Abstract
Clostridium difficile is mainly a nosocomial pathogen and is a significant cause of antibiotic-associated diarrhea. It is also implicated in the majority of cases of pseudomembranous colitis. Recently, advancements in next generation sequencing technology (NGS) have highlighted the extent of damage to the gut microbiota caused by broad-spectrum antibiotics, often resulting in C. difficile infection (CDI). Currently the treatment of choice for CDI involves the use of metronidazole and vancomycin. However, recurrence and relapse of CDI, even after rounds of metronidazole/vancomycin administration is a problem that must be addressed. The efficacy of alternative antibiotics such as fidaxomicin, rifaximin, nitazoxanide, ramoplanin and tigecycline, as well as faecal microbiota transplantation has been assessed and some have yielded positive outcomes against C. difficile. Some bacteriocins have also shown promising effects against C. difficile in recent years. In light of this, the potential for emerging treatment options and efficacy of anti-C. difficile vaccines are discussed in this review.
Abbreviations
| NGS | = | next generation sequencing |
| CDI | = | Clostridium difficile infection |
| PMC | = | pseudomembranous colitis |
| PaLoc | = | pathogenicity locus |
| RNA | = | ribonucleic acid |
| RBS | = | ribosome binding site |
| R027 | = | ribotype 027 |
| CdtLoc | = | binary toxin locus |
| ESCMID | = | European Society of Clinical Microbiology and Infectious Diseases |
| FMT | = | faecal microbiota transplantation |
| IDSA | = | Infectious Diseases Society of America |
| MIC | = | minimum inhibitory concentration |
| SHEA | = | Society for Healthcare Epidemiology of America |
| GIT | = | gastrointestinal tract |
| M21V | = | methionine to valine substitution at residue 21 |
| DPC | = | Dairy Products Collection |
| ATCC | = | American Type Culture Collection |
| NVB | = | Novacta Biosystems Ltd |
| V15F | = | valine to phenylalanine substitution at residue 15 |
| HIV | = | human immunodeficiency virus |
| IgG | = | immunoglobulin G |
| ETEC | = | enterotoxigenic E. coli |
| LTA | = | lipoteichoic acid |
| RBD | = | receptor binding domain |
| DNA | = | deoxyribonucleic acid |
| FDA | = | Food and Drug Administration |
Introduction
Clostridium difficile is a Gram-positive anaerobic sporeformer and is the etiological agent responsible for C. difficile-associated diarrhea. C. difficile was initially considered a harmless commensal of the gastrointestinal tract of infants, when originally isolated in 1935 but its role in nosocomial diarrhea and pseudomembranous colitis (PMC) was appreciated only in the 1970s.Citation1,2 The development of antibiotics for the treatment of infectious diseases in the 20th century has been a significant accomplishment. However, it is ironic that antibiotics, and in particular broad spectrum antibiotics, are the main etiological agents of one of the most notorious nosocomial infections, CDI. CDI has significant financial implications, and an estimated €5000–15000 is spent per CDI case in England and approximately €3 billion per year in the EU in total. The corresponding figure is about €2–4 billion per year in the US.Citation3,4 The majority of C. difficile strains produce toxin A and toxin B, which are responsible for the clinical manifestation of the disease. The recent emergence and widespread dissemination of ‘hypervirulent’ outbreak-associated C. difficile strains have caused problems for clinical practitioners. Furthermore, the ongoing problem of CDI recurrence post-antibiotic therapy, caused by perturbations of the gut microbiota, has encouraged scientists to seek alternative therapeutic options. Perhaps the most potent defense against CDI is the maintenance/restoration of a fully intact gut microbiota, providing protection through a complex process referred to as ‘colonization resistance.’
This review focuses on the successes and failures of current and emerging treatment options for CDI. In particular, we focus on antibiotics and adjunctive therapeutic options which have the potential to replace the current standard metronidazole and/or vancomycin therapy. In this regard, recent in vivo studies and clinical trials conducted with alternative and/or adjunct anti-C. difficile therapeutic options are discussed.
Roles of C. difficile toxin genes and disease
Until recently, investigating the role of C. difficile genes had been problematic. However, advancements in targeted mutagenesis systems have helped this cause.Citation5-7 A 19.6 kb pathogenicity locus (PaLoc) encodes the genes (tcdA and tcdB) for toxins A and B (TcdA and TcdB respectively), along with genes for putative positive and negative regulators of toxin expression (tcdR and tcdC respectively), as well as tcdE, encoding a putative holin protein ().Citation8,9 TcdR functions as an alternative RNA polymerase sigma factor, and thus behaves as a positive regulator of toxin gene expression.Citation10 In contrast, TcdC was initially thought to serve as a negative regulator of toxin production, destabilising the TcdR-holoenzyme, thus hindering transcription of the PaLoc.Citation8 However, in recent times, it has been demonstrated that transcription levels of the genes in the PaLoc and consequent total toxin production barely differs between a wild type C. difficile 630 Δerm strain and its tcdC mutant, suggesting that TcdC may not be a key regulator of toxin expression in the strain.Citation11 It is noteworthy that earlier studies investigating the role of TcdC were in vitro investigations.Citation12,13 The in vivo mechanisms of this protein had remained largely unclear however. A recent in vivo study has now indicated that TcdC may not actually play a key role in C. difficile virulence.Citation14 Furthermore, in another recent study, it was noted that there was no decrease in tcdC expression levels during stationary phase of growth, implying that TcdC may serve a modulatory function instead of a previously-hypothesized repressive function.Citation15 Polymorphisms in the tcdR-tcdB intergenic region as well as in the tcdR ribosome binding site (RBS) in the ‘non-hypervirulent’ VPI 10463 strain (which still produces high levels of toxins) likely results in increased translation of TcdR, consequently leading to read-through transcription of the toxin genes. Such polymorphisms may account for the increased levels of toxins produced by some isolates.Citation15 In addition, the study found that epidemic-associated strains sporulated at an earlier stage and produced a greater number of spores than other non-epidemic-associated isolates. Thus, increased sporulation rates along with high level toxin production may explain the outbreak-associated nature of such ‘hypervirulent strains.’Citation15 In an in vitro study, Vohra & Poxton noted that outbreak-associated R027 strains produced higher amounts of toxins in the logarithmic and stationary growth phases, compared to other ribotypes.Citation16 Moreover, epidemic-associated strains were found to produce more toxins and a greater number of spores relative to R012.Citation16 It was also particularly noteworthy that tcdC expression levels were not attenuated during stationary growth phase, as was previously thought, lending credence to the novel hypothesis that TcdC has a modulatory effect on toxin production, instead of a repressive one. In addition, an elevated level of expression of tcdE in R027 strains highlights its involvement in the release of the toxins. Therefore, it is hypothesized that a combination of factors, including greater toxin production, increased spore formation and increased expression of holin proteins may contribute to the epidemic-associated traits of certain strains.Citation16 Interestingly however, using isogenic strains of C. difficile, Carter et al. showed that a naturally occurring mutation in tcdC is responsible for the hypervirulence of epidemic C. difficile isolates.Citation12 Thus, the precise mechanisms of action of TcdC in vivo have yet to be definitively ascertained.
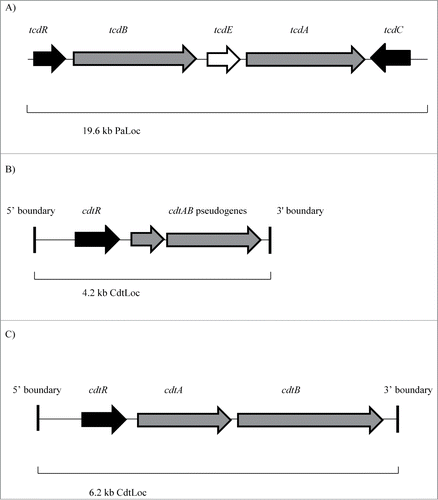
Figure 1. (A) Clostridium difficile pathogenicity locus (PaLoc). Schematic organization of the C. difficile PaLoc, which is 19.6 kb in length. tcdA and tcdB (shaded in gray) are the 2 genes encoding the 2 large C. difficile toxins, TcdA and TcdB respectively. tcdR (shaded in black) encodes a positive regulator of transcription, whereas tcdC (shaded in black) encodes a putative negative regulator/modulatory protein. tcdE (shaded in white) encodes a holin protein. Adapted from J Dupuy et al.Citation8 J Med Microbiol 2008; 57: 685–90 and Carter et al.Citation5 J Bacteriol 2007; 189: 7290–7301. (B) Binary toxin locus (CdtLoc) from C. difficile 630. Schematic organization of the binary toxin locus (4.2 kb in length) from the binary toxin-negative C. difficile 630 strain. The CDT binary toxin-encoding pseudogenes, cdtAB, are shaded in gray. The response regulator gene, cdtR is shaded in black. The highly conserved 5′ and 3′ boundaries are also indicated. Adapted from Carter et al.Citation5 J Bacteriol 2007; 189: 7290–7301. (C) Binary toxin locus (CdtLoc) from C. difficile QCD-32g58. Schematic organization of the binary toxin locus (6.2 kb in length) from the binary toxin-positive C. difficile QCD-32g58 strain. The CDT binary toxin-encoding genes cdtA and cdtB, are shaded in gray. The response regulator gene, cdtR, is shaded in black. The highly conserved 5′ and 3′ boundaries are also indicated. Adapted from Carter et al.Citation5 J Bacteriol 2007; 189: 7290–7301. © American Society for Microbiology. Reproduced by permission of Becky Zwadyk. Permission to reuse must be obtained from the rightsholder.
