Abstract
Growth hormone (GH) supplementation therapy to adults with GH deficiency has beneficial effects on adipose tissue lipid metabolism, improving thus adipocyte functional morphology and insulin sensitivity. However, molecular nature of these effects remains unclear. We therefore tested the hypothesis that lipid-mobilizing adipokine zinc-α2-glycoprotein is causally linked to GH effects on adipose tissue lipid metabolism. Seventeen patients with severe GH deficiency examined before and after the 5-year GH replacement therapy were compared with age-, gender- and BMI-matched healthy controls. Euglycemic hyperinsulinemic clamp was used to assess whole-body and adipose tissue-specific insulin sensitivity. Glucose tolerance was determined by oGTT, visceral and subcutaneous abdominal adiposity by MRI, adipocyte size morphometrically after collagenase digestion, lipid accumulation and release was studied in differentiated human primary adipocytes in association with GH treatment and zinc-α2-glycoprotein gene silencing. Five-year GH replacement therapy improved glucose tolerance, adipose tissue insulin sensitivity and reduced adipocyte size without affecting adiposity and whole-body insulin sensitivity. Adipose tissue zinc-α2-glycoprotein expression was positively associated with whole-body and adipose tissue insulin sensitivity and negatively with adipocyte size. GH treatment to adipocytes in vitro increased zinc-α2-glycoprotein expression (>50%) and was paralleled by enhanced lipolysis and decreased triglyceride accumulation (>35%). Moreover, GH treatment improved antilipolytic action of insulin in cultured adipocytes. Most importantly, silencing zinc-α2-glycoprotein eliminated all of the GH effects on adipocyte lipid metabolism. Effects of 5-year GH supplementation therapy on adipose tissue lipid metabolism and insulin sensitivity are associated with zinc-α2-glycoprotein. Presence of this adipokine is required for the GH action on adipocyte lipid metabolism in vitro.
Abbreviations:
- ACC1, acetyl-CoA carboxylase 1
- BSA, bovine serum albumin
- DGAT, diacylglycerol acyltransferase
- DMEM, Dulbecco's Modified Eagle Medium
- EHC, euglycemic hyperinsulinemic clamp
- FAS, fatty acid synthase
- FABP4, fatty acid binding protein 4
- FBS, fetal bovine serum
- FFA, free fatty acids
- GAPDH, glyceraldehyde-3-phosphate dehydrogenase
- GH, growth hormone
- GLUT4, glucose transporter 4
- HSL, hormone sensitive lipase
- GHD, growth hormone deficiency
- IGF-1, insulin-like growth factor 1
- IRS1, insulin receptor substrate 1
- MRI, magnetic resonance imaging
- oGTT, oral glucose tolerance test
- PPARGC1A, peroxisome proliferator-activated receptor 1 gamma coactivator 1 α
- rhGH, recombinant human growth hormone
- RPL13A, ribosomal protein L13a
- TG, triglycerides
- ZAG, zinc-α2-glycoprotein.
Introduction
Severe GH deficiency in adults is associated with abdominal adiposity, marked adipocyte hypertrophy, reduced muscle mass, strength and physical performance, dyslipidemia, local and systemic inflammation and insulin resistance.Citation1–4 Common obesity and metabolic syndrome are frequently associated with decreased levels of circulating GH and some beneficial effects of low-dose GH treatment to obese individuals with relative GH deficiency were reported.Citation5–7 Long-term GH supplementation to GH deficient adults has been shown to reverse some of the negative metabolic consequences of GH deficiency and reduce metabolic and cardiovascular morbidity among these patients.Citation3,8 However, conflicting data regarding the effect of GH therapy on cardiovascular risk exist.Citation9,10 Our previous study identified profound molecular and morphological changes in subcutaneous adipose tissue of GH deficient adults, pointing at extreme adipocyte hypertrophy and enhanced pro-inflammatory state, indicating defects in adipose tissue lipid metabolism.Citation1 Aim of the presented follow-up study was to learn if GH treatment effectively counteracts aforementioned unfavorable adipose tissue and whole-body phenotypes and to better understand the mechanisms of beneficial GH action in the adipose tissue.
Adipose tissue is a highly dynamic multifunctional organ, producing a wide range of adipokines, modulating many physiological processes in different tissues.Citation11–13 Changes in adipose tissue secretory profile such as found in common obesity or in severe GH deficiency invariably associated with extreme adipocyte hypertrophy, might severely impact the whole-body lipid metabolism, leading to the development of metabolic disease.Citation1,14 Previous reports suggest that adipokine zinc-α2-glycoprotein (ZAG) acts as a modulator of body fat mass by stimulating adipose tissue (AT) lipolysis, lipid oxidation (AT, skeletal muscle) and thermogenesis (brown AT) and by modulating lipogenesis (AT).Citation15–20 ZAG was first isolated from human plasma and subsequently identified as a product of secretory epithelial cells and several malignant tumors.Citation21–23 Later, ZAG was shown to be identical to a lipid-mobilizing factor responsible for loss of fat mass in cancer cachexia.Citation24 In human adipocytes, ZAG was found to be secreted at levels comparable to those of adiponectin with a similar regulatory pattern in obesity.Citation25,26 Production of ZAG in the adipose tissue is elevated proportionally to the degree of weight loss in patients with cancer cachexia.Citation27 Interestingly, administration of ZAG to either lean (NMRI) or obese (ob/ob) mice induced a selective reduction in body fat with no effect on lean body mass, food and water intake.Citation28 ZAG deficient mice display decreased adipose tissue lipolysis and are prone to diet-induced obesity.Citation17 ZAG has also been shown to promote catecholamine-induced lipolysis in adipose tissue of cachectic cancer patients and in 3T3-L1 adipocytes.Citation16 Reduction of ZAG in the adipose tissue of obese individuals is associated with a decrease in lipid-mobilizing capacity and might participate in the development of obesity-related metabolic disease.Citation26
The primary aim of our study was to investigate the effects of 5-year GH replacement therapy to patients with severe GH deficiency on adiposity, whole-body insulin sensitivity and adipose tissue morphologic, metabolic and secretory properties. Hypothesis that adipokine ZAG plays a key role in regulating GH effects on lipid metabolism was tested in vitro in 3T3-L1 and differentiated human primary adipocytes.
