Abstract
The adipose tissue represents a critical and predominant site for the interaction between metabolic and inflammatory responses during health and disease. In the white adipose tissue microenvironment, macrophages/adipocytes cross-talk have been shown to influence the metabolic and inflammatory states of both cell types, and contribute to the development of systemic insulin resistance during obesity. Indeed, the existence of paracrine loops between mature adipocytes and macrophages, especially during obesity-induced stress, involving the release of, and response to, an array of cytokines and regulatory factors, have been extensively studied using several in vitro and in vivo model systems. Published evidence together with recent observations, brought to light the unexpected role of erythropoietin and its receptor in the regulation of white adipose tissue mass, energy homeostasis, and inflammation as demonstrated by erythropoietin effects on adipocyte development and metabolic profile, and macrophage infiltration, cytokine responses, and activation state during diet-induced obesity. In this commentary, we discuss the newly added elements and perspectives to our understanding of the erythropoietin/erythropoietin-receptor axis as a regulator of obesity-induced white adipose tissue inflammation, providing insight into its effects on cytokine responses of macrophages and adipocytes, and possible links to glucose metabolism and insulin resistance.
Obesity-induced insulin resistance is a risk factor for type 2 diabetes. Key to the development of obesity-induced insulin resistance and a hallmark of the metabolic stress are the ongoing chronic inflammation, both systemically and locally in the white adipose tissue.Citation1-4 The latter is largely mediated by macrophages (Mф), whose responses, activation states, and their interactions with adipocytes strongly influence the inflammatory basis of metabolic deterioration. Erythropoietin (EPO), discovered for its indispensable role during erythropoiesis, is a glycoprotein hormone primarily produced in the adult kidneys, and its recombinant form is clinically used for the treatment of anemia in chronic kidney disease including type 2 diabetes patients undergoing renal dialysis.Citation5 EPO expression is induced in response to hypoxia, via the activation of hypoxia inducible factor pathway.Citation6 EPO exerts its function via its receptor EPO-R, a member of the class I cytokine receptor family. In addition to its indispensible role during erythropoiesis, other biological activities for EPO have also been reported.Citation7,8
Beyond erythropoiesis, EPO increases blood flow and oxygen delivery through its effects on the vascular endothelium.Citation9,10 Moreover, EPO has anti-apoptotic and tissue protective effects leading to reduced Mф infiltration and inhibited pro-inflammatory cytokines expression e.g. TNF-α in experimental models of multiple sclerosis and ischemia-induced injury.Citation11,12 In the context of obesity, we and others showed that EPO improved glucose intolerance and decreased body weight and fat mass accumulation.Citation13–16 EPO-R is highly expressed in white adipose tissue by both adipocytes and stromal vascular fraction, where its signaling inhibit adipogenesis, and regulate body weight and fat mass.Citation15,16 EPO-R is also highly expressed in the hypothalamus where its signaling is involved in regulating physical activity and food intake.Citation15,16
Using differentiated 3T3-L1 cell line as an in vitro model system to study adipocytes, it was recently proposed that EPO improves insulin resistance, up-regulates glucose uptake, increases adiponectin, and reduces leptin and TNF-α levels via direct signaling in adipocytes.Citation17 In addition, using mice with adipocyte-specific deletion of EPO receptor, we reported that adipocyte-specific blockade of EPO/EPO-R signaling results in obesity, and decreased glucose tolerance and insulin sensitivity when fed high fat diet.Citation18 Our findings demonstrated that EPO increased oxidative metabolism, fatty acid oxidation, and key metabolic genes in adipocytes, and in white adipose tissue of obese wild-type mice. The increased metabolic activity by EPO was found to be associated with induction of brown fat-like features in white adipocytes, as demonstrated by increases in brown fat gene expression, mitochondrial content, and uncoupled respiration.Citation18 Although this remains controversial, as conflicting evidence exists,Citation19 likely due to differences in the genetic background of the mice used in these studies, our findings strongly suggest that EPO may be a contributing player during adipose energy homeostasis in conjunction with other metabolism co-regulators, providing a potential therapeutic strategy to protect against obesity and metabolic disorders.Citation18 Collectively, these observations summarized above, prompted us to explore additional non-erythroid effects for EPO in the context of adipose tissue microenvironment.