For a long time, the elucidation of the precise functions of TcdA and TcdB using a genetic approach was hampered by a lack of tools to isolate C. difficile isogenic toxin gene mutants. However, Lyras and co-workers as well as Kuehne and coworkers facilitated the study of isogenic mutants.Citation17,18 Syrian golden hamsters were infected with either toxin A− mutants, toxin B− mutants or wild type strains in a study.Citation17 Infection with the wild type strain resulted in the death of 90% of hamsters included in the study. Infection with toxin A− mutants resulted in death of 94% of hamsters, indicating that toxin A is not crucial for disease. Interestingly, infection with toxin B− strains only caused disease in 22% of hamsters, highlighting that toxin B was in fact the main virulence factor rather than toxin A, contrary to previous assumptions. More significantly, the study helped to explain the pathogenicity caused by toxin A−B+ isolates in clinical settings.Citation17
Some C. difficile strains also produce an additional toxin called binary toxin (CDT). Importantly, the genes encoding binary toxin, designated cdtA and cdtB are not part of the PaLoc but are nevertheless found on the chromosome as part of the binary toxin locus (CdtLoc) ().Citation5,19-21 CdtR is a response regulator encoded by CdtLoc and controls binary toxin production.Citation5 Isolates which do not produce binary toxin, such as C. difficile CD37, have a conserved 68 bp sequence in place of the CdtLoc.Citation5 The binary toxin-negative C. difficile 630 strain consists of fused cdtAB pseudogenes as part of a 4.2 kb CdtLoc, whereas binary toxin-positive isolates such as C. difficile QCD-32g58 consist of a 6.2 kb CdtLoc, composed of the 2 binary toxin genes cdtA and cdtB, in addition to the regulatory gene, cdtR (Citation5
Therapeutic Options
Current treatment of CDI and antibiotics
The current treatment modalities for CDI involve the immediate discontinuation of antibiotics given to the patient for other diseases, and commencement of metronidazole and vancomycin administration post-haste. The rates of metronidazole treatment failure are significantly higher in patients who are still on other antibiotics, due to continued perturbations of the competing gut microbiota.Citation21 One study suggested that metronidazole and vancomycin were equally effective for mild cases of CDI, with treatment success rates of 90% and 98% respectively.Citation22 However, for more severe cases, vancomycin was the treatment of choice, as success rates for metronidazole and vancomycin were 76% and 97% respectively, though recurrence rates were similar i.e. 15% of metronidazole-treated patients, compared to 14% for vancomycin.Citation22 In this regard, it must be noted that slightly different success rates are reported for metronidazole and vancomycin against C. difficile, depending on a variety of factors, such as type of study conducted, sample size and geographical location. High failure rates for metronidazole, due to the emergence of the outbreak-associated R027 strains and also a rise in the number of elderly patients in hospitals affected by CDI who are already being treated with other antibiotics, have also been reported.Citation23,24 The treatment for non-epidemic and epidemic C. difficile strains appears to be similar, with metronidazole as the primary treatment choice for mild-moderate cases of CDI, followed by vancomycin for more severe CDI, according to the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) guidelines. Fidaxomicin has already been proven to have promising efficacy as a therapeutic for CDI. Other antibiotics such as ramoplanin, tigecycline and the rifamycin group of antibiotics have shown potent activity against C. difficile and research is ongoing regarding their clinical efficacy against CDI. Fidaxomicin and faecal microbiota transplantation (FMT) is also strongly advocated by ESCMID for recurrent CDI cases.Citation25,26
For mild-moderate CDI, 500 mg of oral metronidazole 3 times a day for 10–14 d is recommended, whereas 125 mg of oral vancomycin 4 times a day for the same duration is indicated for more severe cases of CDI.Citation27 Oral vancomycin, supplemented with intravenous metronidazole if necessary, is recommended for severe CDI by the IDSA. Although oral administration of metronidazole or vancomycin is optimal, metronidazole can also be administered intravenously as it is capable of reaching the intestinal lumen via diffusion across the inflamed colon. A prospective study conducted by Wenisch et al. demonstrated that oral metronidazole (7.4% mortality) was more effective against C. difficile than intravenous metronidazole (38.1% mortality).Citation28 Vancomycin may also be introduced intracolonically or as a retention enema. A dose of 100 mg of the glycopeptide teicoplanin twice a day in addition to metronidazole or vancomycin is recommended by the ESCMID for CDI.Citation29 However, fusidic acid and bacitracin do not seem to be as effective as glycopeptides or metronidazole and thus are contraindicated by ESCMID. Fusidic acid, oral bacitracin and teicoplanin are not recommended in the USA for CDI.Citation29
Alternative vancomycin dosing strategies
Though vancomycin and metronidazole have been used for the treatment of CDI for the last 3 decades, recurrence and relapse of disease still remains a serious problem. About 50% of cases of recurrence of disease are due to relapse, whereby the original C. difficile strain that was culpable for the infection causes symptoms again, due to spores surviving the CDI treatment.Citation30 Alternative dosing regimens for vancomycin have been investigated to circumvent the problem of recurrent CDI. Pulsed vancomycin dosing, which involves short intermittent administration of vancomycin, as well as tapered dosing of vancomycin have proved successful. In a retrospective study conducted by McFarland et al. with a total of 163 recurrent CDI patients, a subset received vancomycin and a smaller subset received tapered doses of vancomycin, whereby vancomycin was decreased incrementally from 500 mg-3 g daily to 125–750 mg daily, over 3 weeks.Citation31 A 31% recurrence rate was noted for this tapered dose strategy compared to recurrence rates of 43–54% with conventional vancomycin dosing for 10 d. Pulsed vancomycin administration of 125–500 mg per day every 2–3 d for 27 d resulted in a 14% recurrence rate.Citation31 Although no randomized controlled trials have assessed such dosing regimens, the IDSA and the SHEA recommend the use of pulsed or tapered vancomycin dosing for second or third recurrences of CDI.Citation27 In cases of extremely severe C. difficile colitis, intracolonic vancomycin administration may have the potential to be used as an adjunct, to ensure higher concentrations of the drug in the colon.Citation32,33 This may involve the use of an intracolonic bolus with an intravenous solution of vancomycin. A rectal retention enema such as 500 mg of vancomycin in 1 L of saline solution may be another option. Kim et al. reported a 70% success rate in a recent trial whereas Apisarnthanarak et al. approximated a success rate of 57–75% for intracolonic vancomycin administration in a review of relevant case series.Citation32,33
Fidaxomicin
Fidaxomicin is an oral macrocyclic antibiotic, produced by Dactylosporangium aurantiacum, targeted against C. difficile.Citation34 One of the main advantages of fidaxomicin is that it is tailored specifically toward C. difficile, with little impact on the commensal gut microbiota and has been shown to inhibit spore formation and toxin production in C. difficile.Citation25,36 This narrow spectrum of action is hugely beneficial as it permits quick restoration of the commensal gut microbiota in CDI patients, and thus decreases the risk of recurrence of disease due to overgrowth of C. difficile.Citation25,36 Another advantage is that it sustains a certain level of antibacterial activity for a more prolonged period, compared to metronidazole and vancomycin.Citation34 Therefore, it is capable of inhibiting C. difficile at concentrations lower than the MIC.
A number of in vitro and in vivo studies have highlighted the potential of fidaxomicin in combating C. difficile. Fidaxomicin MICs against 114 C. difficile isolates were reported to be between 0.008–0.125 μg/ml in a recent study by Rashid et al.Citation37 Fidaxomicin was shown to inhibit C. difficile toxin gene expression and consequent toxin production by measuring total mRNA and protein in another study.Citation38. With respect to the propensity for fidaxomicin resistance development, isolates with attenuated sensitivity to the antibiotic displayed mutations in the rpoB gene as well as the gene CD22120 (a marR homolog) in an in vitro study.Citation39 Encouragingly, since fidaxomicin does not exhibit activity against Gram negatives, the possibility for development of resistance among other enteric bacteria is low.Citation40 Fidaxomicin was shown to interact in a synergistic manner with rifamycins in a study, while at the same time, having a reduced likelihood of resistance development.Citation41 It was also encouraging that no cross resistance with rifamycins was apparent in the in vitro study.Citation41
With respect to in vivo studies, Koon et al. noted that the administration of 20 μM fidaxomicin or 120 μM of OP-1118 (a primary metabolite of fidaxomicin) reduced the level of enteritis caused by TcdA in a model of the mouse ileum.Citation42 Among the most notable histological differences between administration of fidaxomicin and the control group was the decreased cell rounding of colonic CCD-18Co fibroblasts as a result of fidaxomicin and OP-1118. Thus, fidaxomicin may exert anti-inflammatory effects in addition to its anti-C. difficile activity. A study by Chilton et al. showed that fidaxomicin as a first line treatment option was effective at treating CDI in a human gut model, irresponsive to metronidazole or vancomycin.Citation43 It was shown that administration of 200 mg/L fidaxomicin 2 times per day decreased the total viable count of C. difficile as well as the toxin titres below the minimum detection levels, 2 and 4 d after administration respectively. Fidaxomicin also prevented spore recrudescence and had negligible effects on the gut microbiota.Citation43 In addition, fidaxomicin was still detected 21 d after administration in the gut models. The results from the study indicated that fidaxomicin fared better than metronidazole and vancomycin in treating primary and secondary CDI.Citation43
It is promising to note that fidaxomicin has potent antimicrobial activity against outbreak-associated R027 strains as well as non-epidemic-associated strains.Citation44-46 Phase I clinical trials conducted by Shue et al. reported low plasma concentrations of fidaxomicin of less than or equal to 5 ng/ml, with a concomitant near 100% recovery of fidaxomicin and its metabolite in faeces.Citation47 These findings were in accordance with more recent Phase II and Phase III trials which reported that the concentrations of fidaxomicin in faeces were 2000–10000-fold higher than the MIC90 value against C. difficile. Some other clinical trials with CDI patients have shown that fidaxomicin caused fewer recurrences and thus, is indicated for mild, moderate, severe and recurrent CDI.Citation48,49 A Phase II trial by Louie and co-workers assessed the efficacy of several doses of fidaxomicin in treating CDI.Citation50 Response rates of 71%, 80% and 94% were found for patients treated with 100 mg, 200 mg and 400 mg of fidaxomicin respectively. Four patients from the group receiving either 100 mg or 200 mg of fidaxomicin failed therapy, representing an 8.9% treatment failure rate whereas 2 out of 41 patients in the study exhibited recurrence of disease a month after treatment.Citation50 In 2 phase III clinical trials with 1105 CDI patients, treatment with fidaxomicin resulted in comparable initial response rates to vancomycin.Citation51,45 Also, the group of patients treated with fidaxomicin had a 47% lower recurrence rate compared to vancomycin.Citation51 In patients with recurrent CDI, 35.5% of 128 patients receiving vancomycin had a further recurrence whereas only 19.7% had another recurrence when treated with fidaxomicin, as reported in 2 studies.Citation44-46 Although lower recurrence rates have been observed for fidaxomicin compared to vancomycin, the recurrence rates are broadly similar for R027 strains.Citation25,44-46 By using a whole-genome sequencing approach, Eyre et al. showed that fidaxomicin was more effective than vancomycin in preventing relapse of CDI in recent phase III trials.Citation52
Rifamycin antibiotics
The rifamycin subgroup of antibiotics, which include rifaximin, rifampin, rifalazil and others display potent anti-C. difficile activity in vitro.Citation53,54 However, only a handful of reports regarding their clinical use are available.Citation55 In one instance it was reported that 7 patients recovered from CDI with 3 d of rifampin treatment. The dose was 300–600 mg rifampin administered every 12 hours in combination with vancomycin.Citation56 Another study evaluated the effects of metronidazole and rifampin combination therapy, but no beneficial effects were noted compared to metronidazole treatment alone.Citation57 Rifaximin, another rifamycin antibiotic, also shows strong antimicrobial activity against C. difficile in vitro.Citation53 The rates of C. difficile resistance to rifaximin are significantly lower compared to rifampin.Citation55 In an in vitro study, O’Connor and coworkers found that 14 out of 80 C. difficile isolates tested in the study were resistant to rifaximin, whereas rates of metronidazole resistance among C. difficile strains have been reported to vary from 15–35%.Citation58,59
A clinical trial conducted by Johnson et al. showed that 7 of 8 patients administered rifaximin following vancomycin displayed no recurrence of CDI.Citation60 Rifaximin was effective in eradicating symptoms of diarrhea in 3 liver transplant patients, within 36–48 hours of administration.Citation54 A success rate of 64% for rifaximin was reported by Patrick-Basu and co-workers in 25 patients who failed metronidazole treatment.Citation61 A study conducted in a hospital in Houston in the period between May 2007 and September 2011 showed that a rifamycin-resistant strain of C. difficile was found in 49 of 283 (17.3%) patients with CDI, which compared with rates of 34% and 35% rifamycin-resistance in hospital-acquired and community-acquired cases respectively.Citation62 Obuch-Woszczatyński et al.Citation63 documented an outbreak caused by a rifampicin-resistant C. difficile R046 isolate in tuberculosis patients in Poland while Carman et al.Citation64 also reported the isolation of a C. difficile strain resistant to the rifamycin group of antibiotics from a patient being treated with rifaximin for recurrent CDI. The strain was isolated within 32 hours of rifaximin administration and resistance was attributed to single point mutations within the rpoB gene.Citation64
Nitazoxanide
Nitazoxanide is a nitrothiazole benzamide which displays potent antimicrobial activity against intestinal parasites and gastrointestinal tract (GIT) pathogens including C. difficile.Citation65 Musher et al. conducted a randomized, prospective double blind study with hospitalised patients with C. difficile colitis. Patients included in the study were those who had primary CDI (a minimum of 3 unformed stools per day), with symptoms such as abdominal pain, fever or leukocytosis and an enzyme immunoassay indicating CDI.Citation66 89.5% of CDI patients responded to nitazoxanide therapy, which was better than the 82.4% response rate for metronidazole, after a week of therapy in the trial. Furthermore, the sustained response to nitazoxanide a month after therapy was comparable to metronidazole rates.Citation66 The same authors further investigated the efficacy of nitazoxanide in treating CDI patients who had failed metronidazole treatment. Initially, a 74% response rate for nitazoxanide was noted with this patient group. However, subsequent recurrence of disease resulted in a final cure rate of 54% for nitazoxanide in treating CDI patients not responding to metronidazole.Citation67 Another recent prospective double blind randomized study in 2009, also conducted by Musher et al. compared the efficacy of 10 d of nitazoxanide therapy versus 10 d of vancomycin therapy with 50 CDI patients. Patients included in the study were those who had confirmed positive tests for C. difficile toxins, had more than 3 unformed stools within a 24 hour period and presented with at least one of the following: abdominal pain, fever or leukocytosis.Citation68 Response rates of 77% for nitazoxanide and 74% for vancomycin were noted initially. Initial response rates in the study were defined as the absence of any CDI symptoms between days 11–13.Citation68 Among the patient group who completed nitazoxanide and vancomycin therapy, 94% and 87% final response rates were noted respectively. One patient treated with nitazoxanide and 2 patients treated with vancomycin displayed relapse of disease. Although a small sample size was used in the study, acknowledged by the investigators, the findings of the study led to the conclusion that nitazoxanide was comparable to vancomycin in terms of treating CDI.Citation68 A separate case report of a patient with severe recurrent CDI, failing metronidazole and vancomycin therapy successfully treated with 2 weeks of oral nitazoxanide followed by 2 weeks of nitazoxanide and tapered vancomycin administration was also recently documented.Citation69
Tigecycline
Tigecycline, a derivative of minocycline, is a drug which undergoes very little metabolism, resulting in a large percentage of the active compound being excreted in the faeces.Citation70 In a study by Baines et al. using a human gut model, tigecycline prevented the growth of C. difficile and consequent toxin production.Citation71 Similar observations were made by Jump et al. studying tigecycline using a mouse model of infection.Citation72 A few case reports have described the success of tigecycline in combination with other antibiotics, in treating CDI in patients failing conventional metronidazole and vancomycin therapyCitation73,74 while another study has highlighted the success of tigecycline on its own in resolving CDI.Citation70 However, tigecycline has been shown to be a risk factor for CDI in a murine model by causing changes in the composition of the gut microbiota, more specifically by eliciting significant reductions in Bacteroidetes numbers and increases in Proteobacteria numbers.Citation75 Such disruptions of the gut microbiota were shown to predispose to CDI.Citation75 One report however stated that tigecycline administration for as long as 14 d still failed to treat a case of CDI.Citation76 Despite a handful of case reports highlighting the success of tigecycline and rifaximin, the guidelines drafted by the SHEA/IDSA in 2010 do not include tigecycline, rifaximin or linezolid as part of CDI therapeutic options.Citation27
Ramoplanin
Ramoplanin is a lipoglycodepsipeptide antibiotic which was developed as an oral agent for use in patients colonized with vancomycin-resistant enterococci but also exhibits potent anti-C. difficile activity mediated through the inhibition of cell wall synthesis.Citation77,78 Using a hamster model of C. difficile-induced colitis, Jabes and coworkers reported that ramoplanin was a better treatment choice than vancomycin, while in a separate study, Freeman and co-workers found the efficacy of ramoplanin to be comparable to vancomycin in hamster models of infection.Citation79,77 The study by Freeman et al. showed that administration of ramoplanin resulted in the resolution of symptoms in a hamster model of CDI and a reduction in toxin titer in an in vitro gut model.Citation77 The study also showed the superior efficacy of ramoplanin over vancomycin against C. difficile spores, as spores were recovered less often from the ramoplanin-treated hamsters, compared to vancomycin-treated hamsters.Citation77 Doses of 200–400 mg of ramoplanin administered twice a day for 10 d were effective and comparable to vancomycin for the treatment of CDI, according to a phase II trial.Citation80 Although ramoplanin is not yet used to treat CDI, it may eventually become an alternative antibiotic of choice due to its potent anti-C. difficile activity.
