Abstract
Fat grafting popularity continues to rise among plastic surgeons. As a soft tissue filler, adipose tissue had many desirable attributes: it is easy to obtain, autologous, and may reintegrate into recipient sites. However, fat grafting is clinically plagued by unpredictable resorption rates, thus there is much interest in optimizing the procedure of fat grafting for consistent graft volumes. Fat harvesting, a part of fat transfer surgery, involves the removal of adipose tissue from the donor site. Different harvest procedures, such as whole fat excision or liposuction cannulas, result in a range of fat particle volumes, which may play a role in the cellular stability of grafts. The ideal harvesting technique and fat particle diameter is not currently known. This study aims to review the literature on the impact of fat particle size and clinical fat grafting outcomes, to present overarching conclusions, and to provide future directions for study. Current evidence supports excisional methods and larger bore cannulas to minimize cellular damage, preserve the native architecture, and maximize the number of cells within fat particles.
Introduction
Current use of adipose tissue as an injectable includes augmenting tissue volume and correcting contour irregularities, but future applications promise to capture fat's immunomodulatory and regenerative capacity. The benefits of adipose tissue are numerous; it is harvested with minimal donor site morbidity, autologous, and is easily contoured to match the native architecture. Adipose tissue transfer, or fat grafting, has become a common operation in the field of plastic surgery with more than 80,000 patients treated in the US alone with an 11.5% increased from the previous year.Citation1 However, fat grafting also has several shortcomings. Chiefly, reported fat graft retention rates range from 20-80% of at one year.Citation2-6 Absorption of the tissue causes many patients to undergo multiple costly procedures, and prevents the widespread clinical adoption of lipofilling.Citation7-9 Aberrant adipocyte necrosis and subsequent inflammation can also result in other complications such as hematoma, oil cysts, calcifications, or poor aesthetic outcomes.Citation10 The mechanisms and factors influencing graft retention are still unknown.
The unpredictability of fat grafting has lead to a search for an optimized method of harvesting that boosts long-term cellular viability and retention. Kling et al. recently found that 55% of surgeons favor hand held suction over vacuum-, water-, or ultrasonic-assisted harvest with the abdomen as the most common harvest site.Citation11 According to another survey,Citation12 the Coleman technique (54% of surgeons) is the preferred method of adipose harvest, followed by standard liposuction (25%), syringe techniques (16%) and direct excision (5%). The Coleman technique involves a 3-mm blunt cannula attached to a 10-mL Leur-Lok syringe.Citation13 Despite the implementation of the Coleman technique, the most popular standardized lipoharvest technique, the literature still demonstrates variable retention rates of fat grafts with both the Coleman technique and other cannulas.
Research efforts to date have partitioned fat grafting procedures into 3 separate phases: fat harvesting, processing techniques, and fat injection into recipient sites. Fat harvesting is the act of obtaining adipose tissue through excision or the use of the suction cannulas. Processing techniques include centrifugation, filtration, Telfa rolling, or washing the fat in order to remove oil, blood, and other unwanted components in the harvested adipose tissue. Injection techniques vary by the use of different syringes and needles for reintroducing the tissue in the defect area, pattern of reintroduction, and also the effects of pressure, shear, and strain on adipocytes. Within each step of fat grafting, there exist variations in techniques and numerous arguments in favor of one over the other, making it difficult to construct an optimal procedure from start to finish.
With respect to fat harvesting, one parameter that may predict the ability of grafted adipose tissue to survive is the notion of fat particles. Fat particles are defined as intact globules of adipocytes and interconnecting mesenchyme. Particles do not occur in native adipose tissue, but rather are cut and formed during the harvesting step of grafting. En bloc excision of adipose tissue creates the largest fat particles, and liposuction cannulas often create a range of fat particles dependent upon the cannula diameter. It is thought that cannula bores correlates with fat particle size, with larger diameters isolating larger particles.
Historically, Gustav Neuber, a seminal investigator into particle size and fat grafting, reported on his experience of transferring small pieces of upper arm fat to an acquired facial depression. In his study, Neuber cut pieces of fat in “bean or almond size,” which appeared to survive the grafting process.Citation14 Another prominent physician, Lexer, countered that larger pieces of fat gave better results, with grafts of smaller fat lobules inevitably becoming a scar.Citation15 Furthermore, Peer noted that “walnut sized grafts,” appeared to lose less bulk than multiple smaller grafts.Citation16 According to his findings, free fat grafts lost up to 45% of their original volume at one year, which may to attributed to trauma during grafting, such as shearing, or an inability to withstand the new environment, i.e., a lack of blood supply. Guerney studied fat transplants averaging 1.7 mmCitation3 over a period of 12 months and reported that single pieces of transplant were viable for at least one year. However, grafts of similar size divided into smaller pieces only lasted 6 months.Citation17 Again, Peer corroborated that single, walnut sized grafts lost only 45% of their original volume where grafts of smaller particles lost 79% of their weight at 14 months. Here we see the historical studies, which support the notion that the size of adipose particles may have a direct impact on the survival of grafts clinically.