Results
Effects of 5-year GH supplementation to GH deficient adults on adiposity, whole-body and adipose tissue metabolic phenotypes
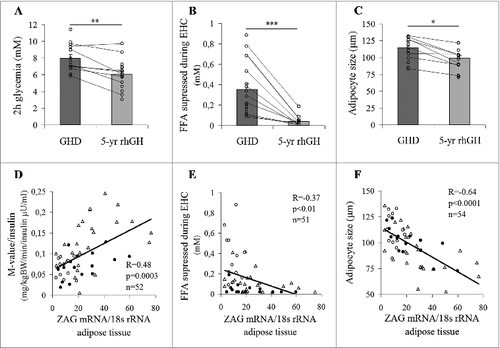
GH treatment-related changes in BMI, waist circumference, visceral and subcutaneous adiposity were analyzed in the entire cohort as well as in 9 patients who underwent both initial and follow-up examination. Considering both analyses we observed that long-term GH supplementation failed to significantly change any of these parameters (). It is however important to note that GH therapy normalized serum IGF-1 to levels found in healthy controls and lowered levels of circulating FFA (). Five-year GH replacement therapy had no effect on the whole-body insulin sensitivity () and fasting glycemia (), however, a significant improvement of glucose tolerance (2h glycemia; ) was observed. Moreover, adipose tissue insulin sensitivity (determined as a degree of insulin-induced FFA suppression during steady state of EHC) was substantially improved by GH treatment () leading to even higher insulin sensitivity than that found in healthy age-, gender- and BMI-matched controls (). In addition, GH therapy significantly reduced the size of subcutaneous adipocytes (∼13%, ), while circulating adiponectin levels were not affected (GHD 10.3 ± 1.1 μg/ml vs. GH treated 12.2 ± 1.8 μg/ml; p = 0.37). It has to be noted that only a tendency toward a decrease in fat cell size (p = 0.056) was observed in a subpopulation of the 9 “real follow-up” patients, likely due to the low patient numbers (). Nevertheless adipose tissue ZAG mRNA was positively associated with the whole-body () and adipose tissue insulin sensitivity (determined as an ability of insulin to suppress FFA during EHC) () and negatively with fat cell size (), suggesting a potential role for this adipokine in mediating GH-induced changes in adipose tissue lipid metabolism and insulin action. Moreover, ZAG protein content was also positively associated with adipose tissue insulin sensitivity (R = −0.36, P < 0.05; R2 = 0.13, P < 0.05). It is important to note that all these associations were at least in part independent on subcutaneous adiposity (M-value: R = 0.41, P < 0.01; fat cell size: R = −0.51, P < 0.001; adipose tissue insulin sensitivity: R = −0.43, P < 0.01; R2 = 0.19, P < 0.01). In addition, no significant differences between adult-onset and childhood-onset GHD patients related to 5-year rhGH treatment were found in any of the measured parameters except the 2 h glycemia and fat cell size, which were decreased in the entire cohort, as well as in childhood-onset GHD patients, but showed only a tendency toward a decrease in adult-onset GHD patients (data not shown).
Table 1. Clinical characteristic of study cohorts
Figure 1. Positive effects of 5-year GH replacement therapy on the whole-body and adipose tissue metabolic phenotypes. The effect of 5-year GH replacement therapy on (A) glucose tolerance (2h glycemia in oral glucose tolerance test), (B) adipose tissue insulin sensitivity (ability of insulin to suppress FFA during EHC) and (C) adipocyte size (lines connect the data points of the “real follow-up” patients). Associations of adipose tissue ZAG gene expression with the (D) whole-body and (E) adipose tissue insulin sensitivity (ability of insulin to suppress FFA during EHC) and (F) adipocyte size (Δ controls; ○ baseline GH naive patients; GH treated GHD patients). GHD, patients with growth hormone deficiency; EHC, euglycemic hyperinsulinemic clamp; FFA, free fatty acids; ZAG, zinc-α2-glycoprotein; rhGH, recombinant human growth hormone; *P < 0.05; **P < 0.01; ***P < 0.001.
GH inhibits lipid accumulation in adipocytes in vitro
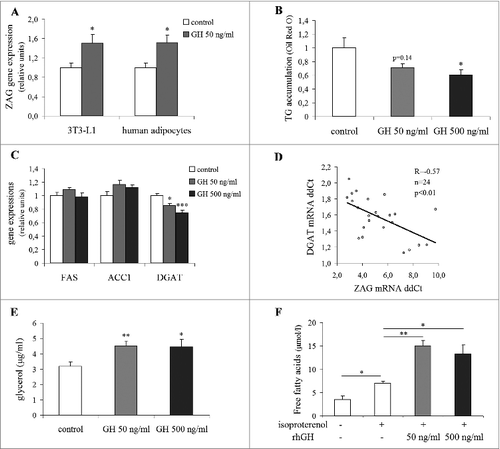
To study the effects of GH on lipid metabolism in adipocytes, we exposed differentiated 3T3-L1 adipocytes to GH for 6 days. Treatment with 50 ng/ml rhGH induced 50% increase in basal ZAG gene expression in 3T3-L1 adipocytes (). More importantly, we have observed that GH-induced increase of ZAG mRNA was paralleled by 30–40% reduction in lipid accumulation (, photo documentation - Supplementary Fig. 2), which was already apparent after 2 d of GH treatment. Lack of the change in gene expression for adiponectin (p = 0.69) and fatty acid binding protein 4 (FABP4; p = 0.20), markers of differentiated adipocytes, indicates that there was no major effect of GH on adipocyte differentiation capacity (data not shown). Decreased lipid accumulation might be at least in part explained by GH-induced reduction in the adipose tissue gene expression of diacylglycerol acyltransferase (DGAT), a key enzyme of triglyceride synthesis (). Furthermore, DGAT mRNA was strongly negatively associated with the expression of ZAG mRNA (), indicating a potential role for this adipokine in mediating the anti-lipogenic effect of GH. This in vitro observation is in line with in vivo findings of 2-fold increased DGAT mRNA in subcutaneous adipose tissue of GH deficient adults, which was fully normalized by GH substitution therapy (Supplementary Fig. 3). GH treatment had no effect on expression of fatty acid synthase (FAS) and acetyl-CoA carboxylase 1 (ACC-1) (). The lipid content-lowering effect might also be explained by stimulatory action of GH on basal lipolysis (). It is important to note that GH promoted isoproterenol-induced lipolysis in 3T3-L1 adipocytes, indicating increased sensitivity to catecholamine-induced lipolysis (). These complex in vitro data indicate that the inhibitory effect of GH on lipid accumulation in adipocytes could be explained by stimulation of lipolytic and inhibition of lipogenic processes and suggest a potential role for ZAG in mediating these effects.