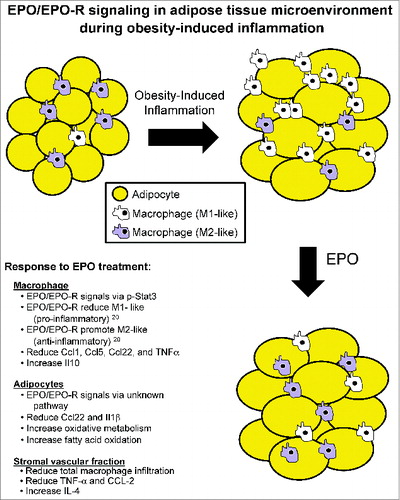
Considering the previously reported links between obesity-induced white adipose tissue inflammation and insulin resistance,Citation1–4 the fact that the anti-inflammatory effects driving EPO-mediated regulation of insulin resistance, and the underlying mechanisms downstream of EPO-R signaling in white adipose tissue Mф remained unknown, we explored EPO/EPO-R contribution to the regulation of obesity-induced adipose tissue inflammation. Using the model of diet-induced obesity in C57BL/6 mice, we found exogenous and endogenous EPO to directly target Mф activity and responses, inhibit white adipose tissue inflammation, and attenuate insulin resistance.Citation20 Our main findings demonstrated that: (i) EPO treatment has an anti-inflammatory effect on white adipose tissue macrophage population during diet-induced obesity in addition to its associated metabolic improvements on glucose tolerance and insulin sensitivity in vivo; (ii) in this model of obesity, EPO treatment reduced pro-inflammatory [M1-like] and increased anti-inflammatory [M2-like] Mф in visceral white adipose tissue depot; in addition (iii) EPO decreased circulating inflammatory monocytes; and lastly (iv) we found the anti-inflammatory effects of EPO to be driven, at least in part, by direct EPO-R response in Mф via Stat3 activation.Citation20 It is noteworthy that EPO effects on M2 but not M1 Mф required interleukin-4 receptor/Stat6 axis, and were not restricted to treatment with exogenous high dose EPO (1000U/kg), but also include endogenous physiological EPO levels as demonstrated by the series of studies conducted using ΔEpoR mouse model where EPO-R expression is restricted to erythroid cells.
These findings have collectively identified EPO/EPO-R signaling as a novel regulator of white adipose tissue inflammation and Mф polarization, and linked such effects to improved glucose metabolism during diet-induced obesity. This is consistent with the critical role of Mф in the development of insulin resistance and glucose intolerance during obesity-induced inflammation.Citation1–4 Previous reports in the literature showed that EPO can (i) inhibit macrophage inflammatory response in the gut and systemically,Citation21 and (ii) promote cholesterol efflux in lipid laden Mф (i.e. foam cells).Citation22 In the white adipose tissue during diet-induced obesity, we show that EPO/EPO-R signaling regulates inflammation and macrophage responses.Citation20 We were surprised to find that EPO promotes the expansion of the white adipose tissue M2-like macrophage population, as a result of increased IL-4 levels that drive their proliferation in situ, where the anti-inflammatory effects of EPO emerged prior to any detectable changes in body weight or composition.Citation20
Obesity-induced inflammation and immune cell infiltration result in elevated levels of cytokines and chemokines such as TNF-α and CCL2, both systemically and locally in WAT, where cells of the stromal vascular fraction including Mф, are known to be the main producing source, particularly in visceral fat depots.Citation2,23–26 Interestingly, Mф infiltration to WAT has been shown to require, and kinetically follow CD8+ T lymphocytes recruitment.Citation27 During inflammation, cytokines such as TNF-α can block insulin receptor signaling via mechanisms that range from reduced activity of Akt, JNK-mediated activation of insulin receptor substrate serine phosphorylation, to the direct degradation of insulin receptor substrate.Citation28,29 Not surprisingly, the inhibition of Mф infiltration and monocytes recruitment to WAT by means of blocking the expression and/or signaling of adhesion molecules such as osteopontin, MGL-1 and β4 integrin or inflammatory chemokines namely CCL2 (MCP-1), have been reported to ameliorate insulin resistance by blunting the inflammatory response.Citation30–33 Whereas, their increased infiltration to WAT, accomplished by targeted tissue specific CCL2 over-expression, resulted in insulin resistance and glucose intolerance.Citation34
