; http://dx.doi.org/10.1126/science.1217718
- Willing BP, Gill N, Finlay BB. The role of the immune system in regulating the microbiota. Gut Microbes 2010; 1:213-23; PMID:21327028; http://dx.doi.org/10.4161/gmic.1.4.12520
- Mantis NJ, Rol N, Corthésy B. Secretory IgA's complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol 2011; 4:603-11; PMID:21975936; http://dx.doi.org/10.1038/mi.2011.41
- Kayama H, Takeda K. Regulation of intestinal homeostasis by innate and adaptive immunity. Int Immunol 2012; 24:673-80; PMID:22962437; http://dx.doi.org/10.1093/intimm/dxs094
- Ivanov II, Honda K. Intestinal commensal microbes as immune modulators. Cell Host Microbe 2012; 12:496-508; PMID:23084918; http://dx.doi.org/10.1016/j.chom.2012.09.009
- Vieira AT, Teixera MM, Martins FS. The role of probiotics and prebiotics in inducing gut immunity. Front Immunol 2013; 4:445; PMID:24376446; http://dx.doi.org/10.3389/fimmu.2013.00445
- Mathias A, Duc M, Favre L, Benyacoub J, Blum S, Corthésy B. Potentiation of polarized intestinal Caco-2 cell responsiveness to probiotics complexed with secretory IgA. J Biol Chem 2010; 285:33906-13; PMID:20729211; http://dx.doi.org/10.1074/jbc.M110.135111
- Zeuthen LH, Fink LN, Frokiaer H. Epithelial cells prime the immune response to an array of gut-derived commensals towards a tolerogenic phenotype through distinct actions of thymic stromal lymphopietin and transforming growth factor-beta. Immunology 2008; 123:197-208; PMID:17655740
- Macpherson AJ, McCoy KD. Stratification and compartimentalization of immunoglobulin responses to commensal intestinal microbes. Semin Immunol 2013; 25:358-63; PMID:24238818; http://dx.doi.org/10.1016/j.smim.2013.09.004
- Wines BD, Hogarth PM. IgA receptors in health and disease. Tissue Antigens 2006; 68:103-14; PMID:16866880; http://dx.doi.org/10.1111/j.1399-0039.2006.00613.x
- Kitamura T, Garofalo RP, Kamijo A, Hammond DK, Oka JA, Caflisch CR, Shenoy N, Casola A, Weigel PH, Goldblum RM. Human intestinal epithelial cells express a novel receptor for IgA. J Immunol 2000; 164:5029-34; PMID:10799857; http://dx.doi.org/10.4049/jimmunol.164.10.5029
- Mathysiak-Budnik T, Moura IC, Arcos-Fajardo M, Lebreton C, Ménard S, Candalh C, Ben-Khalifa K, Dugave C, Tamouza H, van Niel G, et al. Secretory IgA mediates retrotranscytosis of intact gliadin peptides via the transferrin receptor in celiac disease. J Exp Med 2008; 205:143-54; PMID:18166587; http://dx.doi.org/10.1084/jem.20071204
- Moura IC, Arcos-Fajardo M, Sadaka C, Leroy V, Benhamou M, Novak J, Vrtovsnik F, Haddad E, Chintalacharuvu KR, Monteiro RC. Glycosylation and size of IgA1 are essential for interaction with mesangial transferrin receptor in IgA nephropathy. J Am Soc Nephrol 2004; 15:622-34; PMID:14978164; http://dx.doi.org/10.1097/01.ASN.0000115401.07980.0C
- Anderson GJ, Powell LW, Halliday JW. The endocytosis of transferrin by rat intestinal epithelial cells. Gastroenterology 1994; 106:414-22; PMID:8299907
- Lebreton C, Ménard S, Abed J, Moura IC, Coppo R, Dugave C, Monteiro RC, Fricot A, Traore MG, Griffin M, et al. Interactions among secretory immunoglobulin A, CD71, and transgluraminase-2 affect permeability of intestinal epithelial cells to gliadin peptides. Gastroenterology 2012; 143:698-707; PMID:22750506; http://dx.doi.org/10.1053/j.gastro.2012.05.051
- Perrier C, Corthésy B. Gut permeability and food allergies. Clin Exp Allergy 2010; 41:20-8; PMID:21070397; http://dx.doi.org/10.1111/j.1365-2222.2010.03639.x
- Uhlig HH, Powrie F. Dendritic cells and the intestinal microflora: a role for localized mucosal immune responses. J Clin Invest 2003; 112:648-51; PMID:12952911; http://dx.doi.org/10.1172/JCI19545
- Rol N, Favre L, Benyacoub J, Corthésy B. The role of secretory immunglobulin A in the natural sensing of commensal bacterial by mouse Peyer's patch dendritic cells. J Biol Chem 2012; 287:40074-82; PMID:23027876; http://dx.doi.org/10.1074/jbc.M112.405001
- Tsuji NM. Antigen-specific CD4+ regulatory T cells in the intestine. Inflamm Allergy Drug Targets 2006; 5:191-201; PMID:16918482; http://dx.doi.org/10.2174/187152806778256043