Bacteriocins against C. difficile
Bacteriocins are ribosomally synthesized antimicrobial peptides with either narrow spectrum or broad spectrum activity against other bacteria.Citation81 To date, the activity of a few bacteriocins has been assessed against C. difficile. Bacteriocins, due to their ribosomally-synthesized nature, can also be the subject of bioengineering strategies to find derivatives with ameliorated bioactivity against specific bacterial targets, such as C. difficile. Furthermore, some probiotic strains have the ability to produce bacteriocins in situ. Since bacteriocins are currently not used clinically against C. difficile, the development of resistance among target C. difficile strains has not been a problem thus far. When considering the use of bacteriocins as an alternative/adjunctive therapeutic option for CDI, the mode of delivery of the bacteriocin to the colon must be carefully evaluated. Encapsulation of the bacteriocin may be a means to overcome proteases. It must be noted that the anti-C. difficile activities of the bacteriocins described herein are predominantly based on in vitro studies and the in vivo efficacies of the majority of these bacteriocins have yet to be determined.
Thuricin CD
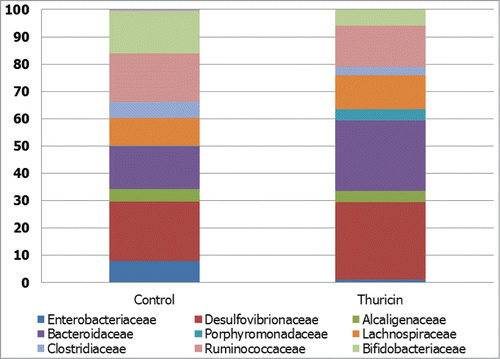
Thuricin CD is a recently discovered bacteriocin with potent narrow spectrum activity against C. difficile.Citation82 The main advantage of thuricin CD is that its antimicrobial activity is largely restricted to C. difficile and has little or no impact on other commensal gut microbes. This was demonstrated using a model of the human distal colon and a high-throughput sequencing approach which revealed that thuricin CD had minimal impact on the numbers of Firmicutes, Bacteroidetes and Proteobacteria, compared to vancomycin and metronidazole which elicited a decrease in Firmicutes and Bacteroidetes numbers, concomitant with an increase in Proteobacteria numbers.()Citation82
Figure 2. Narrow spectrum antimicrobial effects of thuricin CD. The effect of thuricin CD (90 μM) on family-level taxonomic distribution of the microbial communities present in model of the distal colon, expressed as percentage of total assignable sequences. Redrawn from Rea et al.Citation82 Proc Natl Acad Sci U S A 2011; 108: 4639–44. © Proceedings of the National Academy of Sciences of the United States of America. Reproduced by permission of Kay McLaughlin. Permission to reuse must be obtained from the rightsholder.
Nisin and lacticin 3147
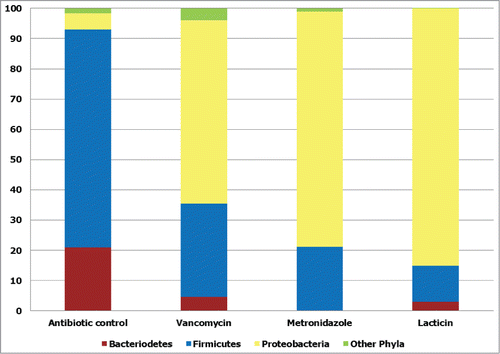
Nisin is a member of the lantibiotic family of bacteriocins with broad spectrum antimicrobial activity against a range of Gram-positive bacteria, including antibiotic-resistant bacteria and food pathogens.Citation83 Studies by Field et al. showed that a bioengineered derivative of nisin A, designated M21V, displayed more potent antimicrobial activity against a variety of Gram-positive pathogens, including C. difficile R027, compared to wild type nisin A.Citation83 Lacticin 3147 is a 2-peptide lantibiotic produced by Lactococcus lactis DPC 3147.Citation84 Unlike thuricin CD, it has a broad spectrum of antimicrobial activity against Gram-positive pathogens. Lacticin 3147 seems to trigger a rapid lysis of log phase C. difficile cells, measured by quantifying the release of acetate kinase. Addition of high lacticin 3147 concentrations of 6 μg/ml results in the decrease of C. difficile ATCC 43593 cell numbers from 10Citation6 cfu/ml to zero in 2 hours. Subsequent studies with lacticin 3147 using a model of the human distal colon showed that it caused a decrease in Firmicutes numbers with a concomitant increase in Proteobacteria numbers, similar to the effects seen with vancomycin and metronidazole.Citation82 ()
Figure 3. Broad spectrum antimicrobial effects of vancomycin, metronidazole and the bacteriocin lacticin 3147. The effects of the broad spectrum antimicrobials vancomycin, metronidazole and lacticin 3147 on phylum level diversity of gut communities in a model of the distal colon, expressed as percentage of total population of assignable tags. Other phyla: Actinobacteria, Spirochaetes, Lentisphaerae, and Tenericutes. Redrawn from Rea et al.Citation82 Proc Natl Acad Sci U S A 2011; 108: 4639–44. © Proceedings of the National Academy of Sciences of the United States of America. Reproduced by permission of Kay McLaughlin. Permission to reuse must be obtained from the rightsholder.
Actagardine and NVB302
Actagardine A is a 19-amino acid lantibiotic with potent antimicrobial activity against Gram-positive bacteria, including C. difficile. A bioengineered V15F derivative of actagardine A exhibited lower minimum inhibitory concentration (MIC) values against the same C. difficile targets compared to the wild type actagardine.Citation85 NVB302 is a semi-synthetic type B lantibiotic, derived from actagardine and is effective against C. difficile. Crowther et al. conducted a recent study investigating the efficacy of NVB302 compared to vancomycin in treating CDI employing an in vitro gut model.Citation86 The gut microbiota count as well as C. difficile viable counts and spores were enumerated following NVB302 and vancomycin administration and a decrease in viable C. difficile counts with vancomycin and NVB302 administration was noted. NVB302 performed better than vancomycin as cytotoxin levels were undetectable 7 d subsequent to NVB302 administration, compared to undetectable cytotoxin levels 5 d after vancomycin instillation. C. difficile spores did not germinate following either vancomycin or NVB302 instillation.Citation86
LFF571 (GE2270 derivative)
GE2270 is a thiopeptide bacteriocin which inhibits translation in bacteria.Citation87 LFF571 is a semi-synthetic derivative of the thiopeptide GE2270, developed by Novartis, which displays antimicrobial activity against a range of Gram-positive bacteria, including C. difficile.Citation88 The in vivo activity of LFF571 against C. difficile was compared with vancomycin in a study with Golden Syrian hamster models.Citation89 LFF571 was administered orally 24 hours post-infection. Doses of 0.2, 1, 2, 5 or 10 mg/kg of LFF571 were used. Administration of 5 mg/kg LFF571 resulted in a 71% initial response rate, whereby 5 out of the 7 hamsters survived after 21 days, while 37.5% of animals survived 21 d when treated with 20 mg/kg vancomycin. In terms of recurrence rates, LFF571 once again fared better than vancomycin. Only 2.2% of hamsters had recurrence at the conclusion of treatment with 5 mg/kg LFF571, whereas 37.8% of hamsters which survived at the termination of treatment with 20 mg/kg vancomycin, experienced recurrence.Citation89
Recently, a randomized double blind trial was conducted investigating the efficacy and safety of LFF571 in healthy volunteers.Citation90 Encouragingly, no serious side effects of LFF571 were noted among 56 volunteers. LFF571 largely remained in the gut with very low concentrations noted in serum (the highest concentration being 3.2 ng/ml in serum in one volunteer). Moreover, LFF571 was tolerated equally well irrespective of single or multiple doses in healthy volunteers participating in the study.Citation90
A summary of MIC values of antibiotics being investigated to replace metronidazole/vancomycin as well as various bacteriocins against C. difficile, as reported in published studies, is included in .