Figure 2. Growth hormone regulates ZAG and inhibits lipid accumulation in 3T3-L1 adipocytes in vitro. (A) The effect of 6-day GH treatment on ZAG gene expression in differentiated 3T3-L1 and primary human adipocytes. (B) The effect of 6-day GH treatment on lipid accumulation and (C) expression of FAS, ACC1, DGAT mRNA in differentiated 3T3-L1 adipocytes. (D) Association of ZAG gene expression with DGAT mRNA. Effect of 6-day GH treatment on (E) basal and (F) isoproterenol-induced lipolysis in differentiated 3T3-L1 adipocytes. Presented data represent an average value of 3 (A, B) or 4 (C, D, E, F) independent experiments. ACC1, acetyl CoA carboxylase 1; DGAT, acyl CoA:diacylglycerol acyltransferase; FAS, fatty acid synthase; GH, growth hormone; ZAG, zinc-α2-glycoprotein; *P < 0.05; **P < 0.01; ***P < 0.001.
Zinc-α2-glycoprotein as a mediator of GH effects on adipocyte lipid metabolism in vitro
To verify the possible involvement of zinc-α2-glycoprotein in mediating GH-induced changes in adipose tissue lipid metabolism and insulin sensitivity, differentiated human primary adipocytes were transfected with ZAG siRNA. Subsequently, both negative control and ZAG silenced adipocytes were exposed to 0, 50 and 500 ng/ml rhGH. Efficiency of ZAG silencing was clearly demonstrated by near elimination of mRNA (−98%) and substantial reduction in protein (−83%) 6 d post-transfection (). GH induced a 51% increase of ZAG mRNA in differentiated primary human adipocytes (). This was paralleled by reduction in lipid accumulation, which could already be seen after 2 d and fully develops after 6 d of GH treatment (∼38% decrease, ). Importantly, this occurred without any decrease in adipocyte differentiation as evidenced by expression of adiponectin, FABP4 and perilipin 1 (data not shown). The essential finding was that ZAG silencing largely abolished the inhibitory effect of GH on lipid accumulation in adipocytes as it increased triglyceride accumulation by 23% () and reduced glycerol release by 19% () in differentiated human primary adipocytes.
Figure 3. Silencing ZAG eliminates GH effects on lipid metabolism in vitro. The effect of ZAG silencing on (A) ZAG gene and protein expression in differentiated human primary adipocytes. Representative western blot shows ZAG protein in siRNA transfected adipocytes when compared to control - scramble transfected cells. The effect of 6-day growth hormone treatment and transfection of ZAG/scramble siRNA on (B, C) lipid accumulation, (D) basal lipolysis (glycerol release in absence of insulin) and (E) insulin sensitivity (glycerol release in presence of 66 nM insulin) in differentiated human primary adipocytes. The results represent an average of 3 (A, B, C, D) or 4 (E) independent experiments. GH, growth hormone; ZAG, zinc-α2-glycoprotein; TG, triglycerides; picture presented in (C) was taken at magnification of 50x; *P < 0.05; **P < 0.01; ***P < 0.001.
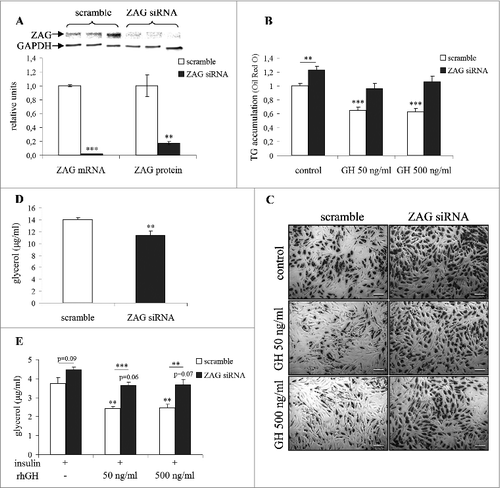
Our next experiment revealed that ZAG silencing significantly decreased insulin-sensitizing effect of GH in adipocytes (). First, we showed that GH treatment enhanced the antilipolytic effect of insulin as exemplified by the suppression of glycerol release in differentiated human primary adipocytes by almost 40% (). Second key finding was that this insulin-sensitizing effect was alleviated by ZAG silencing (). Results of all these in vitro experiments clearly showed that inhibitory action of GH on lipid accumulation in differentiated human primary adipocytes was largely mediated by ZAG.
Combining results of in vitro silencing experiments with outcomes of clinical and ex vivo molecular analyses allowed us to propose, that ZAG might be involved in mediating beneficial effects of GH replacement therapy on adipose tissue metabolic phenotype in patients with severe GH deficiency.