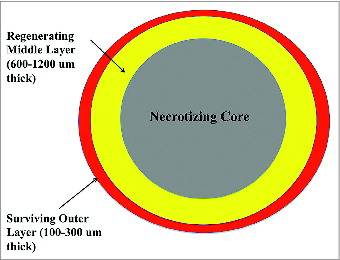
Kato et al.Citation18 recently published a landmark study, underlining the importance of the size of fat particles in grafting. It should be noted that the particle structure of fat does not occur in the native tissue, but rather is created during the harvesting procedure. Within a particle, the adipocytes most likely to survive were located closer to the surface of the lobule. Based on histological findings fat particles could be divided into 3 sections: the layer closest to the surface in which all adipocytes survive, the middle layer where the adipocytes die but are replaced by proliferating stem cells, and a central core dominated by necrosis, oil cysts, and fibrosis (). Demarcation of the surviving outer zone and the regenerating middle occurred at 1-300 um and 600-1200 um from the surface respectively. Prior to the reestablishment of a blood supply, fat particles largely depend on simple diffusion for nutrients. As the diameter of adipose lobules become larger, the central zone of necrosis will theoretically expand according to diffusional limitations. Therefore, one can see that the size of fat particles may ultimately influence how much of the grafted material survives. While the article by Kato et al. hints that smaller fat particles may better maintain volume by diffusion, Del Vecchio argues that smaller particles may lack stromal constituents such a fibroblasts, blood vessels, and connective tissue for structural support of adipocytes and proliferating stem cells.Citation19 Since inosculation, a process by which blood vessels rejoin, requires both recipient site vessels as well as those in the grafted fat, smaller particles does not necessarily mean better. The ideal lobular size must then be large enough to contain adequate mesenchyme while not so thick as to preclude nutritional diffusion.
Figure 1. During the first 3 months of tissue remodeling, adipocytes have 3 different fates depending upon their microenvironment and proximity to the surface of the fat particle. Adipocytes in the outermost layer are able to obtain sufficient nutrients through diffusion and survive. Mature adipocytes within the middle layer are unable to withstand the hypoxic conditions, however stem cells are able to survive and proliferate when blood supply is reestablished. All cells within the core die independent of maturity and eventually undergo cicatrization or form oil cysts.
To date, there is no consensus on the best means of harvesting fat particles from patients. This review article focuses on the various harvesting techniques for fat grafting, with respect to the size of isolated fat particles, and the subsequent impact on graft retention rate and the quality of harvested adipocytes. Fat particle size is rarely tested as a single variable, however cannula diameter functions as a surrogate for fat particle size.
Results
81 articles were located, of which 17 articles met the inclusion criteria. The articles covered in the review are summarized in . While the understanding and evolution of liposuction may mirror the shape and size of fat particle aspirate, key studies in this area typically examine or compare 3 categories of fat harvesting: excision, syringe aspiration, or liposuction cannulas.
Table 1. List of review studies (Alphabetical Order)
Excision of whole fat versus liposuction
Studies investigating excision vs. liposuction have yet to produce definitive results in favor of one of the over. En bloc fat grafting is purported to be less traumatic to adipocytes and promote graft survival,Citation20-22 yet it often requires multiple larger incisions to access the tissue. In a rabbit model, Kononas et al. found that 2 ml of surgically excised fat maintained its volume better than liposuction harvested grafts when transferring fat from the groin to auricular region.Citation21 Guyuron et al.,Citation23 demonstrated that thorough his technique with 1-cc syringe aspiration from abdominal and gluteal harvest sites, graft volume maintenance and patient satisfaction were comparable to procedures using whole fat excision with respect to fat grafting to the face. The same study also found a greater prevalence of fibrosis in the suctioned grafts. An article by Crawford et al.,Citation24 highlighted the importance of minimizing cellular trauma during power-assisted liposuction to increase the number of viable adipocytes in grafts harvested from the hips, potentially pointing to gentler means of harvesting such as excision. Comparing fat harvested from the groin of rabbits, Nguyen et al. found that only 10% of the adipocytes survive after liposuction at −760 mm Hg as opposed to 95% of excised grafts.Citation25
Conversely, Pu et al.Citation26 saw no difference between the cellular architecture of liposuctioned and en bloc removed fat from abdominal depots, but did find decreased enzymatic activity of glycerol-3-phosphate deydrongenase, a surrogate marker for cellular metabolism, in the liposuctioned sample. The study was corroborated by Lalikos et al.,Citation27 which indicated that liposuction does not lead to increased architectural distortion when compared to excised whole fat concerning abdominal harvest sites.