Table 1. Summary of minimum inhibitory concentration values (MIC) of anti-C. difficile antibiotics and bacteriocins. In vitro MICs of alternative antibiotics to metronidazole and vancomycin as well as as in vitro MICs of bacteriocins and bioengineered derivatives thereof against C. difficile strains as reported in the literature
Faecal Microbiota Transplantation
Since the main risk factor for acquiring CDI appears to be the perturbation of the gut microbiota due to broad spectrum antibiotics, and subsequent overgrowth of C. difficile, the restoration of the intestinal microbiota via faecal microbiota transplantation (FMT) seems like an appropriate therapeutic option. FMT is the process of introducing faeces from a healthy donor, in a liquid suspension, into the GIT of a patient.Citation101 Typically, patients considered for FMT are those who have confirmed C. difficile colitis and have had at least 2 relapses following antibiotic therapy. Stool donors are screened for HIV-1, HIV-2, hepatitis A, B, C and faecal samples tested for bacterial/parasitic pathogens such as Salmonella, C. difficile, S. aureus.Citation101,102 The majority of cases of FMT occur through the rectum, but nasogastric, nasoduodenal, nasojejunal instillations are common as well. 250 mg of vancomycin every 8 hours for 4 d and 2 20 mg doses of omeprazole per day for 4 d are administered to the transplant recipient to decrease C. difficile numbers and allow the introduced bacteria to colonize by decreasing acid production in the stomach and consequently elevating the pH, in the event of nasogastric instillations.Citation103-105
The treatment success rate of FMT for recurrent CDI has been reported to be approximately 90% in a study involving 18 subjects, while a response rate of 100% was reported when 12 recurrent CDI patients were treated with FMT in a separate study.Citation101,106 Over the last 5 years, investigators who have used FMT to treat recurrent cases of CDI have reported success rates ranging from 86–100%.Citation102,106-108 The first clinical trial comparing FMT against vancomycin was recently conducted by van Nood et al. and an 81% success rate for faecal transplantation after the first infusion (13/16 cases resolved) was significantly better than the 31% success rate found for vancomycin alone.Citation109 A mix of 33 different bacteria from healthy stool samples was effective in treating CDI in 2 patients, in another recent study.Citation110 Most recently, a retrospective assessment of 31 patients treated with either FMT or rectal bacteriotherapy (RBT) was conducted by Emanuelsson et al.Citation111 The results indicated that overall out of 31 recurrent CDI patients, 74% were treated successfully, defined by a continued lack of symptoms and diarrhea within 3 d of treatment. 70% of patients treated with FMT responded successfully, while the corresponding rates for RBT were 88%.Citation111
FMT can lead to alterations in the composition of the gut microbiota. A rise in Bacteroides, Faecalibacterium and Roseburia numbers with a concomitant decrease in Enterobacteriaeceae in C. difficile patients who are treated with FMT is common.Citation107 Hamilton et al. also noted a rise in Bacteroidetes and Firmicutes numbers in 3 patients treated with FMT.Citation108 An increase in Bacteroidaceae, Porphyromonadaceae and Rikenellaceae families of the Bacteroidetes phylum, accompanied by an increase in Ruminococcaceae and Lachnospiraceae families of the Firmicutes phylum following FMT were noted in a study and it has been hypothesized that the presence of such families is associated with gastrointestinal health.Citation108 Fuentes et al. studied the composition, diversity and changes that take place in the faecal microbiota as a result of FMT by utilizing a phylogenetic microarray platform.Citation112 Compositional analysis of the gut microbiota from faecal samples of 9 recurrent CDI patients was performed and data were analyzed before and after faecal infusion, as well as 10 weeks after therapy. The predominant change in the composition of the gut microbiota went from a low-diversity state composed mainly of Proteobacteria and Bacilli to a more diverse state, predominantly composed of Clostridium and Bacteroides groups as well as butyrate producers.Citation112 The findings of these types of studies could form the basis for the identification of biomarkers which can predict recurrence or resolution of CDI and could help us understand an optimal microbiota composition for targeted therapeutic strategies. FMT has been shown to normalize the composition of faecal bile acid in recurrent CDI cases.Citation113 Faecal samples prior to FMT consisted mainly of primary bile acids and bile salts with extremely low concentrations of secondary bile acids. However, faecal samples post-FMT had an abundance of secondary bile acids, similar to donor samples from non-CDI volunteers.Citation113 Thus, FMT has a major role in restoring faecal microbiota composition as well as metabolic composition. Recurrent CDI patients are unable to metabolize primary bile acids to secondary bile acids.Citation113
Animal trials can also be invaluable in terms of providing information and optimizing successful FMT procedures. Six different bacteria were used to cure C. difficile R027-infected mice, in a recent in vivo trial.Citation114 During CDI resolution, 4 out of the 6 bacterial strains managed to colonize the mice while several other commensals also proliferated, increasing the microbial diversity in doing so. Despite the promising in vivo and clinical trial outcomes, there has been a general reluctance in resorting to FMT among both patients and doctors due to the unattractive nature of the procedure, as well as the extensive screening of donor samples for pathogens that is required prior to transplantation.Citation115 Banking of frozen stool samples which have already been screened for the presence of pathogens, may be a means of expediting the processes involved in performing FMT for CDI cases.
Vaccines for CDI
Earlier vaccine studies
The development of vaccines for CDI has been ongoing for the last 20 years. Torres et al. found that a C. difficile culture filtrate inactivated with formalin was effective in hamsters.Citation116 An inactivated C. difficile toxoid administered intramuscularly or via the rectum also conferred protection to hamsters.Citation117 It was found that an adjuvanated toxoid B vaccine allowed colonization of toxin A−B+ C. difficile strains to occur in hamsters but prevented disease from occurring in a more recent trial.Citation118 A study by Sougioultzis and coworkers reported the effects of an 8-week immunization course with a toxoid vaccine on 3 recurrent CDI patients.Citation119 The authors found significantly elevated levels of serum IgG anti-toxin A and anti-toxin B in 2 of the 3 patients. All 3 patients recovered from recurrent CDI, even after vancomycin treatment was ceased, implying that the injectable toxoid vaccine was effective.Citation119
Sanofi pasteur toxoid vaccine
More recently, a toxoid vaccine has been developed by the Sanofi Pasteur Institute and its development has been fast-tracked by the US FDA since 2010. This Sanofi Pasteur vaccine consists of formalin-inactivated toxins A and B from C. difficile VPI 10463. The primary target group for this Sanofi vaccine is elderly patients who may be immunocompromised and/or hospitalised patients on antibiotics, who are at a greater risk of acquiring CDI. Phase I and Phase II clinical trials with the Sanofi vaccine have already been conducted and results of the Phase II trial are intended to be published shortly (unpublished data of Phase II trial (H-030–012) presented at the 114th General Meeting of the American Society for Microbiology, 19 May 2014, Boston, USA). In 2013, a Phase III clinical trial with a vaccine called Cdiffense was launched by Sanofi, recruiting approximately 15000 volunteers as part of a randomized observer-blind multinational trial, with a total duration of approximately 4.5 y. The aim of this Cdiffense trial, currently ongoing, is to assess the immunogenicity and safety of the toxoid vaccine. Anosova et al. assessed the immunogenicity and protective capabilities of the Sanofi Pasteur toxoid vaccine in a hamster model of CDI.Citation120 The hamsters showed an increase an IgG titres in response to the vaccine. There is a positive correlation between the levels of antibody-toxin binding and neutralisation titres and consequent protection against C. difficile.
Recombinant vaccines
Karczewski et al. investigated the potential of a recombinant toxin fragment vaccine, composed of 2 separate fragments of TcdB, against C. difficile.Citation121 When such recombinant fragments of TcdB were administered to Golden Syrian hamsters, in combination with TcdA, the animals displayed significant IgG responses and strong neutralising antibody titres. Significantly, the recombinant TcdB vaccines investigated in the study were also effective against subsequent challenge with C. difficile spores.Citation121 Spencer et al. also noted in their study that recombinant fragments from TcdA and TcdB provide protection to hamsters against C. difficile.Citation122 Importantly, however, a greater immune response is mounted when the binding domain of TcdB is replaced with the glucosyltransferase domain of the toxin.Citation122
An injectable subunit recombinant protein vaccine for immunogenicity and safety in volunteers was tested in 2011.Citation123 This recombinant vaccine is made up of truncated versions of C. difficile toxins A and B, designated IC84. Intercell IC84v is composed of recombinant fusion proteins of the binding regions of toxins A and B. Both alum-adjuvanated and non-adjuvanated recombinant vaccines were immunogenic in volunteers under 65 y of age.Citation123
Ghose et al. assessed the immunogenic potential of a recombinant fusion protein vaccine consisting of the Salmonella enterica serovar Typhimurium flagellin (FliC) subunit D1 fused to the non-toxic domains of TcdA and TcdB.Citation124 The authors observed that anti-TcdA and anti-TcdB IgG titres were elevated in sera of mice. The outcome of the study proved that a recombinant protein-based vaccine which targets the receptor binding motifs of TcdA and TcdB, when adjuvanated with S. enterica serovar Typhimurium flagellin (FliC) subunit D1, stimulates a quick high-level immune response in a mouse model of infection, provided that the mouse is challenged with the same strain of C. difficile from which the antigens were obtained.Citation124 Such findings could have implications for the development of ‘designer’ recombinant vaccines, using antigens from the most common C. difficile outbreak strains. A plasmid-based approach to produce a recombinant toxoid in a TcdA−TcdB− strain of C. difficile has also been utilised.Citation125 As expected, the TcdA and TcdB toxoids expressed in this plasmid system were significantly less toxic to human IMR-90 cells. Such formalin-inactivated antigens elicited a protective effect in hamster models of CDI.Citation125
Wang et al. developed a chimeric toxin vaccine, consisting of immunogenic epitopes from both TcdA and TcdB.Citation126 In essence, the receptor binding domain of TcdA replaced that of TcdB and the resulting chimeric toxin displayed strong immunogenic potential. The chimera cTxAB was generated by deleting the glucosyltransferase domains of both TcdA and TcdB and was found to be equally effective against laboratory and outbreak-associated strains.Citation126 Thus, a blueprint for design of recombinant toxin chimeras may serve a useful purpose in anti-C. difficile vaccine development. Bertolo et al. created a dual vaccine consisting of PSII from C. difficile fused to the LTB enterotoxin from enterotoxigenic E. coli (ETEC).Citation127 Such a vaccine could play an important role in preventing diarrhea from C. difficile as well as ETEC.
Vaccines based on the polysaccharide glycans and glycoconjugate vaccines
Other investigators have studied the immunogenicity of vaccines based on polysaccharide glycans found on the surface of C. difficile cells, namely PSI, PSII, PSIII.Citation128-132 Adamo et al. noted that mice, hamsters, as well as farm animals had elevated levels of anti-PSII antibodies in response to vaccines containing the polysaccharide.Citation129,132 Interestingly, due to exposure to C. difficile, humans also carry anti-PSII IgA and IgG antibodies. Such antibodies could have great potential in targeting PSII on the outer surface of C. difficile cells. Jiao et al. further studied the importance of C. difficile PSI polysaccharide as a basis for the development of anti-C. difficile vaccines.Citation128 It is apparent that while PSII is found on the cell surface of biofilms of C. difficile, PSI and PSIII are expressed stochastically. The pentasaccharide moiety of PSI, chemically synthesized and fused to an exotoxin B subunit could be an effective vaccine with 2 separate antigens to mount an immune response. Importantly in the study, anti-PSI IgG antibodies were found in sera of horses in response to both the native PSI polysaccharide, as well as the chemically synthesized non-phosphorylated PSI repeating block, thus highlighting the potential for such an approach in vaccine development.Citation128 Novel chemical synthesis protocols to generate large quantities of the pentasaccharide repeating unit and oligosaccharide structures were optimised by Martin et al. to overcome problems with low-level expression of natural PSI.Citation133 Using mouse models, the authors demonstrated that a chemically synthesized PSI repeating unit CRM197 conjugate elicited an immune response. The use of glycan microarrays helped to identify the minimal immunogenic epitopes such as the repeating PSI-pentasaccharide repeating unit, as a basis for vaccine development.Citation133
As PSII is expressed at a higher level by the majority of C. difficile ribotypes in comparison to PSI and PSIII, it has a greater potential to form the basis for a C. difficile vaccine.Citation134 Romano et al. studied effectiveness of recombinant toxin A and B fragments fused to PSII glycoconjugates.Citation134 While anti-PSII IgG levels were stimulated in response to both TcdA_B2 and TcdB_GT, the TcdB_GT induced a higher level of IgG. This increase in IgG titres was encouraging as it proves that glycoconjugate vaccines in combination with different C. difficile antigens can be effective as potential vaccines.Citation134
Cox et al. investigated the role of a lipoteichoic acid-based glycoconjugate as a possible antigen to evoke an immune response.Citation135 Antibodies in response to the conserved lipoteichoic acid (LTA) glycoconjugate were stimulated in mouse and rabbit models. The authors optimised an amino functionality as the conjugation point. Overall, the study highlighted that the surface of C. difficile cells contains highly conserved LTA polymers, which has the potential to act as an antigen to evoke an immune response.Citation135 Oberli et al. chemically synthesized an oligosaccharide-conjugate vaccine composed of the PSII hapten of a C. difficile R027 strain.Citation136 The authors found that immunized mice displayed specific IgG antibodies in serum in response to the synthetic hexasaccharide and diphtheria toxoid variant CRM(197) conjugate vaccine. The IgG antibodies were mounted specifically against the glycan repeating subunit of the conjugate vaccine.Citation136
DNA-based and other types of vaccines
Baliban et al. investigated the effect of cloning the receptor binding domain (RBD) of TcdA and TcdB on protection against C. difficile and vaccination with such DNA sequences elicited high levels of anti-RBD antibodies in mouse models.Citation137 In another study, a gene which encoded the receptor binding domain of TcdA was synthesized and designed for expression in human cells.Citation138 Vaccination of mice with this DNA vaccine stimulated high concentrations of antibodies to be produced and prevented death when inoculated with TcdA.Citation138
A DNA vaccine expressing the glucosyltransferase domain of TcdB has also been developed and the authors found that only a fraction of C. difficile toxin fragments, which included an N-terminal glucosyltransferase domain of TcdB and the C-terminal receptor binding domain of TcdA evoked antibody responses as measured by cytotoxicity assays and/or prevention of death using mouse models.Citation139 Importantly, the antibodies generated in response to the N-terminus of the TcdB DNA vaccine provided a greater degree of protection, when used in conjunction with anti-TcdA antibodies in a mouse model of infection.Citation139 Seregin et al. developed an adenovirus-based vaccine against C. difficile and the investigators observed that the vaccine induced significant and rapid humoral and cellular responses (T-cells) in mouse models.Citation140 This immune response was sufficient to protect mice from subsequent C. difficile challenge. Furthermore, IgG antibodies specific to TcdA in plasma were notable 3 d after vaccination in the immunized mice. In addition to these findings, the authors discovered the main immune-dominant T cell epitopes in TcdA.Citation140 Thus, such an adenovirus-based vaccine also has the potential to be used for CDI.