Discussion
In our work, we describe beneficial effects of 5-year GH replacement therapy on adipose tissue insulin sensitivity and the whole-body glucose metabolism. We also propose a novel mechanism by which GH via its action on zinc-α2-glycoprotein regulates adipose tissue lipid metabolism influencing thus the adipose tissue as well as whole-body insulin sensitivity.
Beneficial effects of GH replacement therapy on adipose tissue morphology and metabolic phenotype
In our previous work we have shown that abdominal obesity in severe GH deficiency is associated with extreme adipocyte hypertrophy, paralleled by a specific shift in the spectrum of produced adipokines, possibly contributing to the negative impact of GH deficiency on the whole-body and adipose tissue insulin sensitivity.Citation1 However, very limited information linking extreme adipocyte hypertrophy with molecular changes related to GH deficiency is available.Citation1,29,30 Five years of GH administration to adults with severe GH deficiency significantly reduced the size of subcutaneous adipocytes, without affecting subcutaneous and visceral adiposity, indicating a possibility of positive effect of GH on adipocyte differentiation. This observation is in line with previous findings in children with GH deficiency.Citation31 GH-induced reduction of adipocyte size was in our hands paralleled by a massive improvement of adipose tissue insulin sensitivity and up-regulation of lipid-mobilizing adipokine zinc-α2-glycoprotein in both adipose tissue and circulation.Citation32 It is well known that GH stimulates lipolysis in adipocytes as well as uptake and oxidation of FFA in skeletal muscle, preventing thus the excessive lipid deposition.Citation33–36 However, the mechanism of GH lipolytic action remains elusive. Since adipose tissue ZAG mRNA and protein levels were increased by GH treatment to GHD individuals in vivo Citation32 as well as in vitro and expression of ZAG was associated with adipocyte size, whole-body and adipose tissue insulin sensitivity independently on subcutaneous adiposity, we hypothesized that GH might exert its effects on lipid metabolism and insulin sensitivity in adipose tissue by regulating this lipid-mobilizing adipokine.
Zinc-α2-glycoprotein mediates GH effects on adipose tissue lipid metabolism
Important role of ZAG in regulation of lipid metabolism was first accentuated in studies with ZAG-deficient mice, which are prone to diet-induced obesity and their adipose tissue is formed by huge hypertrophic adipocytes resistant to catecholamine-induced lipolysis.Citation17 In addition, the direct antiobesity and antidiabetic effect of intravenously or orally administered ZAG has recently been demonstrated in ob/ob mice and Wistar rats with diet-induced obesity.Citation19,20,37,38 Our in vitro experiments clearly showed that GH treatment effectively limited excessive lipid accumulation in both differentiated 3T3-L1 and human primary adipocytes and that this effect was abolished by ZAG silencing. In line with these findings, experiments with ZAG transgenic mice demonstrated reduced expression of key lipogenic enzymes (FAS, ACC1 and DGAT) and increased hormone sensitive lipase (HSL) mRNA in epididymal adipose tissue.Citation15 We found that the in vitro inhibitory effect of GH on lipid accumulation is mediated by reduction in lipid synthesis (lower DGAT expression) and stimulation of lipolysis (higher free fatty acid and glycerol release). This GH action seemed to be abolished by silencing ZAG in 3T-L1 adipocytes (preliminary data in Supplementary Fig. 4). Therefore, it is plausible to think that zinc-α2-glycoprotein mediates at least some of the beneficial effects of GH in adipose tissue by stimulating basal lipolysis and inhibiting excessive lipogenesis. Published in vitro studies have indicated that GH exerts dual action on adipogenesis, and its direction depends on the stage of cell differentiation. GH promoted differentiation (lipid accumulation) of preadipocytes when added in the early-induction phase, but had lipolysis and lipid utilization-promoting effects in mature adipocytes.Citation39 Importantly, this GH effect could not be replicated by IGF-1.Citation39,40 It needs to be emphasized that in our hands no changes of adipocyte differentiation markers were found in GH-treated cells. We have also clearly shown that GH treatment intensified the insulin-induced suppression of lipolysis in adipocytes, which was largely mitigated in the absence of ZAG, emphasizing importance of GH - ZAG axis in the adipose tissue insulin sensitivity. Our previous work with patients differing in the degree of obesity, glucose intolerance and insulin resistance revealed a strong positive associations between ZAG and the whole-body as well as adipose tissue-specific insulin sensitivity.Citation41 In vitro experiment showed that lower sensitivity to antilipolytic action of insulin found in ZAG-silenced adipocytes could directly be mirrored by the adipose tissue insulin resistance of GH deficient adults in vivo which was improved with GH substitution therapy and associated with upregulation of zinc-α2-glycoprotein. Moreover, we have previously shown that silencing ZAG in differentiated human primary adipocytes lowered expression levels of IRS1, SLC2A4 (GLUT4) and PPARGC1A (PGC1α).Citation41 All these results support the notion that GH prevents excessive hypertrophy of adipocytes and the lipolytic, antilipogenic and insulin-sensitizing effects of GH seem to be mediated by zinc-α2-glycoprotein, pointing at a novel ZAG-dependent mechanism of GH action.
Conclusions
Our results demonstrate that 5-year GH replacement therapy improved glucose tolerance and adipose tissue insulin sensitivity and reduced adipocyte size. In addition, we identified zinc-α2-glycoprotein as a key factor in GH beneficial action on adipose tissue lipid metabolism and insulin sensitivity. Zinc-α2-glycoprotein was positively associated with the whole-body and adipose tissue insulin sensitivity and negatively with adiposity and adipocyte size. Silencing zinc-α2-glycoprotein in differentiated human primary adipocytes abolished GH effects on lipid metabolism and insulin sensitivity clearly indicating a potential role for GH - ZAG axis in protecting against the development of obesity and GH deficiency-related metabolic disease.
Patients/Methods/Materials
Study cohorts
All studies were approved by the Local Ethics Committee and conform to the ethical guidelines of the 2000 Helsinki declaration. All study participants provided witnessed-written-informed consent prior entering the study.
