Abstract
Adipose tissue remodeling occurs in obesity, characterized by adipocyte hypertrophy and increased infiltration of macrophages which also shift to a proinflammatory phenotype. Factors derived from these macrophages significantly alter adipocyte function, such as repressing adipogenesis, inducing inflammatory response and desensitizing insulin action. As macrophages produce a cocktail of inflammatory signals, identifying the key factors that mediate the detrimental effects may offer effective therapeutic targets. IL-1β, a major cytokine produced largely by macrophages, is implicated in the development of obesity-associated insulin resistance. In this article, we discuss recent advances in our understanding of the role of IL-1β in macrophage-adipocyte crosstalk in obesity. IL-1β impairs insulin sensitivity in adipose tissue by inhibition of insulin signal transduction. Blocking the activity of IL-1β, its receptor binding or production improves insulin signaling and action in human adipocytes. This is in parallel with a reduction in macrophage-stimulated proinflammatory profile and lipolysis. Targeting IL-1β may be beneficial for protecting against obesity-related insulin resistance at the tissue and systemic levels.
Abbreviations:
- Akt, protein kinase B
- CCL5, chemokine (C-C motif) ligand-5
- GLUT4, glucose transporter 4
- IL-1β, interleukin-1β
- IL-1Ra, interleukin-1 receptor antagonist
- IL-6, interleukin-6
- IL-8, interleukin-8
- IRS1, insulin receptor substrate 1
- MC, macrophage-conditioned
- MCP-1, monocyte chemotactic protein-1
- NFκB, nuclear factor of κ light polypeptide gene enhancer in B-cells
- NLRP3, nucleotide-binding oligomerization domain
- leucine-rich repeat and pyrin
- domain-containing protein 3
- PI3K, phosphoinositide-3-kinase
- SVF, stromal vascular fraction
- TNFα, tumour necrosis factor-alpha
Adipose Tissue Remodeling in Obesity
Central obesity has a close link to the development of ‘metabolic syndrome’ manifested by insulin resistance, glucose intolerance, dyslipidemia and hypertension.Citation1 The metabolic derangements predispose to the development of type 2 diabetes and cardiovascular diseases.Citation1 Although the pathophysiology of obesity-associated metabolic disorders is complex, evidence suggests that chronic low-grade inflammation in white adipose tissue plays a role.Citation2-4 Adipose tissue is composed of mature adipocytes (∼50%) and other cells (∼50%) from the stromal vascular fraction (SVF) which contains preadipocytes, fibroblasts, endothelial cells and immune cells (i.e. macrophages, lymphocytes, mast cells, dendritic cells, eosinophils).Citation5,6 The plasticity of adipose tissue provides a mechanistic base for its pleiotropic functions that influence lipid, endocrine, and immune homeostasis. However, over-nutrition in obesity triggers adipose tissue remodeling that the tissue becomes a site of inflammation. In addition to adipocyte hypertrophy, there is a marked accumulation of macrophages together with other immune cells in adipose tissue.Citation2,Citation7,Citation8 The proportion of macrophages in the SVF is estimated to be increased from ∼10% in lean to ∼40-50% in obese adipose tissue.Citation9 Furthermore, obesity induces a phenotypic switch from the M2 macrophages (producing anti-inflammatory cytokines) to the M1 macrophages (producing pro-inflammatory cytokines), associated with insulin resistance both in mice and humans.Citation10-12 Factors derived from macrophages also influence adipose tissue biology, such as inhibiting adipogenesis, modulating adipokine production, and mounting inflammatory responses.Citation13-18
Macrophage-Adipocyte crosstalk on insulin signaling in adipose tissue
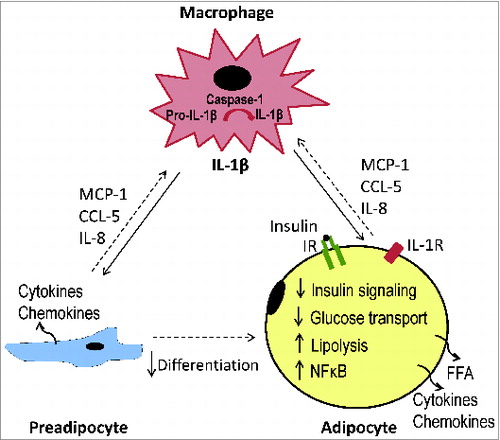
In addition to skeletal muscle and liver, adipose tissue is a key organ that responds to insulin action.Citation19 In obesity the lowered insulin sensitivity in adipocytes could stimulate lipolysis and fatty acid release into the circulation, leading to ectopic fat deposition and ultimately systemic insulin resistance.Citation20 Insulin action requires the activation of insulin signaling pathway; IRS1, one of the major substrates of the insulin receptor, is essential to activate PI3K in response to insulin, which leads to the phosphorylation of protein kinase B (also known as Akt) resulting in an increase in GLUT4 transporters in the plasma membrane.Citation21 The enhanced macrophage-adipocyte crosstalk in obesity, including macrophage-derived factors, has been shown to disrupt insulin action in murine 3T3-L1 adipocytes.Citation22 However, whether it occurs in human adipose tissue together with the potential mediators and mechanisms are largely unknown. Our recent study examined the impact of signals derived from human macrophages on insulin signaling in human adipocytes.Citation23 We observed that macrophage-derived factors impair insulin signaling in human adipocytes as macrophage-conditioned (MC) medium inhibited gene and protein expression of insulin signaling molecules, including IRS1, PI3K p85α, GLUT4 and the phosphorylation of Akt. In contrast, the expression and release of the proinflammatory markers (i.e. IL-6, IL-8, MCP-1, CCL-5) by adipocytes were markedly increased.