Conclusions
The overuse of broad spectrum antibiotics has led to numerous C. difficile outbreaks, especially in North America, Canada and Europe over the last 2 decades. The advent of next-generation sequencing technology in recent years has helped to emphasize the extent of damage caused to the gut microbiota due to broad spectrum antibiotics. The perturbation of the gut microbiota as a result of antibiotics removes the most potent defense against opportunistic pathogens such as C. difficile i.e., the presence of a fully intact gut microbiota. This often leads to a continuous cycle of CDI and recurrence, as further treatment with broad spectrum antibiotics inhibits the restoration of the commensal gut microbiota, leading to acquisition of CDI again. Thus, it is clear that there is an urgent need to develop alternative/adjunctive therapeutic options to metronidazole and vancomycin in order to circumvent this ongoing problem of recurrence of disease. Fidaxomicin has already been proved to have promising efficacy as a therapeutic for CDI. Other antibiotics such as ramoplanin, nitazoxanide, tigecycline and the rifamycin group have shown potent in vitro activity and some promising in vivo results against C. difficile and research is ongoing regarding their clinical efficacy against CDI. Development of these alternative antibiotics is crucial as the overuse of the current antibiotics metronidazole and vancomycin may lead to development of resistance among C. difficile targets. Furthermore, there is an inherent risk with the overuse of vancomycin with respect to the spread of vancomycin-resistant enterococci in hospital environments.
The restoration of the commensal gut microbiota using FMT has also shown encouraging results in recent years. FMT has tremendous potential in this regard, as it directly tackles the root cause of the problem i.e. dysbiosis caused by broad spectrum antibiotics, resolved by restoring the commensal microbiota via faecal transplantation. Perhaps the most elusive therapeutic option over the years to arrest the cyclic pattern of relapse and recurrence of CDI, however, has been a narrow spectrum antimicrobial with potent anti-C. difficile activity and lack of activity against the commensal gut microbiota. It may be the case that a narrow spectrum antimicrobial targeted against C. difficile and/or restoration of the gut microbiota via FMT may eventually prove to be the most effective treatment regimens for CDI. The importance of mounting an effective immune response as a result of C. difficile vaccines must not be overlooked either. Overall, due to the vast number of studies investigating anti-C. difficile therapeutic options in recent years, scientists are closer than ever at finally tackling this notorious infection.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
HM is in receipt of an Irish Research Council for Science, Engineering and Technology EMBARK scholarship. Research in PDC, CH, MRC and RPR laboratories is supported by the Science Foundation of Ireland (SFI)-funded Center for Science, Engineering and Technology and the Alimentary Pharmabiotic Center (APC).
References
- Hall IC, O'Toole E. Intestinal flora in new-born infants with a description of a new pathogenic anaerobe, Bacillus difficilis. Am J Dis Child 1935; 49:390-402; http://dx.doi.org/10.1001/archpedi.1935.01970020105010
- George RH, Symonds JM, Dimock F, Brown JD, Arabi Y, Shinagawa N, Keighley MR, Alexander-Williams J, Burdon DW. Identification of Clostridium difficile as a causative agent of pseudomembranous colitis. BMJ 1978; 1:695; PMID:630301; http://dx.doi.org/10.1136/bmj.1.6114.695
- Dubberke ER, Olsen MA. Burden of Clostridium difficile on the healthcare system. Clin Infect Dis 2012; 55:S88-92; PMID:22752870; http://dx.doi.org/10.1093/cid/cis335
- Vedantam, Clark A, Chu M, McQuade R, Mallozzi M, Viswanathan VK. Clostridium difficile infection: toxins and non-toxin virulence factors, and their contributions to disease establishment and host response. Gut Microbes 2012; 3:121-34; PMID:22555464; http://dx.doi.org/10.4161/gmic.19399
- Carter GP, Lyras D, Allen DL, Mackin KE, Howarth PM, O’Connor JR, Rood JI. Binary toxin production in Clostridium difficile is regulated by CdtR, a LytTR family response regulator. J Bacteriol 2007; 189:7290-301; PMID:17693517; http://dx.doi.org/10.1128/JB.00731-07
- Heap JT, Pennington OJ, Cartman ST, Carter GP, Minton NP. The ClosTron: a universal gene knock-out system for the genus Clostridium. J Microbiol Methods 2007; 70:452-64; PMID:17658189; http://dx.doi.org/10.1016/j.mimet.2007.05.021
- O’Connor JR, Lyras D, Farrow KA, Adams V, Powell DR, Hinds J, Cheung JK, Rood JI. Construction and analysis of chromosomal Clostridium difficile mutants. Mol Microbiol 2006; 61:1335-51; http://dx.doi.org/10.1111/j.1365-2958.2006.05315.x
- Dupuy B, Govind R, Antunes A, Matamouros S. Clostridium difficile toxin synthesis is negatively regulated by TcdC. J Med Microbiol 2008; 57:685-90; PMID:18480323; http://dx.doi.org/10.1099/jmm.0.47775-0
- Carter GP, Rood JI, Lyras D. The role of toxin A and toxin B in the virulence of Clostridium difficile. Trends Microbiol 2012; 20:21-9; PMID:22154163; http://dx.doi.org/10.1016/j.tim.2011.11.003
- Mani, Dupuy B. Regulation of toxin synthesis in Clostridium difficile by an alternative RNA polymerase sigma factor. Proc Natl Acad Sci U S A 2001; 98:5844-9; PMID:11320220; http://dx.doi.org/10.1073/pnas.101126598
- Bakker D, Smits WP, Kuijper EJ, Corver J. TcdC does not significantly repress toxin expression in Clostridium difficile 630Δerm. PLoS One 2012; 7:e43247; PMID:22912837; http://dx.doi.org/10.1371/journal.pone.0043247
- Carter GP, Douce GR, Govind R, Howarth PM, Mackin KE, Spencer J, Buckley AM, Antunes A, Kotsanas D, Jenkin GA, Dupuy B, Rood JI, Lyras D. The anti-sigma factor TcdC modulates hypervirulence in an epidemic BINAP11487;027 clinical isolate of Clostridium difficile. PLoS Pathog 2011; 7:e1002317; PMID:22022270; http://dx.doi.org/10.1371/journal.ppat.1002317
- Matamouros S, England P, Dupuy B. Clostridium difficile toxin expression is inhibited by the novel regulator TcdC. Mol Microbiol 2007; 64:1274-88; PMID:17542920; http://dx.doi.org/10.1111/j.1365-2958.2007.05739.x
- Murray R, Boyd D, Levett PN, Mulvey MR, Alfa M. Truncation in the tcdC region of the Clostridium difficile PathLoc of clinical isolates does not predict increased biological activity of Toxin B or Toxin A. BMC Infect Dis 2009; 9:103; PMID:19558711; http://dx.doi.org/10.1186/1471-2334-9-103
- Merrigan M, Venugopal A, Mallozzi M, Roxas B, Viswanathan VK, Johnson S, Gerding DN, Vedantam G. Human hypervirulent Clostridium difficile strains exhibit increased sporulation as well as robust toxin production. J Bacteriol 2010; 192:4904-11; PMID:20675495; http://dx.doi.org/10.1128/JB.00445-10
- Vohra P, Poxton IR. Comparison of toxin and spore production in clinically relevant strains of Clostridium difficile. Microbiology 2011; 157:1343-53; PMID:21330434; http://dx.doi.org/10.1099/mic.0.046243-0
- Lyras LD, O'Connor JR, Howarth PM, Sambol SP, Carter GP, Phumoonna T, Poon R, Adams V, Vedantam G, Johnson S, et al. Toxin B is essential for virulence of Clostridium difficile. Nature 2009; 458:1176-9; PMID:19252482; http://dx.doi.org/10.1038/nature07822
- Kuehne SA, Cartman ST, Heap JT, Kelly ML, Cockayne A, Minton NP. The role of toxin A and toxin B in Clostridium difficile infection. Nature 2010; 467:711-3; PMID:20844489; http://dx.doi.org/10.1038/nature09397
- Geric B, Carman RJ, Rupnik M, Genheimer CW, Sambol SP, Lyerly DM, Gerding DN, Johnson S. Binary toxin-producing, large clostridial toxin-negative Clostridium difficile strains are enterotoxic but do not cause disease in hamsters. J Infect Dis 2006; 193:1143-50; PMID:16544255; http://dx.doi.org/10.1086/501368
- Perelle S, Gibert M, Bourlioux P, corthier G, Popoff MR. Production of a complete binary toxin (actin-specific ADP-ribosyltransferase) by Clostridium difficile CD196. Infect Immunol 1997; 65:1402-7
- Modena S, Gollamudi S, Friedenberg F. Continuation of antibiotics is associated with failure of metronidazole for Clostridium difficile-associated diarrhea. J Clin Gastroenterol 2006; 40:49-54; PMID:16340634; http://dx.doi.org/10.1097/01.mcg.0000190761.80615.0f
- Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis 2007; 45:302-7; PMID:17599306; http://dx.doi.org/10.1086/519265
- Fernandez A, Anand G, Friedenberg F. Factors associated with failure of metronidazole in Clostridium difficile-associated disease. J Clin Gastroenterol 2004; 38:414-8; PMID:15100520; http://dx.doi.org/10.1097/00004836-200405000-00005
- Zilberberg MD, Shorr AF, Micek ST, Doherty JA, Kollef MH. Clostridium difficile-associated disease and mortality among the elderly critically ill. Crit Care Med 2009; 37:2583-9; PMID:19623053; http://dx.doi.org/10.1097/CCM.0b013e3181ab8388
- Cornely OA, Miller MA, Louie TJ, Crook DW, Gorbach SL. Treatment of first recurrence of Clostridium difficile infection: fidaxomicin vs. vancomycin. Clin Infect Dis 2012; 55:S154-61; PMID:22752865; http://dx.doi.org/10.1093/cid/cis462
- Karadsheh Z, Sule S. Fecal transplantation for the treatment of recurrent Clostridium difficile infection. N Am J Med Sci 2013; 5:339-43; PMID:23923106; http://dx.doi.org/10.4103/1947-2714.114163
- Cohen SH, Gerding DN, Johnson S, Kelly CP, Loo VG, McDonald LC, Pepin J, Wilcox MH. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol 2010; 31:431-55; PMID:20307191; http://dx.doi.org/10.1086/651706
- Wenisch JM, Schmid D, Kuo HW, Allerberger F, Michl V, Tesik P, Tucek G, Laferl H, Wenisch C. Prospective observational study comparing three different treatment regimes in patients with Clostridium difficile infection. Antimicrob Agents Chemother 2012; 56:1974-8; PMID:22252830; http://dx.doi.org/10.1128/AAC.05647-11
- Bauer MP, Kuijper EJ, van Dissel JT. European Society of Clinical Microbiology and Infectious Diseases (ESCMID): treatment guidance document for Clostridium difficile infection (CDI). Clin Microbiol Infect 2009; 15:1067-79; PMID:19929973; http://dx.doi.org/10.1111/j.1469-0691.2009.03099.x
- Barbut F, Richard A, Hamadi K, Chomette V, Burghoffer B, Petit JC. Epidemiology of recurrences or reinfections of Clostridium difficile-associated diarrhea. J Clin Microbiol 2000; 38:2386-8; PMID:10835010
- McFarland LV, Elmer GW, Surawicz CM. Breaking the cycle: treatment strategies for 163 cases of recurrent Clostridium difficile disease. Am J Gastroenterol 2002; 97:1769-75; PMID:12135033; http://dx.doi.org/10.1111/j.1572-0241.2002.05839.x
- Kim PK, Huh HC, Cohen HW, Feinberg EJ, Ahmad S, Coyle C, Teperman S, Boothe H. Intracolonic vancomycin or severe Clostridium difficile colitis. Surg Infect (Larchmt) 2013; 14:532-9; PMID:23560732; http://dx.doi.org/10.1089/sur.2012.158
- Apisarnthanarak A, Razavi B, Mundy LM. Adjunctive intracolonic vancomycin for severe Clostridium difficile colitis: case series and review of the literature. Clin Infect Dis 2002; 35:690-6; PMID:12203166; http://dx.doi.org/10.1086/342334
- Johnson AP, Wilcox MH. Fidaxomicin: a new option for the treatment of Clostridium difficile infection. J Antimicrob Chemother 2012; 67:2788-92; PMID:22865382; http://dx.doi.org/10.1093/jac/dks302
- Babakhani F, Bouillaut L, Gomez A, Sears P, Nguyen L, Sonenshein AL. Fidaxomicin inhibits spore production in Clostridium difficile. Clin Infect Dis 2012; 55:162-9; http://dx.doi.org/10.1093/cid/cis453