Adults with untreated GH deficiency (cohort 1)
Seventeen adults with untreated GH deficiency and 17 healthy age-, gender- and BMI-matched controls were included. GH deficiency was diagnosed as a peak of GH response <3 μg/l in an insulin tolerance test. Eleven patients had adult-onset GHD due to ablation of pituitary macroadenoma (n = 8), craniopharyngeoma (n = 2), or as a result of the Sheehan's syndrome (n = 1). Six patients had childhood-onset GHD of congenital/idiopathic origin. Patients with the adult-onset GHD diagnosed on average at the age of 27 ± 3 y were demonstrably GH naive for approximately 5 y. Patients with the childhood-onset GHD were treated with recombinant human GH (rhGH) until the age of 18. Duration of their GHD before entering the baseline study in 2005 lasted for 4.9 ± 1.7 y. Insulin sensitivity index (M-value) was in GHD patients 25% lower than in healthy age-matched controls (GHD 8.00 ± 0.50 vs. controls 6.01 ± 0.44 mg/kg BW/min). Detailed characteristics of the study population and design are summarized in and Supplementary Figure 1.
Adults with GH deficiency after 5 y of GH replacement therapy (cohort 2)
Nine initially examined patients were willing to undergo the follow-up examination. The cohort was supplemented with 8 GH deficient patients who were not fully phenotyped in 2005 ( and Supplementary Fig. 1). All participants with GH deficiency were receiving rhGH (0.3–0.8 mg/day) for 5 y, resulting in normalization of IGF-1 levels (). All patients were suffering from multiple pituitary deficiencies and received adequate hormone replacement therapy which was assessed regularly (last examination <3 months before study) by measuring serum free-thyroid hormones, testosterone/estradiol, and urinary free-cortisol. Patients with malignant or vascular disease, diabetes, hypertension or lipid-lowering treatment were excluded. Two groups of healthy control individuals were age-, gender- and BMI-matched to cohort 1 and 2, respectively. Eight healthy individuals were participating to the study in both time points (Supplementary Fig. 1).
Clinical phenotyping
Body weight, height and waist circumference were measured. Body composition was assessed by bioelectric impedance (BF511, Omron Healthcare LTD, Muko, Japan). In vivo insulin sensitivity was determined by the euglycemic hyperinsulinemic clamp (EHC) as described previously.Citation42
Magnetic resonance imaging
Abdominal fat distribution was measured in all individuals by MRI using GRE sequence, TR: 134ms, TE: 2.38/5.24ms on a 1.5T Magnetom Symphony MRI scanner (Siemens, Munich, Germany), as described previously.Citation42 Area of subcutaneous and visceral abdominal adipose tissue depots was evaluated from 1 slice centered in the umbilical region between the L4 and L5 vertebrae.
Tissue collection and isolation of adipocytes
Subcutaneous adipose tissue samples (∼500 mg) were taken by needle biopsy from abdominal region in the fasted state as described previously.Citation1 Samples were immediately rinsed, blotted and (i) frozen in liquid nitrogen and stored at −80°C or used to isolate adipocytes by collagenase digestion. Adipocyte diameter was determined histomorphometrically. Microscopic images were analyzed with Image Tool software (UTHSCSA, San Antonio, TX, USA). Average diameter of 100 cells from each adipocyte suspension was calculated.
Cell culture experiments
3T3-L1 preadipocytes were maintained in Dulbecco's Modified Eagle Medium (DMEM) containing 10% fetal bovine serum (FBS) and 1% Penicillin/Streptomycin (growth medium; Gibco, Carlsbad, CA, USA) in 5% CO2 and 37°C. Differentiation was induced by isobutyl-methylxanthine (0.5 mM), dexamethasone (1 μM) and insulin (1 μg/ml; all from Sigma, St. Louis, MA, USA). After 48h, cells were maintained in growth medium containing insulin (1 μg/ml) and after 96 h in growth medium.Citation43 To study effects of GH, rhGH (0, 50 and 500 ng/ml; Genotropin, Pfizer, New York, NY, USA) was added to the media from day 5. Adipocytes were kept in culture until day 10 and serum-starved for 14h before harvesting for RNA. To assess sensitivity of 3T3-L1 adipocytes to lipolytic stimuli, cells were exposed for 30 minutes to 10 μM isoproterenol in Krebs-Ringer buffer with 0.5% fatty acid-free bovine serum albumin (BSA; Sigma, USA) and free fatty acids (FFA) were measured (Wako, Dusseldorf, Germany).
Differentiated human primary adipocytes
Human white preadipocytes were obtained from abdominal subcutaneous adipose tissue collected perioperatively from lean (BMI = 23 kg.m-2) 47 y old Caucasian female and cultured as previously described.Citation44 Differentiation of confluent preadipocytes (passage 5) was induced by supplementing the adipocyte medium (DMEM-HamF12, 33 μM biotine, 17 μM D-panthotenate, 66 nM insulin, 1 nM T3, 10 μg/ml transferrine, 50 μg/ml gentamycine and 100 U/ml Penicillin/Streptomycin; all from Sigma, USA) with rosiglitazone (1 μM), dexamethasone (1 μM) and isobutyl-methylxanthine (0.25 mM). After 6 d of differentiation, cells were maintained in adipocyte medium until day 12. Medium was replaced every other day. Effects of GH were studied by addition of rhGH (0, 50 and 500 ng/ml) from day 7. Medium was replaced 14 hours before harvesting (for RNA and media) with fresh adipocyte medium.
Transfection experiments
Differentiated primary human adipocytes (day 7) were transfected with human ZAG siRNA (30 nM) or non-targeting control (On-Target plus siRNA, Thermo Fisher Scientific, Waltham, MA, USA) using Lipofectamine (Invitrogen, Carlsbad, CA, USA). Silencing of ZAG was confirmed at both mRNA and protein levels with aid of real-time PCR and immunoblotting, respectively (). After 24h, transfection mix was replaced by adipocyte medium containing 0, 50 or 500 ng/ml of rhGH and cells were kept in culture until day 12. For lipolytic and lipid accumulation assays, insulin-free medium was utilized. Medium was replaced every other day and eventually collected to measure glycerol.