IL-1β and insulin resistance
Identification of the major factors that mediate the detrimental effect of macrophages on adipocytes is challenging but it may offer potential therapeutic targets. Several macrophage-secreted factors are implicated in metainflammation, including TNFα and IL-6. TNFα has been shown to induce insulin resistance in rodents and block insulin actions in 3T3-L1 adipocytes.Citation22, Citation24-26 Using human preadipocytes, neutralization of TNFα attenuated macrophage-stimulated increase in IL-6 mRNA while had a modest effect on adipogenic marker aP2.Citation16 In our recent study, simultaneously blocking TNFα and IL-1β had no additive effect on the expression of insulin signaling proteins.Citation23 IL-6 was found to be overexpressed in fat cells from insulin-resistant subjects and induce insulin resistance in 3T3-L1 adipocytes.Citation27 It remains to be investigated whether IL-6 acts as a key mediator in macrophage-adipocyte crosstalk. Macrophage-derived IL-1F6 and IL-1F8, members of the IL-1 family, was reported to stimulate gene expression of IL-6 and IL-8 in mature human adipocytes albeit both are less potent than IL-1β.Citation28 The involvement of IL-1F6 and IL-1F8 in adipose tissue inflammation and insulin sensitivity in obesity is not yet known.
IL-1β, a major proinflammatory cytokine, is produced mostly by macrophages and biologically active IL-1β is formed through cleavage of pro-IL-1β by caspase-1 activated via the NLRP3 inflammasome.Citation29 IL-1β production by adipose tissue macrophages is caspase-1-dependent in humans.Citation30 Growing evidence suggests that IL-1β is critically involved in the translation of obesity-associated inflammation into insulin resistance in rodent models.Citation31,32 IL-1β is also released by human adipose tissue but primarily from the nonfat cells, and the release is enhanced in obesity.Citation33,34 High dose of IL-1β (20 ng/ml) has been shown to decrease protein expression of IRS-1 and GLUT4 mRNA in murine 3T3-L1 adipocytes.Citation35, 36 Our recent work demonstrated that in human adipocytes IL-1β at a lower dose (2 ng/ml) repressed insulin signal transduction by reducing the expression of signaling (i.e. IRS1, PI3K p85α, pAkt) and glucose transporter (GLUT4) proteins.Citation23 We also found in a previous study that macrophage-induced production of matrix metalloproteinase 1 and 3 by preadipocytes is mediated by IL-1β.Citation37 These findings suggest that IL-1β could have a central role in macrophage-adipocyte crosstalk which blocks insulin action in human adipose tissue.
IL-1β as a mediator of macrophage-adipocyte crosstalk
In our recent work, we showed that IL-1β is required for the inhibitory effect of macrophages on insulin signaling in human adipocytes.Citation23 Blocking IL-1β activity, with a neutralizing antibody, substantially reduced effects of MC medium on expression profile of genes involved in insulin signaling, insulin sensitivity and glucose metabolism in adipocytes. Furthermore, IL-1β depletion abolished macrophage-induced inhibition on expression of insulin signaling proteins (IRS1, PI3K p85α and GLUT4 and Akt phosphorylation) and glucose consumption, suggesting that IL-1β blockade can restore insulin signal transduction in human adipocytes. This was supported by the data that inhibition of IL-1β receptor binding in adipocytes with an IL-1 receptor antagonist (IL-1Ra) restored expression of signaling and glucose transport proteins suppressed by MC medium. To substantiate the importance of IL-1β in MC medium, we used a caspase-1 inhibitor to block IL-1β production by macrophages. The protein expression of insulin signaling molecules was partially (for IRS1, PI3K p85α) or totally (for GLUT4) reversed in adipocytes exposed to the MC medium. This is in parallel with the attenuation of macrophage-stimulated proinflammatory profile and lipolysis.
Although the mechanisms by which IL-1β mediates macrophage-adipocyte crosstalk desensitizing insulin action remain to be clarified, local inflammation in adipose tissue could be vital.Citation4 It is evident that macrophage-derived factors potently stimulate the production of proinflammatory cytokines/chemokines (i.e. IL-6, MCP-1, CCL5, IL-8) by adipocytes as well as preadipocytes.Citation16,Citation23,Citation38 The overproduction of chemoattractants may promote monocyte migration and M1 macrophages polarization.Citation39-42 Cytokine IL-6 and TNFα are known to reduce insulin signaling and insulin-stimulated glucose uptake in 3T3-L1 adipocytes.Citation24-27 Interestingly, we observed that overproduction of proinflammatory markers can be largely reversed by antagonizing IL-1β activity, IL-1 receptors or blocking IL-1β production.Citation23 Thus, the detrimental effects of macrophage-derived IL-1β on insulin signaling in adipocytes could be mediated via upregulated inflammatory responses, especially over-production of proinflammatory cytokines/chemokines ().