Distinct from adipocyte survival after harvesting, Huss and KratzCitation28 examined the proliferative capacity of preadipocytes isolated from excised fat or harvested using 5-mm diameter Toomey cannula. Subcutaneous adipose tissue was taken from either abdominal or breast depots. Adipose progenitor cells were isolated from equal weights of excised or liposuctioned samples and subsequently cultured. At a time-point of 120 hours, cell counts showed a significantly higher number in the excised group than fat obtained from the Toomey cannula.
Comparison of cannulas with different aperture sizes
Concerning the effects of cannula sizes on fat graft outcomes, Shiffman and Mirrafati used cannulas ranging from 2.5–3.7 mm in diameter, various suction pressures, and injection needles to discern if there was an effect on cellular viability levels.Citation29 The only significant result was that cellular damage, greater than 10 percent, occurred when a vacuum pressure of −700 mmHg was exceeded.
Ozoy et al. compared a 4-, 3-, and 2-mm-diameter cannulas on cellular viability levels of aspirate from 6 abdominoplasty patients. The prospective study found the highest level of viability with the use of a 4-mm cannula, supporting that a larger bore cannula may minimize cell damage and resorption while improving graft survival.Citation30 Erdim et al. removed fat tissue from the abdomen of 10 consecutive patients using 6-, 4-, and 2-mm liposuction cannulas.Citation31 The viability of fat grafts was assessed using colleganase digestion, supravital staining, and adipocyte counting using a haemocytometer. The results demonstrated a higher number of viable adipocytes from the aspirate occurred with the 6-mm cannula as opposed to the 2- and 4-mm cannulas (P < 0.001 and P < 0.01, respectively). No significant difference was detected in adipocyte viability when the 2- and 4-mm cannulas were compared, however the use of collagenase digestion may serve as a confounding error. Echoing the findings by Ozoy et al., a larger cannula diameter was shown to increase cellular viability.
Contrasting a 2-mm diameter syringe aspiration against a 4-mm diameter Triport power-assisted liposuction cannula, Leong et al. evaluated the metabolic state of fat harvested from abdominal depots using an alamarBlue, a compound which is change colors upon reduction in an active cell, and differentiation potential under medium with known adipogenic agents.Citation32 The study claimed that each group yielded viable cells with no significant difference in terms of metabolism and adipogenesis.
A study by Kirkham et al. harvested human adipose tissue from the abdomen and flank using a 5-mm and 3-mm cannulas in patients undergoing elective liposuction.Citation33 The aspirate was collected under uniform negative pressure (−25 mm Hg), and individual grafts of approximately 1 gram were injected in the flanks of nude mice using a 14-gauge needle. The grafts were analyzed by weight and histology at 6 weeks. The fat lobules in the 5-mm group retained 25% more of their original than the 3-mm fat grafts (P < 0.01). Histologic images showed more nucleated adipocytes along with less cellular rupture and debris than the 5-mm group. Furthermore, the 5-mm group demonstrated less infiltrate and fibrosis than the 3-mm group. As a result, the study recommended the use of larger aspiration cannulas in order to improve graft quality and longevity.
Gonzalez et al. tested the efficacy of 2 different methods of fat harvesting in an animal model.Citation34 Tissue from Zucker rats was aspirated using either a 3-mm liposuction cannula with a 60-cc syringe or a 2-mm blunt needle with a 10-cc syringe adapted to a fine-needle aspiration apparatus. After processing, adipocytes from each group were plated and expanded in vitro. Cell counts, MTT proliferation assay, G3PDH activity, and Oil Red O staining were used to assess cell viability. The study found that higher pressures and a larger oil layer, a marker for cellular trauma, occurred in the 3-mm liposuction cannula. Cellular proliferation was increased in the 2-mm blunt needle aspiration group (p = 0.017). The study ultimately purported that fat viability was higher using the fine-needle apparatus as means of aspiration, however the results could be due larger negative pressure generated from the 60-cc syringe than the 10-cc syringe and not directly to differences in cannula diameters.