- Tannock GW, Munro K, Taylor C, Lawley B, Young W, Byrne B, Emery J, Louie T. A new macrocyclic antibiotic, fidaxomicin (OPT-80), causes less alteration to the bowel microbiota of Clostridium difficile-infected patients than does vancomycin. Microbiology 2010; 156:3354-9; PMID:20724385; http://dx.doi.org/10.1099/mic.0.042010-0
- Rashid MU, Dalhoff A, Weintraub A, Nord CE. In vitro activity of MCB3681 against Clostridium difficile strains. Anaerobe 2014; 28C:216-9; http://dx.doi.org/10.1016/j.anaerobe.2014.07.001
- Babakhani F, Bouillaut L, Sears P, Sims C, Gomez A, Sonenshein AL. Fidaxomicin prevents toxin production in Clostridium difficile. J Antimicrob Chemother 2013; 68: 515-22; http://dx.doi.org/10.1093/jac/dks450
- Leeds JA, Sachdeva M, Mullin S, Barnes SW, Ruzin A. In vitro selection, via serial passage, of Clostridium difficile mutants with reduced susceptibility to fidaxomicin or vancomycin. J Antimicrob Chemother 2014; 69:41-4; PMID:23887866; http://dx.doi.org/10.1093/jac/dkt302
- Juang P, Hardesty JS. Role of fidaxomicin for the treatment of Clostridium difficile infection. J Pharm Pract 2013; 26:491-7; PMID:24064437; http://dx.doi.org/10.1177/0897190013499526
- Babakhani F, Seddon J, Sears P. Comparative microbiological studies of transcription inhibitors fidaxomicin and the rifamycins in Clostridium difficile. Antimicrob Agents Chemother 2014; 58:2934-7; PMID:24550338; http://dx.doi.org/10.1128/AAC.02572-13
- Koon HW, Ho S, Hing TC, Cheng M, Chen X, Ichikawa Y, Kelly CP, Pothoulakis C. Fidaxomicin inhibits Clostridium difficile toxin A-mediated enteritis in the mouse ileum. Antimicrob Agents Chemother 2014; 58:4642-50; PMID:24890583; http://dx.doi.org/10.1128/AAC.02783-14
- Chilton CH, Crowther GS, Freeman J, Todhunter SL, Nicholson S, Longshaw CM, Wilcox MH. Successful treatment of simulated Clostridium difficile infection in a human gut model by fidaxomicin first line and after vancomycin or metronidazole failure. J Antimicrob Chemother 2014; 69:451-62; PMID:24003182; http://dx.doi.org/10.1093/jac/dkt347
- Louie TJ, Miller MA, Mullane KM, Weiss K, Lentnek A, Golan Y, Gorbach S, Sears P, Shue YK. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med 2011; 364:422-31; PMID:21288078; http://dx.doi.org/10.1056/NEJMoa0910812
- Johnson S, Crook DW, Cornely OA. High KP, Miller M, Gorbach SL. Randomized clinical trial in Clostridium difficile infection confirms superiority of fidaxomicin over vancomycin. Gastroenterol 2010; 139: e17; http://dx.doi.org/10.1053/j.gastro.2010.05.063
- Crook DW, Walker AS, Kean Y, Weiss K, Cornely OA, Miller MA, Esposito R, Louie TJ, Stoesser NE, Young BC, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection: meta-analysis of pivotal randomized controlled trials. Clin Infect Dis 2012; 55:S93-103; PMID:22752871; http://dx.doi. org/10.1093/cid/cis499.
- Shue YK, Sears PS, Shangle S, Walsh RB, Lee C, Gorbach SL, Okumu F, Preston RA. Safety, tolerance, and pharmacokinetic studies of OPT-80 in healthy volunteers following single and multiple oral doses. Antimicrob Agents Chemother 2008; 52:1391-1395; PMID:18268081; http://dx.doi.org/10.1128/AAC.01045-07
- Sullivan KM, Spooner LM. Fidaxomicin: a macrocyclic antibiotic for the management of Clostridium difficile infection. Ann Pharmacother 2010; 44:352-9; PMID:20071495; http://dx.doi.org/10.1345/aph.1M351
- Poxton IR. Fidaxomicin: a new macrocyclic, RNA polymerase-inhibiting antibiotic for the treatment of Clostridium difficile infections. Future Microbiol 2010; 5:539-48; PMID:20353295; http://dx.doi.org/10.2217/fmb.10.20
- Louie T, Miller M, Donskey C, Mullane K, Goldstein EJ. Clinical outcomes, safety, and pharmacokinetics of OPT-80 in a phase 2 trial with patients with Clostridium difficile infection. Antimicrob Agents Chemother 2009; 53:223-8; PMID:18955525; http://dx.doi.org/10.1128/AAC.01442-07
- Johnson S, Gerding DN, Louie TJ, Ruiz NM, Gorbach SL. Sustained clinical response as an endpoint in treatment trials of Clostridium difficile-associated diarrhea. Antimicrob Agents Chemother 2012; 56:4043-5; PMID:22615287; http://dx.doi.org/10.1128/AAC.00605-12
- Eyre DW, Babakhani F, Griffiths D, Seddon J, Del Ojo Elias C, Gorbach SL, Peto TE, Crook DW, Walker AS. Whole-genome sequencing demonstrates that fidaxomicin is superior to vancomycin for preventing reinfection and relapse of infection with Clostridium difficile. J Infect Dis 2014; 209:1446-51; PMID:24218500; http://dx.doi.org/10.1093/infdis/jit598
- Hecht DW, Galang MA, Sambol SP, Osmolski JR, Johnson S, Gerding DN. In vitro activities of 15 antimicrobial agents against 110 toxigenic Clostridium difficile clinical isolates collected from 1983 to 2004. Antimicrob Agents Chemother 2007; 51:2716-9; PMID:17517836; http://dx.doi.org/10.1128/AAC.01623-06
- Neff G, Zacharias V, Kaiser TE, Gaddis A, Kemmer N. Rifaximin for the treatment of recurrent Clostridium difficile infection after liver transplantation: a case series. Liver Transpl 2010; 16:960-3; PMID:20677286; http://dx.doi.org/10.1002/lt.22092
- Garey KW, Salazar M, Shah D, Rodrigue R, DuPont HL. Rifamycin antibiotics for treatment of Clostridium difficile-associated diarrhea. Ann Pharmacother 2008; 42:827-35; PMID:18430792; http://dx.doi.org/10.1345/aph.1K675
- Nomura K, Matsumoto Y, Yoshida N, Taji S, Wakabayashi N, Mitsufuji S, Horiike S, Morita M, Okanoue T, Taniwaki M. Successful treatment with rifampin for fulminant antibiotic-associated colitis in a patient with non-Hodgkin's lymphoma. World J Gastroenterol 2004; 10:765-6; PMID:14991957
- Lagrotteria D, Holmes S, Smieja M, Smaill F, Lee C. Prospective, randomized inpatient study of oral metronidazole versus oral metronidazole and rifampin for treatment of primary episode of Clostridium difficile-associated diarrhea. Clin Infect Dis 2006; 43:547-52; PMID:16886144; http://dx.doi.org/10.1086/506354
- O'Connor JR, Galang MA, Sambol SP, Hecht DW, Vedantam G, Gerding DN, Johnson S. Rifampin and rifaximin resistance in clinical isolates of Clostridium difficile. Antimicrob Agents Chemother 2008; 52:2813-7; PMID:18559647; http://dx.doi.org/10.1128/AAC.00342-08
- Lynch T, Chong P, Zhang J, Hizon R, Du T, Graham MR, Beniac DR, Booth TF, Kibsey P, Miller M, et al. Characterization of a stable, metronidazole-resistant Clostridium difficile clinical isolate. PLoS One 2013; 8:e53757; PMID:23349739; http://dx.doi.org/10.1371/journal.pone.0053757
- Johnson S, Schriever C, Galang M, Kelly CP, Gerding DN. Interruption of recurrent Clostridium difficile-associated diarrhea episodes by serial therapy with vancomycin and rifaximin. Clin Infect Dis 2007; 33:846-8; http://dx.doi.org/10.1086/511870
- Patrick-Basu P, Dinani A, Rayapudi K, Pacana T, Shah NJ, Hampole H, Krishnaswamy NV, Mohan V. Rifaximin therapy for metronidazole-unresponsive Clostridium difficile infection: a prospective pilot trial. Therap Adv Gastroenterol 2010; 3:221-5; PMID:21180604; http://dx.doi.org/10.1177/1756283X10372985
- Huang JS, Jiang ZD, Garey KW, Lasco T, Dupont HL. Use of rifamycin drugs and development of infection by rifamycin-resistant strains of Clostridium difficile. Antimicrob Agents Chemother 2013; 57:2690-3; PMID:23545528; http://dx.doi.org/10.1128/AAC.00548-13
- Obuch-Woszczatyński P, Dubiel G, Harmanus C, Kuijper E, Duda U, Wultańska D, van Belkum A, Pituch H. Emergence of Clostridium difficile infection in tuberculosis patients due to a highly rifampicin-resistant PCR ribotype 046 clone in Poland. Eur J Clin Microbiol Infect Dis 2013; 32:1027-30; PMID:23443474; http://dx.doi.org/10.1007/s10096-013-1845-5
- Carman RJ, Boone JH, Grover H, Wickham KN, Chen L. In vivo selection of rifamycin-resistant Clostridium difficile during rifaximin therapy. Antimicrob Agents Chemother 2012; 56:6019-20; PMID:22908175; http://dx.doi.org/10.1128/AAC.00974-12
- Anderson VR, Curran MP. Nitazoxanide: a review of its use in the treatment of gastrointestinal infections. Drugs 2007; 67:1947-67; PMID:17722965; http://dx.doi.org/10.2165/00003495-200767130-00015
- Musher DM, Logan N, Hamill RJ, Dupont HL, Lentnek A, Gupta A, Rossignol JF. Nitazoxanide for the treatment of Clostridium difficile colitis. Clin Infect Dis 2006; 43:421-7; PMID:16838229; http://dx.doi.org/10.1086/506351
- Musher DM, Logan N, Mehendiratta V, Melgarejo NA, Garud S, Hamill RJ. Clostridium difficile colitis that fails conventional metronidazole therapy: response to nitazoxanide. J Antimicrob Chemother 2007; 59:705-10; PMID:17337513; http://dx.doi.org/10.1093/jac/dkl553
- Musher DM, Logan N, Bressler AM, Johnson DP, Rossignol JF. Nitazoxanide versus vancomycin in Clostridium difficile infection: a randomized, double blind study. Clin Infect Dis 2009; 48:e41-6; PMID:19133801; http://dx.doi.org/10.1086/596552
- Rafiullah F, Kanwal S, Majeed UM, Korsten MA, Cheema FH, Luthra M, Sohail MR. Successful use of nitazoxanide in the treatment of recurrent Clostridium difficile infection. BMJ Case Rep 2011; pii:bcr0420114123; PMID:22674696
- Herpers BL, Vlaminckx B, Burkhardt O, Blom H, Biemond-Moeniralam HS, Hornef M, Welte T, Kuijper EJ. Intravenous tigecycline as adjunctive or alternative therapy for severe refractory Clostridium difficile infection. Clin Infect Dis 2009; 48:1732-5; PMID:19435431; http://dx.doi.org/10.1086/599224
- Baines SD, Saxton K, Freeman J, Wilcox MH. Tigecycline does not induce proliferation or cytotoxin production by epidemic Clostridium difficile strains in a human gut model. J Antimicrob Chemother 2006; 58:1062-5; PMID:17030519; http://dx.doi.org/10.1093/jac/dkl364
- Jump RL, Li Y, Pultz MJ, Kypriotakis G, Donskey CJ. Tigecycline exhibits inhibitory activity against Clostridium difficile in the colon of mice and does not promote growth or toxin production. Antimicrob Agents Chemother 2011; 55:546-9; PMID:21135181; http://dx.doi.org/10.1128/AAC.00839-10
- Lao D II, Chiang T, Gomez E. Refractory Clostridium difficile infection successfully treated with tigecycline, rifaximin, and vancomycin. Case Rep Med 2012; 70291; PMID:22829841; http://dx.doi.org/10.1155/2012/702910
- Lu CL, Liu CY, Liao CH, Huang YT, Wang HP, Hsueh PR. Severe and refractory Clostridium difficile infection successfully treated with tigecycline and metronidazole. Int J Antimicrob Agents 2010; 35:311-2; PMID:20045292; http://dx.doi.org/10.1016/j.ijantimicag.2009.11.008
- Bassis CM, Theriot CM, Young VB. Alteration of the murine gastrointestinal microbiota by tigecycline leads to increased susceptibility to Clostridium difficile infection. Antimicrob Agents Chemother 2014; 58:2767-74; PMID:24590475; http://dx.doi.org/10.1128/AAC.02262-13
- Kopterides P, Papageorgiou C, Antoniadou A, Papadomichelakis E, Tsangaris I, Dimopoulou I, Armaganidis A. Failure of tigecycline to treat severe Clostridium difficile infection. Anaesth Intensive Care 2010; 38:755-8; PMID:20715744
- Freeman J, Baines SD, Wilcox MH. Comparison of the efficacy of ramoplanin vs vancomycin in both in vitro and in vivo models of clindamycin-induced Clostridium difficile infection. J Antimicrob Chemother 2005; 56:717-25; PMID:16143709; http://dx.doi.org/10.1093/jac/dki321
- Fulco P, Wenzel RP. Ramoplanin: a topical lipoglycodepsipeptide antibacterial agent. Expert Rev Anti Infect Ther 2006; 4:939-45; PMID:17181409; http://dx.doi.org/10.1586/14787210.4.6.939
- Jabes D, Candiani C, Riva S, Mosconi G. Superior efficacy of short treatment duration of ramoplanin over vancomycin in the hamster model of C. difficile-associated colitis, abstr. B-328. Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother 2003; American Society for Microbiology, Washington, DC
- Pullman J, Prieto J, Leach TS. Ramoplanin vs. vancomycin in the treatment of Clostridium difficile diarrhea: A Phase II study (abstract). Presented at: 44th Interscience Conference Antimicrob Agents Chemother 2004.