Lipid accumulation
Adipocytes fixed with 10% formaldehyde were stained with 0.3% Oil Red O (Sigma, USA). After extensive washing, Oil Red O bound to lipid droplets was extracted to isopropanol and quantified spectrophotometrically. An empty well staining provided blank measurement.
Western blot analysis
Cells were homogenized in RIPA containing protease inhibitors Complete (Roche, Basel, Switzerland), aprotinin, pepstatin A, leupeptin and phosphatase inhibitor cocktails (Sigma, USA). Proteins (40 μg) were separated, transferred to Immobilon-FL membrane (Millipore, Billerica, MA, USA) and incubated with anti-ZAG antibody (sc-13585; Santa Cruz Biotechnology, Santa Cruz, CA, USA) diluted 1:500 in Odyssey blocking buffer (Li-Cor Biosciences, Lincoln, NE, USA) overnight at 4°C. Odyssey Infrared Imaging System (Li-Cor Biosciences, USA) was used for signal detection and evaluation. ZAG protein levels were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH, 1:1000; MAB374, Chemicon, Millipore), which was stably expressed.
RNA isolation and real-time PCR
Total RNA was extracted from subcutaneous adipose tissue (∼150 mg) and cultured adipocytes using Trizol (Invitrogen, USA). Traces of genomic DNA were removed by DNase treatment (Qiagen, Valencia, CA, USA). RNA was reverse transcribed (High Capacity RNA to cDNA kit; Applied Biosystems, Carlsbad, CA, USA) and cDNA was used for qRT-PCR on ABI-7900HT (Applied Biosystems, USA) to measure expression of specific genes with either TaqMan® Gene Expression Assays (Applied Biosystems, USA) or using sybr green and specific set of primers designed with Primer Express 3.0 (Supplementary Table). 18S rRNA, GAPDH and RPL13a were stably expressed in subcutaneous adipose tissue and cultured adipocytes and used as internal reference genes (Supplementary Table). All the analyses were performed in duplicates, calibration curve was used and PCR efficiency was optimized for every set of primers.
Biochemical assays
Fasting serum was used for biochemical analyses. ZAG and adiponectin concentrations were both determined with ELISA kits from BioVendor (Modrice, Czech Republic). Glucose was measured using the glucose oxidase method (Beckman Coulter, Brea, CA, USA), IGF-1 and insulin with IRMA (Immunotech, Marseille, France); GH, triglycerides, total & HDL cholesterol with diagnostic assays from Roche (Switzerland), free fatty acids in serum and media with kit by Randox (London, UK) and glycerol in conditioned media with the Free Glycerol Reagent (Sigma, USA).
Statistical analyses
Differences between the 2 groups were analyzed by Student's t-test, more than 2 groups differences were evaluated with ANOVA followed by the Tukey post hoc test. Pearson correlations between ZAG, clinical and molecular parameters were calculated. Every single data set was tested for normality of distribution by the Shapiro-Wilk test and by evaluating the normal probability plot. Logarithmic transformation or non-parametric methods were used where appropriate. Stepwise multiple regression analysis was used to reveal associations independent on BMI (JMP, SAS Institute, USA). The data are reported as mean ± SEM, with P < 0.05 indicating statistical significance.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
973772_Supplementary_Materials.zip
Download Zip (588.1 KB)Acknowledgments
Authors would like to express their gratitude to all those who contributed to this work (in alphabetical order) A. Banarova, V. Belan, A. Dlesk, J. Fedeles, R. Imrich, Z. Kunakova, M. Kuzma, A. Mitkova, A. Penesova, M. Pura, L. Rossmeislova, M. Srbecky and L. Straub as well as to all study volunteers for their cooperative attitude and genuine interest in our work and in their own metabolic health.
Funding
This work was supported by Pfizer Global Investigator Initiated Research Grant, EFSD New Horizons, Slovak Research and Development Agency Grant 0122-06; Scientific Grant Agency of the Slovak Academy of Sciences grants # 2/0198/11 and 2/0174/12 and National Scholarship Program of the Slovak Republic.