A prospective study by Fisher et al. used a Shippert Biplane Cannula with a 3-mm outer diameter to aspirate human adipose tissue from the flank depot at a negative pressure of 430 mm Hg.Citation35 The adipose tissue was collected using an inline Tissu-Trans Filtron canister with filter pore sizes of 500 and 800 um. Unfiltered and filtered sample of the material remaining in the filter after removal of the filtrate was collected. The aspirated tissue was aliquoted to determine the average fat parcel size and to inject into a nude mouse model for graft retention studies. To discern the average parcel size, fat from the different groups of filter sizes was suspended in saline and imaged using light microscopy. Average parcel sizes of the groups were determined using Image J software. Average sizes for the unfiltered, 500 um, and 800 um filter in the suction assisted lipoaspiration were approximately 2.5 mm, 5.9 mm, and 3.2 mm respectively. At 6 weeks, fat grafts implanted in the mouse model after filtration resulted in significantly higher retention rate (78% of original volume) against the unfiltered adipose tissue (31% volume retention) with a p-value < 0.0001. Interestingly, a t-test between the 500 um and 800 um groups did not give a statistically significant result (p = 0.0826).
Nano- and microfat grafting
With respect to relatively delicate areas of the face such as eyelids and lips, a novel technique of micro and nanofat grafting can be used, typically with harvesting cannulas as small as 0.7 mm in diameter.Citation36-39 Tonnard et al highlighted the clinical application of the micro and nanofat grafts and sought to determine the cellular content of nanofat grafts.Citation40 Briefly, microfat particles were harvested from the abdomen using a cannula with 1-mm diameter. An amount of the microfat was sheared into finer particles using a Leur-to-Leur connector with 2 10-cc syringes. The nanoparticles were then filtered and collected. Macrofat particles were also harvested using a standard 3-mm cannula to serve as controls. To assess viability, samples were stained with calcein AM/ propidium iodide and evaluated for tissue quality. The study provided micrographs, which showed normal cellular architecture and sparse nonviable cells for both the macro and microfat particles. The nanofat grafts were devoid of adipocytes and the native architecture was disrupted. However, the nanografts retained a rich supply of adipose stem cells, which were similar to the macro and micro samples in terms of stem cell proliferation and differentiation. Several clinical cases using nanofat grafts resulted in improved skin quality 6 months post-operatively. Therefore, the author suggests that while nanografts do not contain viable adipocytes, the high content of stems cells in nanografts may be clinically useful in skin rejuvenations.
“Core” fat grafting techniques
Aside from the traditional of fat particles in grafting, Fagrell et al. experimented with cylindrical fat grafting in a rabbit model.Citation20 In this technique, the end of a 1-ml syringe is removed and replaced with a metal sleeve. The plunger was then pulled back until the 1-ml syringe is filled with fat. The internal sleeve diameter measured 4.5 mm, which gives the dimensions of the cylinder as 4.5 mm in diameter and approximately 63 mm in length. Using direct excision, traditional liposuction, or the experimental cylindrical fat graft technique, adipose tissue was harvested from a scapular fat pad and injected into either the scalp or gluteal region. At 6 months, the change in volume of experimental fat cylinders was contrasted against 1 mg of excised fat and 1 ml of lipoaspirate at 6 months. The study found that there was no statistical difference in the percent change of weight between the excised and cylindrical grafts. However, the weight of the aspirated fat was significantly less, and displayed less surviving adipocytes than the excised or cylindrical groups. To the end of core fat grafting, Rubin et al.,Citation41 advocated the use of a 4-mm cannula during core fat harvesting, and Nordstrom et al. investigated the “spaghetti” graft using a 3-mm cannula filter system to harvest from abdominal depots, similar to Fagrell's cylindrical shape.Citation42
Discussion
The use of adipose-based grafts is a well-known technique throughout the medical community. In fact, the first case of fat transplantation within a plastic surgery procedure began with Neuber in 1893.Citation14 Not limited to contour deformities, fat grafts can be used in the case of vocal cord augmentation, breast augmentation or reconstruction, cleft palate repairs, fat pad enhancement, and rejuvenation of the face or hand among other therapies.Citation43
A lack of consistent volume retention results caused fat graphs to fall largely out of favor in plastic surgery until the latter half of the 20th century. Indeed, there has been a recent resurgence of adipose tissue for grafting for the purpose of soft tissue reconstruction for a plethora of indications. Variable retention rates notwithstanding, autologous fat grafting has many desirable traits as an “ideal” soft tissue filler that other materials such as silicone, collagen, or hyaluronic acid do not; adipose is relatively abundant, easily obtainable, and biocompatible with the ability to integrate into recipient tissue. The clinical advantages of adipose tissue lead to search for an optimized technique of fat grafting to minimize, if not eliminate, unpredictable graft loss. Granted, surgeons have been prone to overcorrect soft tissue deficits in anticipation of graft resorption,Citation25,44,45 but this is an imperfect solution; the exact amount of additional tissue to add is debatable and arbitrary at times.