- Cotter PD, Hill C, Ross RP. Bacteriocins: developing innnate immunity for food. Nat Rev Microbiol 2005; 3:777-88; PMID:16205711; http://dx.doi.org/10.1038/nrmicro1273
- Rea MC, Dobson A, O'Sullivan O, Crispie F, Fouhy F, Cotter PD, Shanahan F, Kiely B, Hill C, Ross RP. Effect of broad- and narrow-spectrum antimicrobials on Clostridium difficile and microbial diversity in a model of the distal colon. Proc Natl Acad Sci U S A 2011; 108:4639-44; PMID:20616009; http://dx.doi.org/10.1073/pnas.1001224107
- Field D, Quigley L, O'Connor PM, Rea MC, Daly K, Cotter PD, Hill C, Ross RP. Studies with bioengineered nisin peptides highlight the broad-spectrum potency of nisin V. Microb Biotechnol 2010; 3:473-86; PMID:21255345; http://dx.doi.org/10.1111/j.1751-7915.2010.00184.x
- Rea MC, Clayton E, O'Connor PM, Shanahan F, Kiely B, Ross RP, Hill C. Antimicrobial activity of lacticin 3147 against clinical Clostridium difficile strains. J Med Microbiol 2007; 56:940-6; PMID:17577060; http://dx.doi.org/10.1099/jmm.0.47085-0
- Boakes S, Ayala T, Herman M, Appleyard AN, Dawson MJ, Cortés J. Generation of an actagardine A variant library through saturation mutagenesis. Appl Microbiol Biotechnol 2012; 95:1509-17; PMID:22526797; http://dx.doi.org/10.1007/s00253-012-4041-0
- Crowther GS, Baines SD, Todhunter SL, Freeman J, Chilton CH, Wilcox MH. Evaluation of NVB302 versus vancomycin activity in an in vitro human gut model of Clostridium difficile infection. J Antimicrob Chemother 2013; 68:168-76; PMID:22966180; http://dx.doi.org/10.1093/jac/dks359
- Selva E, Beretta G, Montanini N, Saddler GS, Gastaldo L, Ferrari P, Lorenzetti R, Landini P, Ripamonti F, Goldstein BP et al. Antibiotic GE2270 a: a novel inhibitor of bacterial protein synthesis. Isolation and characterization. J Antibiot (Tokyo) 1991; 44:693-701; PMID:1908853; http://dx.doi.org/10.7164/antibiotics.44.693
- LaMarche MJ, Leeds JA, Amaral A, Brewer JT, Bushell SM, Deng G, Dewhurst JM, Ding J, Dzink-Fox J, Gamber G, Jain A et al. Discovery of LFF571: an investigational agent for Clostridium difficile infection. J Med Chem 2012; 55:2376-87; PMID:22315981; http://dx.doi.org/10.1021/jm201685h
- Trzasko A, Leeds JA, Praestgaard J, Lamarche MJ, McKenney D. Efficacy of LFF571 in a hamster model of Clostridium difficile infection. Antimicrob Agents Chemother 2012; 56:4459-62; PMID:22644020; http://dx.doi.org/10.1128/AAC.06355-11
- Ting LS, Praestgaard J, Grunenberg N, Yang JC, Leeds JA, Pertel P. A first-in-human, randomized, double-blind, placebo-controlled, single- and multiple-ascending oral dose study to assess the safety and tolerability of LFF571 in healthy volunteers. Antimicrob Agents Chemother 2012; 56:5946-51; PMID:22964250; http://dx.doi.org/10.1128/AAC.00867-12
- Ackermann G, Löffler B, Adler D, Rodloff. In vitro activity of OPT-80 against Clostridium difficile. Antimicrob Agents Chemother 2004; 48:2280-2; PMID:15155234; http://dx.doi.org/10.1128/AAC.48.6.2280-2282.2004
- Credito K, Appelbaum P. Activity of OPT-80, a novel macrocycle, compared with those of eight other agents against selected anaerobic species. Antimicrob Agents Chemother 2004; 48:4430-4; PMID:15504874; http://dx.doi.org/10.1128/AAC.48.11.4430-4434.2004
- Finegold S, Molitoris D, Vaisanen M, Song Y, Liu C, Bolanos M. In vitro activities of OPT-80 and comparator drugs against intestinal bacteria. Antimicrob Agents Chemother 2004; 48:4898-902; PMID:15561877; http://dx.doi.org/10.1128/AAC.48.12.4898-4902.2004
- Anton PM, O'Brien M, Kokkotou E, Eisenstein B, Michaelis A, Rothstein D, Paraschos S, Kelly CP, Pothoulakis C. Rifalazil treats and prevents relapse of Clostridium difficile-associated diarrhea in hamsters. Antimicrob Agents Chemother 2004; 48:3975-9; PMID:15388461; http://dx.doi.org/10.1128/AAC.48.10.3975-3979.2004
- McVay CS, Rolfe RD. In vitro and in vivo activities of nitazoxanide against Clostridium difficile. Antimicrob Agents Chemother 2000; 44:2254-8; PMID:10952564; http://dx.doi.org/10.1128/AAC.44.9.2254-2258.2000
- Edlund C, Sabouri S, Nord CE. Comparative in vitro activity of BAY 12-8039 and five other antimicrobial agents against anaerobic bacteria. Eur J Clin Microbiol Infect Dis 1998; 17:193-5; PMID:9665302; http://dx.doi.org/10.1007/BF01691117
- Betriu C, Rodriguez-Avial I, Sanchez BA, Gomez M, Alvarez J, Picazo J. In vitro activities of tigecycline (GAR-936) against recently isolated clinical bacteria in Spain. Antimicrob Agents Chemother 2002; 46:892-5; PMID:11850282; http://dx.doi.org/10.1128/AAC.46.3.892-895.2002
- Peláez T, Alcalá L, Alonso R, Martín-López A, García-Arias V, Marín M, Bouza E. In vitro activity of ramoplanin against Clostridium difficile, including strains with reduced susceptibility to vancomycin or with resistance to metronidazole. Antimicrob Agents Chemother 2005; 49:1157-9; http://dx.doi.org/10.1128/AAC.49.3.1157-1159.2005
- Mathur H, O'Connor PM, Hill C, Cotter PD, Ross RP. Analysis of anti-Clostridium difficile activity of thuricin CD, vancomycin, metronidazole, ramoplanin, and actagardine, both singly and in paired combinations. Antimicrob Agents Chemother 2013; 57:2882-6; PMID:23571539; http://dx.doi.org/10.1128/AAC.00261-13
- Citron DM, Tyrrell KL, Merriam CV, Goldstein EJ. Comparative in vitro activities of LFF571 against Clostridium difficile and 630 other intestinal strains of aerobic and anaerobic bacteria. Antimicrob Agents Chemother 2012; 56:2493-503; PMID:22290948; http://dx.doi.org/10.1128/AAC.06305-11
- Aas J, Gessert CE, Bakken JS. Recurrent Clostridium difficile colitis: case series involving 18 patients treated with donor stool administered via a nasogastric tube. Clin Infect Dis 2003; 36:580-5; PMID:12594638; http://dx.doi.org/10.1086/367657
- Bakken JS, Borody T, Brandt LJ, Brill JV, Demarco DC, Franzos MA, Kelly C, Khoruts A, Louie T, Martinelli LP, Moore TA et al. Treating Clostridium difficile infection with fecal microbiota transplantation. Clin Gastroenterol Hepatol 2011; 9:1044-9; PMID:21871249; http://dx.doi.org/10.1016/j.cgh.2011.08.014
- Rohlke F, Stollman N. Fecal microbiota transplantation in relapsing Clostridium difficile infection. Therap Adv Gastroenterol 2012; 5:403-20; PMID:23152734; http://dx.doi.org/10.1177/1756283X12453637
- Russell G, Kaplan J, Ferraro M, Michelow IC. Fecal bacteriotherapy for relapsing Clostridium difficile infection in a child: a proposed treatment protocol. Pediatrics 2010; 126:e239-42; PMID:20547640; http://dx.doi.org/10.1542/peds.2009-3363
- Cramer JP, Burchard GD, Lohse AW. Old dogmas and new perspectives in antibiotic-associated diarrhea. Med Klin (Munich) 2008; 103:325-38; PMID:18484219; http://dx.doi.org/10.1007/s00063-008-1040-0
- Yoon SS, Brandt LJ. Treatment of refractoryrecurrent C. difficile-associated disease by donated stool transplanted via colonoscopy: a case series of 12 patients. J Clin Gastroenterol 2010; 44:562-6; PMID:20463588; http://dx.doi.org/10.1097/MCG.0b013e3181dac035
- Shahinas D, Silverman M, Sittler T, Chiu C, Kim P, Allen-Vercoe E, Weese S, Wong A, Low DE, Pillai DR. Toward an understanding of changes in diversity associated with fecal microbiome transplantation based on 16S rRNA gene deep sequencing. Mbio 2012; 3:e00338-12; PMID:23093385; http://dx.doi.org/10.1128/mBio.00338-12
- Hamilton MJ, Weingarden AR, Unno T, Khoruts A, Sadowsky MJ. High-throughput DNA sequence analysis reveals stable engraftment of gut microbiota following transplantation of previously frozen fecal bacteria. Gut Microbes 2013; 4:125-35; PMID:23333862; http://dx.doi.org/10.4161/gmic.23571
- van Nood E, Vrieze A, Nieuwdorp M, Fuentes S, Zoetendal EG, de Vos WM, Visser CE, Kuijper EJ, Bartelsman JF, Tijssen JG, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med 2013; 368:407-15; PMID:23323867; http://dx.doi.org/10.1056/NEJMoa1205037
- Petrof E, Gloor G, Vanner S, Weese S, Carter D, Daigneault M, Brown E, Schroeter K, Allen-Vercoe E. Stool substitute transplant therapy for the eradication of Clostridium difficile infection: ‘RePOOPulating’ the gut. Microbiome 2013; 1:3; PMID:24467987; http://dx.doi.org/10.1186/2049-2618-1-3
- Emanuelsson F, Claesson BE, Ljungström L, Tvede M, Ung KA. Faecal microbiota transplantation and bacteriotherapy for recurrent Clostridium difficile infection: a retrospective evaluation of 31 patients. Scand J Infect Dis 2014; 46:89-97; PMID:24354958; http://dx.doi.org/10.3109/00365548.2013.858181
- Fuentes S, van Nood E, Tims S, Heikamp-de Jong I, Ter Braak CJ, Keller JJ, Zoetendal EG, de Vos WM. Reset of a critically disturbed microbial ecosystem: faecal transplant in recurrent Clostridium difficile infection. ISME J 2014; 8:1621-33; PMID:24577353; http:; http://dx.doi.org/10.1038/ismej.2014.13