As previously indicated, signaling of certain inflammatory mediators such as TNF-α has been adversely implicated in the impairment of glucose metabolism by interference with insulin receptor signaling.Citation2,35 Considering our observations that Mф are EPO responsive, and the key contribution of Mф to the development of obesity-induced insulin resistance and glucose intolerance,Citation1–4 we propose that a direct contribution of their EPO/EPO-R signaling is one of the pathways driving the observed improvement of glucose metabolism, possibly via the inhibition of white adipose tissue inflammation. One key piece of evidence in support of this is the fact that EPO/EPO-R signaling down-regulates TNF-α and CCL-2 protein levels, in addition to down-regulating the gene expression of Ccl1, Ccl2, Ccl3 and Ccl22 in stromal vascular fraction,Citation20 thus linking the positive effects of EPO/EPO-R on systemic insulin sensitivity and glucose tolerance to reduced inflammatory response. These studies were performed using mouse inflammatory cytokines and receptors RT2 profiler PCR array (Qiagen), which profiles the expression of 84 key genes mediating the inflammatory response. The exact molecular networks driving this link, such as the role of JAK/STAT pathway and Akt activation downstream of EPO-R signaling, including the possibility of insulin sensitivity regulation by directly affecting signaling downstream of insulin receptor require further studies.
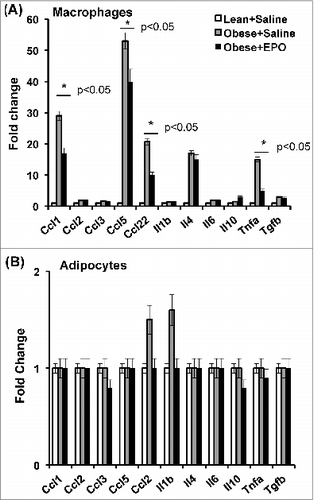
As part of our efforts to delineate the effects of EPO/EPO-R signaling on the ongoing chronic inflammation in the white adipose tissue inflammation microenvironment, we characterized the effect of in vivo EPO treatment on cytokine/chemokine gene expression profiles in Mф versus adipocytes sorted from the visceral fat of obese animals, using mouse inflammatory cytokines and receptors RT2 profiler PCR array, ex vivo after treatment. As expected, EPO treatment of obese animals resulted in reduced gene expression of Ccl1, Ccl5, Ccl22, and Tnfα, while increasing the expression of Il10 in their white adipose tissue Mф when compared to saline treated controls (). In contrast, the effect of EPO treatment on the cytokine/chemokine gene expression of adipocytes was limited, at the tested time points, with marked effects only on Ccl22 and Il1β (). These findings further underscore the inhibitory effects for EPO on obesity-induced inflammation, especially on Mф, in the white adipose tissue microenvironment.
Figure 1. Male C57BL/6 wild type mice with obesity induced by HFD feeding for 12 wks beginning at 6–8 weeks of age, were treated with (black bar) or without (gray bar) EPO (1000U/kg) every 48 hr during the final 2 weeks of the study. Lean mice treated with saline (Lean+Saline; open bar) were used as negative controls. Adipocytes were separated from stromal vascular fraction cells using perigonadal fat, and Mф were purified by FACS from stromal vascular fraction cells using F4/80. Expression of inflammatory cytokine and chemokine genes in adipocytes (A), and Mф (B), were analyzed by qRT-PCR; expression levels are normalized to β-actin, and fold change in expression are relative to negative control Lean+Saline. mRNA levels were quantified. Data represent observations from 3 independent experiments with similar results plotted as mean+SEM for n=4 per group, where statistical significance is *P < 0 .05.