The aim of this review was to present data concerning the process of fat harvesting and to assess if an optimum cannula size to isolate sustainable fat particles exists. The relative paucity of on the subject is evident. Furthermore a clear favorite for cannula size and particle diameter has yet to emerge in the literature. Using similar graft volumes, some studies seem to favor the excision of whole fat over liposuction, whereas others do not note a difference. One prevalent trend in the reviewed literature is that excisional methods and using larger bore cannulas to harvest reduce the occurrence of cellular rupture and preserve the native architecture. This sentiment was echoed by Campbell et al, who found an inverse relationship between cellular damage and the diameter of the instrument to extract fat.Citation46 Accompanying basic science evaluations of fat particles in terms of cellular metabolism, apoptosis, and angiogenesis will help to further our understanding of the connection between cellular behavior and clinical results such a graft retention rates.
Perhaps the most prevalent theories regarding long-term volume maintenance are the “cell survival theory”Citation22 and the “host replacement theory.”Citation47-49 As Peer described, the cell survival theory states that the cellular population of grafts consists of adipocytes that survive transplantation initially through diffusion and then recipient tissue angiogenesis. Akin to the mechanisms underlying skin grafting, plastic surgeons have interpreted this theory to mean that increased cellular viability in grafted materials correlates to the long-term survival and hence volume retention.Citation33 The host replacement theory stipulates that most grafted adipocytes eventually die, and the remaining extracellular matrix serves as a scaffold for donor and recipient replacement cells, which rejuvenate the tissue. While the 2 theories may occur in tandem, current literature supports the cell survival theory, as evidenced by the addition of adipose-derive stem cells to lipoaspirate.Citation50 Studies proclaiming the use of larger-bore cannulas on the basis of reduced cellular trauma also maintain the cell replacement theory as the explanation behind in vivo graft persistence.Citation51 However, the exact microenvironments within and surrounding fat particles have yet to be elucidated, and further studies are needed on both a clinical and basic science level.
Future directions
Presently, there is much evidence that attests to the success of fat grafting and patient satisfaction with outcomes.Citation5,7,52 However, the variability in graft retention often necessitates multiple fat grafting procedures to replace lost grafts. Thus the continued optimization of fat grafting technique is not a superfluous task. The majority of current literature compares already established cannulas with fixed diameters, while the ideal lobular size is still unknown. Perhaps, it would be most sensible to first understand the underlying principles governing adipocyte survival and adipose derived stem cells differentiation within fat grafts. The Adipose Stem Cell Center at the University of Pittsburgh is currently examining how fat lobules of different sizes respond to conditions of hypoxia. Another aim of the research department is to measure volume retention rates in animal models used grafts comprised of various fat particles sizes. Based on these results, a cannula with the ideal diameter to isolate particles could be designed. Although continued studies of fat particle isolation methods are needed, it is only one parameter within the whole technique of fat grafting and as such cannot alone lead to an optimized process of fat grating. Graft quality over time also depends upon determining the proper method of processing the suctioned fat and injecting it into the defect area. Achieving reproducible and long lasting grafts requires a deep understanding of adipocytes from a biological and clinical perspective. Although daunting, the task is worthwhile; human adipose tissue has the potential to be the gold standard for replacement material for any soft tissue reconstruction.
Conclusion
This review has summarized the published data on the appropriate fat harvesting techniques for lipografting, and those methods may isolate a variety of fat particle sizes. Results thus far have failed to produce an absolute ideal lobule measurement and harvesting cannula for minimizing tissue resorption. A wide range of volume retention hinders the use of autologous adipose tissue. Twenty years after the modern resurgence of fat grafting, scientists and surgeons alike are attempting to provide insights into the behavior of adipocytes so that patients need not experience graft loss and undergo several procedures in the future.
Methods
A systematic review was performed of English medical literature located in the PubMed and Google Scholar databases, as well as site-specific searches for the journals Plastic and Reconstructive Surgery and the Annals of Plastic of Surgery. Search terms included the keywords “fat grafting,” “fat particle,” “fat particle size,” “liposuction,” “fat grafting,” “lipofilling,” and “cannula size.” The search was conducted in February of 2014. The goal of the comprehensive search was to locate articles discussing different techniques for the harvesting of autologous fat.
Articles retrieved from the study were read in their entirety, and the corresponding bibliographies were examined for additional studies. Articles were included in the study based upon the inclusion and exclusion criteria listed in . All articles harvested from subcutaneous adipose tissue depots, and articles concerning visceral adipose tissue depots were excluded. Original articles had to specifically note which cannula was used in the lipografting procedure and also provide either long-term clinical follow up data or a cellular attributes of the aspirate. Patient satisfaction with the procedure was taken into account when stated, but not necessary for an article's inclusion. All studies that mentioned the harvesting technique and cannula type or size used in the fat harvesting step were included, regardless of the article type (observational study, retrospective studies, case series, clinical trial, etc). Articles that assessed adipocyte viability or biomarkers but did not inject the adipose tissue into subjects were also included in the review. Studies using animal fat and studies on fat grafting for applications outside of plastic surgery were also reviewed and included. Data concerning the comparative effect between traditional liposuction, ultra-sound assisted, and laser-assisted were not included.