- Mathias A, Corthésy B. N-glycans on secretory component: mediators of the interaction between secretory IgA and gram-positive commensals sustaining mucosal homeostasis. Gut Microbes 2011; 2:287-93; PMID:22067937; http://dx.doi.org/10.4161/gmic.2.5.18269
- Duc M, Johansen FE, Corthésy B. Antigen binding to secretory immunoglobulin A results in decreased sensitivity to intestinal proteases and increased binding to cellular Fc receptors. J Biol Chem 2010; 285:953-60; PMID:19910466; http://dx.doi.org/10.1074/jbc.M109.059220
- Wei M, Shinkura R, Doi Y, Maruya M, Fagarasan S, Honjo T. Mice carrying a knock-in mutation of Aicda resulting in a defect in somatic hypermutation have impaired gut homeostasis and compromised mucosal defense. Nat Immunol 2011; 12:264-70; PMID:21258321; http://dx.doi.org/10.1038/ni.1991
- Kato LM, Kawamoto S, Maruya M, Fagarasan S. Gut TFH and IgA: key players for regulation of bacterial communities and immune homeostasis. Immunol Cell Biol 2014; 92:49-56; PMID:24100385; http://dx.doi.org/10.1038/icb.2013.54
- Jimenez E, Fernandez J, Marin ML, Martin R, Odriozola JM, Nueno-Palop C, Narbad A, Olivares M, Xaus J, Rodriguez JM. Isolation of commensal bacteria from umbilical cord blood of healthy neonates born by cesarean section. Curr Microbiol 2005; 51:270-4; PMID:16187156; http://dx.doi.org/10.1007/s00284-005-0020-3
- Moles L, Gomez M, Heilig H, Bustos G, Fuentes S, de Wos W, Fernandez J, Rodriguez JM, Jimenez E. Bacterial diversity in meconium of preterm neonates and evolution of their fecal microbiota during the first month of life. PLoS One 2013; 8:e66986; PMID:23840569; http://dx.doi.org/10.1371/journal.pone.0066986
- Wopereis H, Oozeer R, Knipping K, Belzer C, Knol J. The first thousand days - intestinal microbiology of early life: establishing a symbiosis. Pediatr Allergy Immunol 2014; PMID:24899389; http://dx.doi.org/10.1111pai.12232
- Isolauri E. Development of healthy gut microbiota early in life. J Paediatr Child Health 2012; 48(Suppl 3):1-6; PMID:22681492; http://dx.doi.org/10.1111/j.1440-1754.2012.02489.x
- Nauta AJ, Ben Amor K, Knol J, Garssen J, van der Beek EM. Relevance of pre- and postnatal nutrition to development and interplay between the microbiota and metabolic and immune system. Am J Clin Nutr 2013; 98:586S-93S; PMID:23824726; http://dx.doi.org/10.3945/ajcn.112.039644
- Nylund L, Satokari R, Salminen S, de Vos WM. Intestinal microbiota during early life - impact on health and disease. Proc Nutr Soc 2014; 5:1-13; PMID:24902044; http://dx.doi.org/10.1017/S0029665114000627
- Kato LM, Kawamoto S, Maruya M, Fagarasan S. The role of the adaptive immune system in regulation of gut microbiota. Immunol Rev 2014; 260:67-75; PMID:24942682; http://dx.doi.org/10.1111/imr.12185
- Macpherson AJ, Mc Coy KD, Johansen FE, Brandtzaeg P. The immune geography of IgA induction and function. Mucosal Immunol 2008; 1:11-22; PMID:19079156; http://dx.doi.org/10.1038/mi.2007.6
- Mohan R, Koebnick C, Schildt J, Mueller M, Radke M, Blaut M. Effect of Bifidobacterium lactis Bb12 supplementation on body weight, fecal pH, acetate, lactate, calprotectin, and IgA in preterm infants. Pediatr Res 2008; 64:418-22; PMID:18552710; http://dx.doi.org/10.1203/PDR.0b013e318181b7fa
- Diana J, Moura IC, Vaugier C, Gestin A, Tissandie E, Beaudoin L, Corthésy B, Hocini H, Lehuen A, Monteiro RC. Secretory IgA induces tolerogenic dendritic cells through SIGNR1 dampening autoimmunity in mice. J Immunol 2013; 191:2335-43; PMID:23926325; http://dx.doi.org/10.4049/jimmunol.1300864
- Rogier EW, Frantz AL, Bruno ME, Wedlund L, Cohen DA, Stromberg AJ, Kaetzel CS. Secretory antibodies in breast milk promote long-term intestinal homeostasis by regulating the gut microbiota and host geneS expression. Proc Natl Acad Sci USA 2014; 111:3074-9; PMID:24569806; http://dx.doi.org/10.1073/pnas.1315792111
- Mirpuri J, Raetz M, Sturge CR, Wilhelm CL, Benson A, Savani RC, Hooper LV, Yarovinsky F. Proteobacteria-specific IgA regulates maturation of the intestinal microbiota. Gut Microbes 2014; 5:28-39; PMID:24637807; http://dx.doi.org/10.4161/gmic.26489
- Slack E, Balmer ML, Fritz JH, Hapfelmeyer S. Functional flexibility of intestinal IgA - broadening the fine line. Front Immunol 2012; 3:100; PMID:22563329; http://dx.doi.org/10.3389/fimmu.2012.00100