Mechanisms other than anti-inflammatory activity during EPO-mediated regulation of glucose metabolism in diet-induced obesity also contribute to EPO response, particularly in skeletal muscle. It has been suggested that a shift to increased fat oxidation in muscle contributes to the observed normalization of glucose sensitivity with EPO.Citation13 In the white adipose tissue, we found that EPO-mediated improvement of glucose metabolism was associated with increased oxidative metabolism, fatty acid oxidation, and key metabolic genes in adipocytes in vitro and in vivo.Citation18 Lastly, the possibility that EPO can directly influence insulin production by pancreatic β-islet cells, or directly regulate insulin receptor signaling is also of interest, particularly in light of the observed negative correlation between serum insulin levels and EPO/EPO-R signaling.Citation20 However, β-cell function as measured by insulin production is not affected by EPO in experimental models of type 1 and 2 diabetes,Citation36 which argues against the involvement for EPO signaling in regulation of β-cell function.
Summary and Conclusion
Herein we propose a model to summarize the effects of EPO/EPO-R signaling on the adipose tissue microenvironment during obesity-induced inflammation (), providing insight into the role of EPO/EPO-R axis in the regulation of Mф subsets, and cytokine/chemokine responses in Mф vs. adipocytes ( and ). Our findings extend the non-erythroid activity of EPO to encompass effects on macrophage infiltration, subset composition, and cytokine/chemokine responses in white fat, with potential implications in clinical practice. Although, our understanding of the exact molecular mechanisms driving the contribution of adipocytes and/or Mф responses to EPO at the metabolic and inflammatory interface, in particular the connection between inflammation regulation and insulin resistance, remains at its infancy, the new roles for EPO and EPO-R in regulating inflammation and metabolism in the white adipose tissue microenvironment surely redefines the boundaries of EPO biology within the territory of immunometabolism.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (ZIA/DK025102).
References
- Zeyda M, Stulnig T: Obesity, inflammation and insulin resistance-a mini-review. Gerontology 2009; 55: 379-86; PMID:19365105; http://dx.doi.org/10.1159/000212758
- Olefsky JM, Glass CK: Macrophages, inflammation, and insulin resistance. Annu Rev Physiol 2010; 72:219-46; PMID:20148674; http://dx.doi.org/10.1146/annurev-physiol-021909-135846
- Lumeng CN, Saltiel AR: Inflammatory links between obesity and metabolic disease. J Clin Invest 2011; 121(6): 2111-7; PMID:21633179; http://dx.doi.org/10.1172/JCI57132
- Han JM and Levings MK. Immune regulation in obesity-associated adipose inflammation. J Immunol 2013; 191(2):527-32; PMID:23825387; http://dx.doi.org/10.4049/jimmunol.1301035
- Schneider A, Schneider MP, Scharnagl H, Jardine AG, Wanner C, Drechsler C. Predicting erythropoietin resistance in hemodialysis patients with type 2 diabetes. BMC Nephrol 2013; 14: 67; PMID:23521816; http://dx.doi.org/10.1186/1471-2369-14-67
- Semenza GL, Wang GL. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol, 1992; 12: 5447-54
- Noguchi CT, Wang L, Rogers HM, Teng R, Jia Y. Survival and proliferation roles of erythropoietin beyond erythroid lineage. Expert Rev Mol Med 2008; 10:e36; PMID:19040789; http://dx.doi.org/10.1017/S1462399408000860
- Zhang Y, Wang L, Dey S, Alnaeeli M, Suresh S, Rogers H, Teng R, Noguchi CT. Erythropoietin action in stress response, tissue maintenance and metabolism. Int J Mol Sci 2014; 15(6):10296-333; PMID:24918289; http://dx.doi.org/10.3390/ijms150610296