Table 2. Inclusion and exclusion criteria
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- The American Society for Aesthetic Plastic Surgery. Cosmetic surgery national data bank statistics 2013. Available from http://www.surgery.org/sites/default/files/Stats2013_4.pdf
- Delay E, Garson S, Tousson G, Sinna R. Fat injection to the breast: technique, results, and indications based on 880 procedures over 10 years. Aesthet Surg J 2009; 29:360-76; PMID:19825464; http://dx.doi.org/10.1016/j.asj.2009.08.010
- Niechajev I, Sevcuk O. Long-term results of fat transplantation: clinical and histologic studies. Plast Reconstr Surg 1994; 94:496-506; PMID:8047602; http://dx.doi.org/10.1097/00006534-199409000-00012
- Park S, Kim B, Shin Y. Correction of superior sulcus deformity with orbital fat anatomic repositioning and fat graft applied to retro-orbicularis oculi fat for Asian eyelids. Aesthet Plast Surg 2011; 35:162-70; PMID:20835821; http://dx.doi.org/10.1007/s00266-010-9574-y
- Zocchi M, Zuliani F. Bicompartmental breast lipostructuring. Aesthet Plast Surg 2008; 32:313-28; PMID:18188638; http://dx.doi.org/10.1007/s00266-007-9089-3
- Gallego S, Ramírez F, Echeverri A. Magnetic resonance imaging assessment of gluteal fat grafts. Aesthet Plast Surg 2006; 30:460-8; PMID:16855890; http://dx.doi.org/10.1007/s00266-005-0202-1
- Illouz YG, Sterodimas A. Autologous fat transplantation to the breast: a personal technique with 25 years of experience. Aesthet Plast Surg 2009; 33:706-15; PMID:19495856; http://dx.doi.org/10.1007/s00266-009-9377-1
- Sterodimas A, de Faria J, Nicaretta B, Boriani F. Autologous fat transplantation versus adipose-derived stem cell–enriched lipografts a study. Aesthet Surg J 2011; 31:682-93; PMID:21813882; http://dx.doi.org/10.1177/1090820X11415976
- Xie Y, Zheng DN, Li QF, Gu B, Liu K, Shen GX, Pu LL. An integrated fat grafting technique for cosmetic facial contouring. J Plast, Reconstr Aesthet Surg 2010; 63:270-6; http://dx.doi.org/10.1016/j.bjps.2008.11.016
- Hörl H, Feller A-M, Biemer E. Technique for liposuction fat reimplantation and long-term volume evaluation by magnetic resonance imaging. Ann Plast Surg 1991; 26:248-58; PMID:2029135; http://dx.doi.org/10.1097/00000637-199103000-00007
- Kling RE, Mehrara BJ, Pusic AL, Young VL, Hume KM, Crotty CA, Rubin JP. Trends in autologous fat grafting to the breast: a national survey of the American society of plastic surgeons. Plast Reconstr Surg 2013; 132:35-46; PMID:23806907; http://dx.doi.org/10.1097/PRS.0b013e318290fad1
- Kaufman MR, Bradley JP, Dickinson B, Heller JB, Wasson K, O'Hara C, Huang C, Gabbay J, Ghadjar K, Miller TA. Autologous fat transfer national consensus survey: trends in techniques for harvest, preparation, and application, and perception of short-and long-term results. Plast Reconstr Surg 2007; 119:323-31; PMID:17255689; http://dx.doi.org/10.1097/01.prs.0000244903.51440.8c
- Jauffret J, Champsaur P, Robaglia-Schlupp A, Andrac-Meyer L, Magalon G. Arguments in favor of adipocyte grafts with the SR Coleman technique. Ann De Chirur Plast Et Esth 2001, 46:31-8; PMID:11233732; http://dx.doi.org/10.1016/S0294-1260(01)80006-6
- Neuber G. Fettransplantation. Chir Kongr Verhandl Deutsche Gesellschaft für Chirurgie. 1893; 22:66.
- Lexer E. Ueber freie fettransplantation. Klin Therap Wehnschr 1911; 18:53.
- Peer LA. The neglected free fat graft. Plast Reconstr Surg 1956; 18:233-50; PMID:13379008; http://dx.doi.org/10.1097/00006534-195610000-00001
- Gurney CE. Experimental Study of the Behavior of Free Fat Transplants. University of Minnesota, 1937; 3:679-92.