- Weingarden AR, Chen C, Bobr A, Yao D, Lu Y, Nelson VM, Sadowsky MJ, Khoruts A. Microbiota transplantation restores normal fecal bile acid composition in recurrent Clostridium difficile infection. Am J Physiol Gastrointest Liver Physiol 2014; 306:G310-9; PMID:24284963; http://dx.doi.org/10.1152/ajpgi.00282.2013
- Lawley TD, Clare S, Walker AW, Stares MD, Connor TR, Raisen C, Goulding D, Rad R, Schreiber F, Brandt C, Deakin LJ et al. Targeted restoration of the intestinal microbiota with a simple, defined bacteriotherapy resolves relapsing Clostridium difficile disease in mice. PLoS Pathog 2012; 8:e1002995; PMID:23133377; http://dx.doi.org/10.1371/journal.ppat.1002995
- Zipursky TI, Sidorsky CA, Freedman MN, Sidorsky KB. Kirkland. Patient attitudes toward the use of fecal microbiota transplantation in the treatment of recurrent Clostridium difficile infection. Clin Infect Dis 2012; 55:1652-8; PMID:22990849; http://dx.doi.org/10.1093/cid/cis809
- Torres JF, Lyerly DM, Hill JE, Monath TP. Evaluation of formalin-inactivated Clostridium difficile vaccines administered by parenteral and mucosal routes of immunization in hamsters. Infect Immun 1995; 12:4619-27
- Giannasca PJ, Zhang ZX, Lei WD, Boden JA, Giel MA, Monath TP, Thomas WD Jr. Serum antitoxin antibodies mediate systemic and mucosal protection from Clostridium difficile disease in hamsters. Infect Immun 1999; 2:527-38
- Siddiqui F, O'Connor JR, Nagaro K, Cheknis A, Sambol SP, Vedantam G, Gerding DN, Johnson S. Vaccination with parenteral toxoid B protects hamsters against lethal challenge with toxin A-negative, toxin B-positive Clostridium difficile but does not prevent colonization. J Infect Dis 2012; 205:128-33; PMID:22124129; http://dx.doi.org/10.1093/infdis/jir688
- Sougioultzis S, Kyne L, Drudy D, Keates S, Maroo S, Pothoulakis C, Giannasca PJ, Lee CK, Warny M, Monath TP, et al. Clostridium difficile toxoid vaccine in recurrent C. difficile-associated diarrhea. Gastroenterology 2005; 128:764-70; PMID:15765411; http://dx.doi.org/10.1053/j.gastro.2004.11.004
- Anosova NG, Brown AM, Li L, Liu N, Cole LE, Zhang J, Mehta H, Kleanthous H. Systemic antibody responses induced by a two-component Clostridium difficile toxoid vaccine protect against C. difficile-associated disease in hamsters. J Med Microbiol 2013; 62:1394-404; PMID:23518659; http://dx.doi.org/10.1099/jmm.0.056796-0
- Karczewski J, Zorman J, Wang S, Miezeiewski M, Xie J, Soring K, Petrescu I, Rogers I, Thiriot DS, Cook JC, et al. Development of a recombinant toxin fragment vaccine for Clostridium difficile infection. Vaccine 2014; 32:2812-8; PMID:24662701; http://dx.doi.org/10.1016/j.vaccine.2014.02.026
- Spencer J, Leuzzi R, Buckley A, Irvine J, Candlish D, Scarselli M, Douce GR. Vaccination against Clostridium difficile using toxin fragments: Observations and analysis in animal models. Gut Microbes 2014; 5:225-32; PMID:24637800; http://dx.doi.org/10.4161/gmic.27712
- Gerding DN. Clostridium difficile infection prevention: biotherapeutics, immunologics, and vaccines. Discov Med 2012; 13:75-83; PMID:22284786
- Ghose C, Verhagen JM, Chen X, Yu J, Huang Y, Chenesseau O, Kelly CP, Ho DD. Toll-like receptor 5-dependent immunogenicity and protective efficacy of a recombinant fusion protein vaccine containing the nontoxic domains of Clostridium difficile toxins A and B and Salmonella enterica serovar typhimurium flagellin in a mouse model of Clostridium difficile disease. Infect Immun 2013; 81:2190-6; PMID:23545305; http://dx.doi.org/10.1128/IAI.01074-12
- Donald RG, Flint M, Kalyan N, Johnson E, Witko SE, Kotash C, Zhao P, Megati S, Yurgelonis I, Lee PK, et al. A novel approach to generate a recombinant toxoid vaccine against Clostridium difficile. Microbiology 2013;159:1254-66; PMID:23629868; http://dx.doi.org/10.1099/mic.0.066712-0
- Wang H, Sun X, Zhang Y, Li S, Chen K, Shi L, Nie W, Kumar R, Tzipori S, Wang J, et al. A chimeric toxin vaccine protects against primary and recurrent Clostridium difficile infection. Infect Immun 2012; 80:2678-88; PMID:22615245; http://dx.doi.org/10.1128/IAI.00215-12
- Bertolo L, Boncheff AG, Ma Z, Chen YH, Wakeford T, Friendship RM, Rosseau J, Weese JS, Chu M, Mallozzi M, et al. Clostridium difficile carbohydrates: glucan in spores, PSII common antigen in cells, immunogenicity of PSII in swine and synthesis of a dual C. difficile-ETEC conjugate vaccine. Carbohydr Res 2012; 354:79-86; PMID:22533919; http://dx.doi.org/10.1016/j.carres.2012.03.032
- Jiao Y, Ma Z, Hodgins D, Pequegnat B, Bertolo L, Arroyo L, Monteiro MA. Clostridium difficile PSI polysaccharide: synthesis of pentasaccharide repeating block, conjugation to exotoxin B subunit, and detection of natural anti-PSI IgG antibodies in horse serum. Carbohydr Res 2013; 378:15-25; PMID:23597587; http://dx.doi.org/10.1016/j.carres.2013.03.018
- Adamo R, Romano MR, Berti F, Leuzzi R, Tontini M, Danieli E, Cappelletti E, Cakici OS, Swennen E, Pinto V, et al. Phosphorylation of the synthetic hexasaccharide repeating unit is essential for the induction of antibodies to Clostridium difficile PSII cell wall polysaccharide. ACS Chem Biol; 7:1420-8; PMID:22620974; http://dx.doi.org/10.1021/cb300221f
- Bertolo L, Boncheff AG, Ma Z, Chen YH, Wakeford T, Friendship RM, Rosseau J, Weese JS, Chu M, Mallozzi M, et al. Clostridium difficile carbohydrates: glucan in spores, PSII common antigen in cells, immunogenicity of PSII in swine and synthesis of a dual C. difficile-ETEC conjugate vaccine. Carbohydr Res 2012;354:79-86; PMID:22533919; http://dx.doi.org/10.1016/j.carres.2012.03.032
- Ganeshapillai J, Vinogradov E, Rousseau J, Weese JS, Monteiro MA. Clostridium difficile cell-surface polysaccharides composed of pentaglycosyl and hexaglycosyl phosphate repeating units. Carbohydr Res 2008;343:703-10; PMID:18237724; http://dx.doi.org/10.1016/j.carres.2008.01.002
- Monteiro MA, Ma Z, Bertolo L, Jiao Y, Arroyo L, Hodgins D, Mallozzi M, Vedantam G, Sagermann M, Sundsmo J, Chow H. Carbohydrate-based Clostridium difficile vaccines. Expert Rev Vaccines 2013; 12:421-31; PMID:23560922; http://dx.doi.org/10.1586/erv.13.9
- Martin CE, Broecker F, Oberli MA, Komor J, Mattner J, Anish C, Seeberger PH. Immunological evaluation of a synthetic Clostridium difficile oligosaccharide conjugate vaccine candidate and identification of a minimal epitope. J Am Chem Soc 2013; 135:9713-22; PMID:23795894; http://dx.doi.org/10.1021/ja401410y
- Romano MR, Leuzzi R, Cappelletti E, Tontini M, Nilo A, Proietti D, Berti F, Costantino P, Adamo R, Scarselli M. Recombinant Clostridium difficile toxin fragments as carrier protein for PSII surface polysaccharide preserve their neutralizing activity. Toxins (Basel) 2014; 6:1385-96; PMID:24759173
- Cox AD, St Michael F, Aubry A, Cairns CM, Strong PC, Hayes AC, Logan SM. Investigating the candidacy of a lipoteichoic acid-based glycoconjugate as a vaccine to combat Clostridium difficile infection. Glycoconj J 2013; 30:843-55; PMID:23974722; http://dx.doi.org/10.1007/s10719-013-9489-3
- Oberli MA, Hecht ML, Bindschädler P, Adibekian A, Adam T, Seeberger PH. A possible oligosaccharide-conjugate vaccine candidate for Clostridium difficile is antigenic and immunogenic. Chem Biol 2011;18:580-8; PMID:21609839; http://dx.doi.org/10.1016/j.chembiol.2011.03.009
- Baliban SM, Michael A, Shammassian B, Mudakha S, Khan AS, Cocklin S, Zetner I, Latimer BP, Bouillaut L, Hunter M, et al. An optimized, synthetic DNA vaccine encoding the toxin A and toxin B receptor binding domains of Clostridium difficile induces protective antibody responses in vivo. Infect Immun 2014; pii:IAI.01950-14
- Gardiner DF, Rosenberg T, Zaharatos J, Franco D, Ho DD. A DNA vaccine targeting the receptor-binding domain of Clostridium difficile toxin A. Vaccine 2009; 27:3598-604; PMID:19464540; http://dx.doi.org/10.1016/j.vaccine.2009.03.058
- Jin K, Wang S, Zhang C, Xiao Y, Lu S, Huang Z. Protective antibody responses against Clostridium difficile elicited by a DNA vaccine expressing the enzymatic domain of toxin B. Hum Vaccin Immunother 2013; 9:63-73; PMID:23143772; http://dx.doi.org/10.4161/hv.22434
- Seregin SS, Aldhamen YA, Rastall DP, Godbehere S, Amalfitano A. Adenovirus-based vaccination against Clostridium difficile toxin A allows for rapid humoral immunity and complete protection from toxin A lethal challenge in mice. Vaccine 2012; 30:1492-501; PMID:22200503; http://dx.doi.org/10.1016/j.vaccine.2011.12.064