- Kertesz N, Wu J, Chen TH, Sucov HM, Wu H. The role of erythropoietin in regulating angiogenesis. Dev Biol 2004; 276: 101-10; PMID:15531367; http://dx.doi.org/10.1016/j.ydbio.2004.08.025
- Beleslin-Cokic BB, Cokic VP, Yu X, Weksler BB, Schechter AN, Noguchi CT. Erythropoietin and hypoxia stimulate erythropoietin receptor and nitric oxide production by endothelial cells. Blood 2004; 104(7):2073-80; PMID:15205261; http://dx.doi.org/10.1182/blood-2004-02-0744
- Villa P, Bigini P, Mennini T, Agnello D, Laragione T, Cagnotto A, Viviani B, Marinovich M, Cerami A, Coleman TR, et al. Erythropoietin selectively attenuates cytokine production and inflammation in cerebral ischemia by targeting neuronal apoptosis. J Exp Med 2003; 198:971-5; PMID:12975460; http://dx.doi.org/10.1084/jem.20021067
- Yuan R, Maeda Y, Li W, Lu W, Cook S, Dowling P. Erythropoietin: a potent inducer of peripheral immuno/inflammatory modulation in autoimmune EAE. PLoS One 2008; 3:e1924; PMID:18382691; http://dx.doi.org/10.1371/journal.pone.0001924
- Hojman P, Brolin C, Gissel H, Brandt C, Zerahn B, Pedersen BK, Gehl J. Erythropoietin over-expression protects against diet-induced obesity in mice through increased fat oxidation in muscles. PLoS One 2009; 4(6):e5894; PMID:19521513; http://dx.doi.org/10.1371/journal.pone.0005894
- Katz O, Stuible M, Golishevski N, Lifshitz L, Tremblay ML, Gassmann M, Mittelman M, Neumann D. Erythropoietin treatment leads to reduced blood glucose levels and body mass: insights from murine models. J Endocrinol 2010; 205(1):87-95; PMID:20061512; http://dx.doi.org/10.1677/JOE-09-0425
- Foskett A, Alnaeeli M, Wang L, Teng R, Noguchi CT. The effects of erythropoietin dose titration during high-fat diet-induced obesity. J Biomed Biotechnol 2011; 2011:373781; PMID:21541227
- Teng R, Gavrilova O, Suzuki N, Chanturiya T, Schimel D, Hugendubler L, Mammen S, Yver DR, Cushman SW, Mueller E, et al. Disrupted erythropoietin signalling promotes obesity and alters hypothalamus proopiomelanocortin production. Nat Commun 2011; 2:520; PMID:22044999
- Pan Y, Shu JL, Gu HF, Zhou DC, Liu XL, Qiao QY, Fu SK, Gao FH, Jin HM. Erythropoietin improves insulin resistance via the regulation of its receptor-mediated signaling pathways in 3T3L1 adipocytes. Mol Cell Endocrinol 2013; 10; 367(1-2):116-23; PMID:23313788; http://dx.doi.org/10.1016/j.mce.2012.12.027
- Wang L, Teng R, Di L, Rogers H, Wu H, Kopp JB, Noguchi CT. PPARα and Sirt1 mediate erythropoietin action in increasing metabolic activity and browning of white adipocytes to protect against obesity and metabolic disorders. Diabetes 2013; 62(12):4122-31; PMID:23990359; http://dx.doi.org/10.2337/db13-0518
- Luk CT, Shi SY, Choi D, Cai EP, Schroer SA, Woo M. In vivo knockdown of adipocyte erythropoietin receptor does not alter glucose or energy homeostasis. Endocrinology 2013; 154(10):3652-9; PMID:23885016; http://dx.doi.org/10.1210/en.2013-1113
- Alnaeeli M, Raaka BM, Gavrilova O, Teng R, Chanturiya T, Noguchi CT. Erythropoietin signaling: A novel regulator of white adipose tissue inflammation during diet-induced obesity. Diabetes 2014; 63(7):2415-31; PMID:24647735; http://dx.doi.org/10.2337/db13-0883
- Nairz M, Schroll A, Moschen AR, Sonnweber T, Theurl M, Theurl I, Taub N, Jamnig C, Neurauter D, Huber LA, et al. Erythropoietin contrastingly affects bacterial infection and experimental colitis by inhibiting nuclear factor-κB-inducible immune pathways. Immunity 2011; 34(1):61-74; PMID:21256055; http://dx.doi.org/10.1016/j.immuni.2011.01.002