- Kato H, Mineda K, Eto H, Doi K, Kuno S, Kinoshita K, Kanayama K, Yoshimura K. Degeneration, regeneration, and cicatrization after fat grafting: dynamic total tissue remodeling during the first 3 months. Plast Reconstr Surg 2014; 133:303e-13e; PMID:24572875; http://dx.doi.org/10.1097/PRS.0000000000000066
- Del Vecchio D, Rohrich RJ. A classification of clinical fat grafting: different problems, different solutions. Plast Reconstr Surg 2012; 130:511-22; PMID:22929236; http://dx.doi.org/10.1097/PRS.0b013e31825dbf8a
- Fagrell D, Eneström S, Berggren A, Kniola B. Fat cylinder transplantation: an experimental comparative study of three different kinds of fat transplants. Plast Reconstr Surg 1996; 98:90-6; PMID:8657793; http://dx.doi.org/10.1097/00006534-199607000-00014
- Kononas TC, Bucky LP, Hurley C, May Jr JW. The fate of suctioned and surgically removed fat after reimplantation for soft-tissue augmentation: a volumetric and histologic study in the rabbit. Plast Reconstr Surg 1993; 91:763-8; PMID:8460177; http://dx.doi.org/10.1097/00006534-199304001-00001
- Peer LA. Loss of weight and volume in human fat grafts: with postulation of a" cell survival theory". Plast Reconstr Surg 1950; 5:217-30; http://dx.doi.org/10.1097/00006534-195003000-00002
- Guyuron B, Majzoub RK. Facial augmentation with core fat graft: a preliminary report. Plast Reconstr Surg 2007; 120:295-302; PMID:17572578; http://dx.doi.org/10.1097/01.prs.0000264399.40701.71
- Crawford JL, Hubbard BA, Colbert SH, Puckett CL. Fine tuning lipoaspirate viability for fat grafting. Plast Reconstr Surg 2010; 126:1342-8; PMID:20885257; http://dx.doi.org/10.1097/PRS.0b013e3181ea44a9
- May Jr JW. Comparative study of survival of autologous adipose tissue taken and transplanted by different techniques (Discussion). Plast Reconstr Surg 1990; 85:387-9; http://dx.doi.org/10.1097/00006534-199001000-00057
- Pu LL, Cui X, Fink BF, Cibull ML, Gao D. The viability of fatty tissues within adipose aspirates after conventional liposuction: a comprehensive study. Ann Plast Surg 2005; 54:288-92; PMID:15725835
- Lalikos JF, Li Y-Q, Roth TP, Doyle JW, Matory WE, Lawrence WT. Biochemical assessment of cellular damage after adipocyte harvest. J Surg Res 1997; 70:95-100; PMID:9228935; http://dx.doi.org/10.1006/jsre.1997.5090
- Huss FR, Kratz G. Adipose tissue processed for lipoinjection shows increased cellular survival in vitro when tissue engineering principles are applied. Scand J Plast Reconstr Surg Hand Surg 2002; 36:166-71; PMID:12141205; http://dx.doi.org/10.1080/028443102753718050
- Shiffman MA, Mirrafati S. Fat transfer techniques: the effect of harvest and transfer methods on adipocyte viability and review of the literature. Dermatol Surg 2001; 27:819-26; PMID:11553171
- Özsoy Z, Kul Z, Bilir A. The role of cannula diameter in improved adipocyte viability: a quantitative analysis. Aesthet Surg J 2006; 26:287-9; http://dx.doi.org/10.1016/j.asj.2006.04.003
- Erdim M, Tezel E, Numanoglu A, Sav A. The effects of the size of liposuction cannula on adipocyte survival and the optimum temperature for fat graft storage: an experimental study. J Plast, Reconstr Aesthet Surg 2009; 62:1210-4; http://dx.doi.org/10.1016/j.bjps.2008.03.016
- Leong DT, Hutmacher DW, Chew FT, Lim T-C. Viability and adipogenic potential of human adipose tissue processed cell population obtained from pump-assisted and syringe-assisted liposuction. J Dermatol Sci 2005; 37:169-76; PMID:15734286; http://dx.doi.org/10.1016/j.jdermsci.2004.11.009
- Kirkham JC, Lee JH, Medina III MA, McCormack MC, Randolph MA, Austen Jr WG. The impact of liposuction cannula size on adipocyte viability. Ann Plast Surg 2012; 69:479-81; PMID:22964677; http://dx.doi.org/10.1097/SAP.0b013e31824a459f