References
- Ukropec J, Penesova A, Skopkova M, Pura M, Vlcek M, Radikova Z, Imrich R, Ukropcová B, Tajtáková M, Koska J, et al. Adipokine protein expression pattern in growth hormone deficiency predisposes to the increased fat cell size and the whole body metabolic derangements. J Clin Endocrinol Metab 2008; 93:2255-62; PMID:18334583; http://dx.doi.org/10.1210/jc.2007-2188
- Svensson J, Fowelin J, Landin K, Bengtsson BA, Johansson JO. Effects of seven years of GH-replacement therapy on insulin sensitivity in GH-deficient adults. J Clin Endocrinol Metab 2002; 87:2121-7; PMID:11994351; http://dx.doi.org/10.1210/jcem.87.5.8482
- Moller N, Jorgensen JO. Effects of growth hormone on glucose, lipid, and protein metabolism in human subjects. Endocr Rev 2009; 30:152-77; PMID:19240267; http://dx.doi.org/10.1210/er.2008-0027
- Widdowson WM, Gibney J. The effect of growth hormone (GH) replacement on muscle strength in patients with GH-deficiency: a meta-analysis. Clin Endocrinol (Oxf) 2010; 72:787-92; PMID:19769614; http://dx.doi.org/10.1111/j.1365-2265.2009.03716.x
- Johannsson G, Marin P, Lonn L, Ottosson M, Stenlof K, Bjorntorp P, Sjöström L, Bengtsson BA. Growth hormone treatment of abdominally obese men reduces abdominal fat mass, improves glucose and lipoprotein metabolism, and reduces diastolic blood pressure. J Clin Endocrinol Metab 1997; 82:727-34; PMID:9062473
- Mekala KC, Tritos NA. Effects of recombinant human growth hormone therapy in obesity in adults: a meta analysis. J Clin Endocrinol Metab 2009; 94:130-7; PMID:18940879; http://dx.doi.org/10.1210/jc.2008-1357
- Garten A, Schuster S, Kiess W. The insulin-like growth factors in adipogenesis and obesity. Endocrinol Metab Clin North Am 2012; 41:283-95; v-vi; PMID:22682631; http://dx.doi.org/10.1016/j.ecl.2012.04.011
- Arafat AM, Mohlig M, Weickert MO, Schofl C, Spranger J, Pfeiffer AF. Improved insulin sensitivity, preserved beta cell function and improved whole-body glucose metabolism after low-dose growth hormone replacement therapy in adults with severe growth hormone deficiency: a pilot study. Diabetologia 2010; 53:1304-13; PMID:20372873; http://dx.doi.org/10.1007/s00125-010-1738-4
- Claessen KM, Appelman-Dijkstra NM, Adoptie DM, Roelfsema F, Smit JW, Biermasz NR, Pereira AM. Metabolic profile in growth hormone-deficient (GHD) adults after long-term recombinant human growth hormone (rhGH) therapy. J Clin Endocrinol Metab 2013; 98:352-61; PMID:23162104; http://dx.doi.org/10.1210/jc.2012-2940
- Burger AG, Monson JP, Colao AM, Klibanski A. Cardiovascular risk in patients with growth hormone deficiency: effects of growth hormone substitution. Endocr Pract 2006; 12:682-9; PMID:17229667; http://dx.doi.org/10.4158/EP.12.6.682
- Mohamed-Ali V, Goodrick S, Rawesh A, Katz DR, Miles JM, Yudkin JS, Klein S, Coppack SW. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. J Clin Endocrinol Metab 1997; 82:4196-200; PMID:9398739
- Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 1993; 259:87-91; PMID:7678183; http://dx.doi.org/10.1126/science.7678183
- Ahima RS, Flier JS. Adipose tissue as an endocrine organ. Trends Endocrinol Metab 2000; 11:327-32; PMID:10996528; http://dx.doi.org/10.1016/S1043-2760(00)00301-5
- Rosen ED, Spiegelman BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nature 2006; 444:847-53; PMID:17167472; http://dx.doi.org/10.1038/nature05483
- Gong FY, Zhang SJ, Deng JY, Zhu HJ, Pan H, Li NS, Shi YF. Zinc-alpha2-glycoprotein is involved in regulation of body weight through inhibition of lipogenic enzymes in adipose tissue. Int J Obes (Lond) 2009; 33:1023-30; PMID:19621019; http://dx.doi.org/10.1038/ijo.2009.141
- Islam-Ali B, Khan S, Price SA, Tisdale MJ. Modulation of adipocyte G-protein expression in cancer cachexia by a lipid-mobilizing factor (LMF). Br J Cancer 2001; 85:758-63; PMID:11531264; http://dx.doi.org/10.1054/bjoc.2001.1992
- Rolli V, Radosavljevic M, Astier V, Macquin C, Castan-Laurell I, Visentin V, Guigné C, Carpéné C, Valet P, Gilfillan S, et al. Lipolysis is altered in MHC class I zinc-alpha(2)-glycoprotein deficient mice. FEBS Lett 2007; 581:394-400; PMID:17234189; http://dx.doi.org/10.1016/j.febslet.2006.12.047
- Russell ST, Tisdale MJ. Effect of a tumour-derived lipid-mobilising factor on glucose and lipid metabolism in vivo. Br J Cancer 2002; 87:580-4; PMID:12189560; http://dx.doi.org/10.1038/sj.bjc.6600493
- Russell ST, Tisdale MJ. Studies on the antiobesity effect of zinc-alpha2-glycoprotein in the obob mouse. Int J Obes (Lond) 2011; 35:345-54; PMID:20697416; http://dx.doi.org/10.1038/ijo.2010.150
- Russell ST, Tisdale MJ. Role of beta-adrenergic receptors in the anti-obesity and anti-diabetic effects of zinc-alpha2-glycoprotien (ZAG). Biochim Biophys Acta 2012; 1821:590-9; PMID:22227600; http://dx.doi.org/10.1016/j.bbalip.2011.12.003
- Burgi W, Schmid K. Preparation and properties of Zn-alpha 2-glycoprotein of normal human plasma. J Biol Chem 1961; 236:1066-74; PMID:13689030
- Diez-Itza I, Sanchez LM, Allende MT, Vizoso F, Ruibal A, Lopez-Otin C. Zn-alpha 2-glycoprotein levels in breast cancer cytosols and correlation with clinical, histological and biochemical parameters. Eur J Cancer 1993; 29A:1256-60; PMID:8343263; http://dx.doi.org/10.1016/0959-8049(93)90068-Q