- Lu KY, Ching LC, Su KH, Yu YB, Kou YR, Hsiao SH, Huang YC, Chen CY, Cheng LC, Pan CC, et al. Erythropoietin suppresses the formation of macrophage foam cells: role of liver X receptor alpha. Circulation 2010; 121(16): 1828-37; PMID:20385932; http://dx.doi.org/10.1161/CIRCULATIONAHA.109.876839
- Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Speigelman BM. Increased adipose tissue expression of tumor necrosis factor- in human obesity and insulin resistance. J Clin Invest 1995; 95: 2409-15; PMID:7738205; http://dx.doi.org/10.1172/JCI117936
- Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 2003; 112: 1796-808; PMID:14679176; http://dx.doi.org/10.1172/JCI200319246
- Xu H, Banres GT, Yang Q, Tan G, Yan D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 2003; 112: 1821-1830; PMID:14679177; http://dx.doi.org/10.1172/JCI200319451
- Heilbronn LK, Campbell LV. Adipose tissue macrophages, low grade inflammation and insulin resistance in human obesity. Curr Pharm Des 2008; 14: 1225-30; PMID:18473870; http://dx.doi.org/10.2174/138161208784246153
- Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, Otsu M, Hara K, Ueki K, Sugiura S, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med 2009; 15(8):914-20; PMID:19633658; http://dx.doi.org/10.1038/nm.1964
- Aguirre V, Werner ED, Giraud J, Lee YH, Shoelson SE, White MF. Phosphorylation of Ser307 in insulin receptor substrate-1 blocks interaction with the insulin receptor and inhibits insulin action. J Biol Chem 2002; 277: 1531-7; PMID:11606564; http://dx.doi.org/10.1074/jbc.M101521200
- Shoelson, SE, Lee J, Goldine AB. Inflammation and insulin resistance. J Clin Invest 2006; 116: 1793-108; PMID:16823477; http://dx.doi.org/10.1172/JCI29069
- Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, Kitazawa S, Miyachi H, Maeda S, Egashira K, et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance and hepatic steatosis in obesity. J Clin Invest 2006; 116(6):1494-505; PMID:16691291; http://dx.doi.org/10.1172/JCI26498
- Feral CC, Neels JG, Kummer C, Slepak M, Olefsky JM, Ginsberg MH. Blockade of 4 integrin signaling ameliorates the metabolic consequences of high-fat diet-induced obesity. Diabetes 2008; 57: 1842-51; PMID:18426864; http://dx.doi.org/10.2337/db07-1751
- Westcott DJ, Delproposto JB, Geletka LM, Wang T, Singer K, Saltiel AR, Lumeng CN. MGL1 promotes adipose tissue inflammation and insulin resistance by regulating 7/4hi monocytes in obesity. J Exp Med 2009; 206(13):3143-56; PMID:19995956; http://dx.doi.org/10.1084/jem.20091333
- Kiefer FW, Zeyda M, Gollinger K, Pfau B, Neuhofer A, Weichhart T, Säemann MD, Geyeregger R, Schlederer M, Kenner L, et al. Neutralization of osteopontin inhibits obesity-induced inflammation and insulin resistance. Diabetes 2010; 59(4):935-46; PMID:20107108; http://dx.doi.org/10.2337/db09-0404
- Kamei N, Tobe K, Suzuki R, Ohsugi M, Watanabe T, Kubota N, Ohtsuka-Kowatari N, Kumagai, K, Sakamoto K, Kobayashi M. et al. Overexpression of monocyte chemoattractant protein-1 in adipose tissues causes macrophage recruitment and insulin resistance. J Biol Chem 2006; 281(36):26602-14; PMID:16809344; http://dx.doi.org/10.1074/jbc.M601284200
- Lumeng CN, Deyoung SM, Bodzin JL, Saltiel AR. Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes 2007; 56(1):16-23; PMID:17192460; http://dx.doi.org/10.2337/db06-1076
- Choi D, Schroer SA, Lu SY, Wang L, Wu X, Liu Y, Zhang Y, Gaisano HY, Wagner KU, Wu H, et al. Erythropoietin protects against diabetes through direct effects on pancreatic beta cells. J Exp Med 2010; 207:2831-42; PMID:21149549; http://dx.doi.org/10.1084/jem.20100665