- Gonzalez AM, Lobocki C, Kelly CP, Jackson IT. An alternative method for harvest and processing fat grafts: an in vitro study of cell viability and survival. Plast Reconstr Surg 2007; 120:285-94; PMID:17572577; http://dx.doi.org/10.1097/01.prs.0000264401.19469.ad
- Fisher C, Grahovac TL, Schafer ME, Shippert RD, Marra KG, Rubin JP. Comparison of harvest and processing techniques for fat grafting and adipose stem cell isolation. Plast Reconstr Surg 2013; 132:351-61; PMID:23584621; http://dx.doi.org/10.1097/PRS.0b013e3182958796
- Dasiou-Plakida D. Fat injections for facial rejuvenation: 17 years experience in 1720 patients. J Cosmet Dermatol 2003; 2:119-25; PMID:17163916; http://dx.doi.org/10.1111/j.1473-2130.2004.00060.x
- Nguyen P, Desouches C, Gay A, Hautier A, Magalon G. Development of micro-injection as an innovative autologous fat graft technique: the use of adipose tissue as dermal filler. J Plast, Reconstr Aesthet Surg 2012; 65:1692-9; http://dx.doi.org/10.1016/j.bjps.2012.06.014
- Trepsat F. [Midface reshaping with micro-fat grafting]. Ann De Chir Plast Et esthet 2009; 54:435-43; PMID:19446945; http://dx.doi.org/10.1016/j.anplas.2009.03.008
- Mazzola RF. Fat injection: from filling to regeneration. St. Louis, MO: Quality Medical Publishing, 2009; 373-422.
- Tonnard P, Verpaele A, Peeters G, Hamdi M, Cornelissen M, Declercq H. Nanofat grafting: basic research and clinical applications. Plast Reconstr Surg 2013; 132:1017-26; PMID:23783059; http://dx.doi.org/10.1097/PRS.0b013e31829fe1b0
- Rubin A, Hoefflin SM. Fat purification: Survival of the fittest. Plast Reconstr Surg 2002; 109:1463-4; PMID:11965014; http://dx.doi.org/10.1097/00006534-200204010-00049
- Nordström RE, Wang J, Fan J. " Spaghetti" fat grafting: a new technique. Plast Reconstr Surg 1997; 99:917-8; PMID:9047222; http://dx.doi.org/10.1097/00006534-199703000-00058
- Gutowski KA, Force AFGT. Current applications and safety of autologous fat grafts: a report of the ASPS fat graft task force. Plast Reconstr Surg 2009; 124:272-80; PMID:19346997; http://dx.doi.org/10.1097/PRS.0b013e3181a09506
- Sawhney C, Banerjee T, Chakravarti R. Behaviour of dermal fat transplants. British J Plast Surg 1969; 22:169-76; PMID:4891594; http://dx.doi.org/10.1016/S0007-1226(69)80061-5
- Peer LA. Transplantation of fat. In: Converse JM (Ed.), Reconstructive plastic surgery. Philadelphia, PA: Saunders, 1977;251-61.
- Campbell G-L, Laudenslager N, Newman J. The effect of mechanical stress on adipocyte morphology and metabolism. Am J Cosmet Surg 1987; 4:89-94
- Doldere JH, Thompson EW, Slavin J, Trost N, Cooper-White JJ, Cao Y, O'Connor AJ, Penington A, Morrison WA, Abberton KM. Long-term stability of adipose tissue generated from a vascularized pedicled fat flap inside a chamber. Plast Reconstr Surg 2011; 127:2283-92; PMID:21617462; http://dx.doi.org/10.1097/PRS.0b013e3182131c3e
- Eto H, Kato H, Suga H, Aoi N, Doi K, Kuno S, Yoshimura K. The fate of adipocytes after nonvascularized fat grafting: evidence of early death and replacement of adipocytes. Plast Reconstr Surg 2012; 129:1081-92; PMID:22261562; http://dx.doi.org/10.1097/PRS.0b013e31824a2b19
- Peer LA. Cell survival theory versus replacement theory. Plast Reconstr Surg 1955; 16:161-8; PMID:13266544; http://dx.doi.org/10.1097/00006534-195509000-00001
- Billings Jr E, May Jr JW. Historical review and present status of free fat graft autotransplantation in plastic and reconstructive surgery. Plast Reconstr Surg 1989; 83:368-81; PMID:2643129; http://dx.doi.org/10.1097/00006534-198902000-00033
- Marques A, Brenda E, Saldiva PH, Amarante MT, Ferreira MC. Autologous fat grafts: a quantitative and morphometric study in rabbits. Scand J Plast Reconstr Surg Hand Surg 1994; 28:241-7; PMID:7899832; http://dx.doi.org/10.3109/02844319409022006
- Coleman SR. Structural fat grafts: the ideal filler? Clin Plast Surg 2001; 28:111-9; PMID:11248861