- Tada T, Ohkubo I, Niwa M, Sasaki M, Tateyama H, Eimoto T. Immunohistochemical localization of Zn-alpha 2-glycoprotein in normal human tissues. J Histochem Cytochem 1991; 39:1221-6; PMID:1918940; http://dx.doi.org/10.1177/39.9.1918940
- Todorov PT, McDevitt TM, Meyer DJ, Ueyama H, Ohkubo I, Tisdale MJ. Purification and characterization of a tumor lipid-mobilizing factor. Cancer Res 1998; 58:2353-8; PMID:9622074
- Bao Y, Bing C, Hunter L, Jenkins JR, Wabitsch M, Trayhurn P. Zinc-alpha2-glycoprotein, a lipid mobilizing factor, is expressed and secreted by human (SGBS) adipocytes. FEBS Lett 2005; 579:41-7; PMID:15620688; http://dx.doi.org/10.1016/j.febslet.2004.11.042
- Mracek T, Ding Q, Tzanavari T, Kos K, Pinkney J, Wilding J, Trayhurn P, Bing C. The adipokine zinc-alpha2-glycoprotein (ZAG) is downregulated with fat mass expansion in obesity. Clin Endocrinol (Oxf) 2010; 72:334-41; PMID:19549246; http://dx.doi.org/10.1111/j.1365-2265.2009.03658.x
- Mracek T, Stephens NA, Gao D, Bao Y, Ross JA, Ryden M, Arner P, Trayhurn P, Fearon KC, Bing C. Enhanced ZAG production by subcutaneous adipose tissue is linked to weight loss in gastrointestinal cancer patients. Br J Cancer 2011; 104:441-7; PMID:21245862; http://dx.doi.org/10.1038/sj.bjc.6606083
- Hirai K, Hussey HJ, Barber MD, Price SA, Tisdale MJ. Biological evaluation of a lipid-mobilizing factor isolated from the urine of cancer patients. Cancer Res 1998; 58:2359-65; PMID:9622075
- Agha A, Monson JP. Modulation of glucocorticoid metabolism by the growth hormone - IGF-1 axis. Clin Endocrinol (Oxf) 2007; 66:459-65; PMID:17371460
- Paulsen SK, Pedersen SB, Jorgensen JO, Fisker S, Christiansen JS, Flyvbjerg A, Richelsen B. Growth hormone (GH) substitution in GH-deficient patients inhibits 11beta-hydroxysteroid dehydrogenase type 1 messenger ribonucleic acid expression in adipose tissue. J Clin Endocrinol Metab 2006; 91:1093-8; PMID:16368752; http://dx.doi.org/10.1210/jc.2005-1694
- Bonnet F, Vanderschueren-Lodeweyckx M, Eeckels R, Malvaux P. Subcutaneous adipose tissue and lipids in blood in growth hormone deficiency before and after treatment with human growth hormone. Pediatr Res 1974; 8:800-5; PMID:4370413; http://dx.doi.org/10.1203/00006450-197409000-00005
- Balaz M, Ukropcova B, Kurdiova T, Gajdosechova L, Vlcek M, Janakova Z, Fedeles J, Pura M, Gasperikova D, Smith SR, et al. Growth hormone regulates zinc-alpha2-glycoprotein, adipokine linked to insulin sensitivity. Obesity (Silver Spring) 2014; PMID:25098857; http://dx.doi.org/10.1002/oby.20856
- Moller N, Jorgensen JO, Schmitz O, Moller J, Christiansen J, Alberti KG, Orskov H. Effects of a growth hormone pulse on total and forearm substrate fluxes in humans. Am J Physiol 1990; 258:E86-91; PMID:2405702
- Rabinowitz D, Klassen GA, Zierler KL. Effect of human growth hormone on muscle and adipose tissue metabolism in the forearm of man. J Clin Invest 1965; 44:51-61; PMID:14254256; http://dx.doi.org/10.1172/JCI105126
- Marcus C, Bolme P, Micha-Johansson G, Margery V, Bronnegard M. Growth hormone increases the lipolytic sensitivity for catecholamines in adipocytes from healthy adults. Life Sci 1994; 54:1335-41; PMID:8190005; http://dx.doi.org/10.1016/0024-3205(94)00512-5
- Ottosson M, Lonnroth P, Bjorntorp P, Eden S. Effects of cortisol and growth hormone on lipolysis in human adipose tissue. J Clin Endocrinol Metab 2000; 85:799-803; PMID:10690893
- Russell ST, Tisdale MJ. Studies on the anti-obesity activity of zinc-alpha2-glycoprotein in the rat. Int J Obes (Lond) 2011; 35:658-65; PMID:20856251; http://dx.doi.org/10.1038/ijo.2010.193
- Russell ST, Tisdale MJ. Role of beta-adrenergic receptors in the oral activity of zinc-alpha2-glycoprotein (ZAG). Endocrinology 2012; 153:4696-704; PMID:22903615; http://dx.doi.org/10.1210/en.2012-1260
- Tominaga S, Morikawa M, Osumi T. Growth hormone has dual stage-specific effects on the differentiation of 3T3-L1 preadipocytes. J Biochem 2002; 132:881-9; PMID:12473190; http://dx.doi.org/10.1093/oxfordjournals.jbchem.a003301
- Morikawa M, Green H, Lewis UJ. Activity of human growth hormone and related polypeptides on the adipose conversion of 3T3 cells. Mol Cell Biol 1984; 4:228-31; PMID:6700589
- Balaz M, Vician M, Janakova Z, Kurdiova T, Surova M, Imrich R, Majercikova Z, Penesova A, Vlcek M, Kiss A, et al. Subcutaneous adipose tissue zinc-alpha2-glycoprotein is associated with adipose tissue and whole-body insulin sensitivity. Obesity (Silver Spring) 2014; 22:1821-9; PMID:24753506; http://dx.doi.org/10.1093/10.1002/oby.20764
- Kurdiova T, Balaz M, Vician M, Maderova D, Vlcek M, Valkovic L, Srbecky M, Imrich R, Kyselovicova O, Belan V, et al. Are skeletal muscle & adipose tissue Fndc5 gene expression and irisin release affected by obesity, diabetes and exercise? In vivo & in vitro studies. J Physiol 2013; 592:1091-107; PMID:24297848; http://dx.doi.org/10.1113/jphysiol.2013.264655
- Meissburger B, Stachorski L, Roder E, Rudofsky G, Wolfrum C. Tissue inhibitor of matrix metalloproteinase 1 (TIMP1) controls adipogenesis in obesity in mice and in humans. Diabetologia 2011; 54:1468-79; PMID:21437772; http://dx.doi.org/10.1007/s00125-011-2093-9
- Malisova L, Kovacova Z, Koc M, Kracmerova J, Stich V, Rossmeislova L. Ursodeoxycholic Acid but not tauroursodeoxycholic Acid inhibits proliferation and differentiation of human subcutaneous adipocytes. PLoS One 2013; 8:e82086; PMID:24312631; http://dx.doi.org/10.1371/journal.pone.0082086