Targeting IL-1β in obesity
In rodent models of obesity and diabetes, blocking IL-1β reduces hyperglycemia and tissue inflammation.Citation43-46 In patients with type 2 diabetes, IL-1β antagonism or IL-1 receptor antagonist (anakinra) also show beneficial effects on glucose control and β-cell function, with a reduction in circulating proinflammatory markers.Citation47-49 Since adipose tissue inflammation links obesity to insulin resistance, studies on the tissue specific effects of IL-1β inhibition in humans are required. Targeting IL-1β might put a brake on the vicious cycle of macrophage infiltration and escalated inflammatory response in adipose tissue, thereby improving insulin sensitivity at tissue and also systemic levels.
Disclosure of Potential Conflicts of Interest
The author has no conflicts of interest to declare.
Acknowledgment
The author thanks colleagues at the Obesity Biology Research Group, University of Liverpool, for their contribution to the research program.
Funding
This work was supported in part by the Medical Research Council (G0801226).
References
- Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009; 120:1640-5.
- Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 2003; 112:1821-30.
- Odegaard JI, Chawla A. Pleiotropic actions of insulin resistance and inflammation in metabolic homeostasis. Science 2013; 339:172-7.
- Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest 2011; 121:2111-7.
- Gesta S, Tseng YH, Kahn CR. Developmental origin of fat: tracking obesity to its source. Cell 2007; 131:242-56.
- Bertola A, Ciucci T, Rousseau D, Bourlier V, Duffaut C, Bonnafous S, et al. Identification of adipose tissue dendritic cells correlated with obesity-associated insulin-resistance and inducing Th17 responses in mice and patients. Diabetes 2012; 61:2238-47.
- Chawla A, Nguyen KD, Goh YP. Macrophage-mediated inflammation in metabolic disease. Nat Rev Immunol 2011; 11:738-49.
- Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW, Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 2003; 112:1796-808.
- McNelis JC, Olefsky JM. Macrophages, immunity, and metabolic disease. Immunity 2014; 41:36-48.
- Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest 2007; 117:175-84.
- Fujisaka S, Usui I, Bukhari A, Ikutani M, Oya T, Kanatani Y, et al. Regulatory mechanisms for adipose tissue M1 and M2 macrophages in diet-induced obese mice. Diabetes 2009; 58:2574-82.
- Wentworth JM, Naselli G, Brown WA, Doyle L, Phipson B, Smyth GK, et al. Pro-inflammatory CD11c+CD206+ adipose tissue macrophages are associated with insulin resistance in human obesity. Diabetes 2010; 59:1648-56.
- Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol 2011; 11:98-107.
- Constant VA, Gagnon A, Landry A, Sorisky A. Macrophage-conditioned medium inhibits the differentiation of 3T3-L1 and human abdominal preadipocytes. Diabetologia 2006; 49:1402-11.
- Gao D, Trayhurn P, Bing C. Macrophage-secreted factors inhibit ZAG expression and secretion by human adipocytes. Mol Cell Endocrinol 2010; 325:135-42.
- Lacasa D, Taleb S, Keophiphath M, Miranville A, Clement K. Macrophage-secreted factors impair human adipogenesis: involvement of proinflammatory state in preadipocytes. Endocrinology 2007; 148:868-77.
- Gagnon A, Foster C, Landry A, Sorisky A. The role of interleukin 1beta in the anti-adipogenic action of macrophages on human preadipocytes. J Endocrinol 2013; 217:197-206.
- Kotnik P, Keuper M, Wabitsch M, Fischer-Posovszky P. Interleukin-1beta downregulates RBP4 secretion in human adipocytes. PLoS ONE 2013; 8:e57796.
- Hotamisligil GS. Molecular mechanisms of insulin resistance and the role of the adipocyte. Int J Obes Relat Metab Disord 2000; 24 Suppl 4:S23-7.
- Smith U. Impaired (‘diabetic’) insulin signaling and action occur in fat cells long before glucose intolerance-is insulin resistance initiated in the adipose tissue? Int J Obes Relat Metab Disord 2002; 26:897-904.
- Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol 2006; 7:85-96.
- Lumeng CN, Deyoung SM, Saltiel AR. Macrophages block insulin action in adipocytes by altering expression of signaling and glucose transport proteins. Am J Physiol Endocrinol Metab 2007; 292:E166-74.
- Gao D, Madi M, Ding C, Fok M, Steele T, Ford C, et al. Interleukin-1beta mediates macrophage-induced impairment of insulin signaling in human primary adipocytes. Am J Physiol Endocrinol Metab 2014; 307:E289-304.
- Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 1993; 259:87-91.
- Stephens JM, Lee J, Pilch PF. Tumor necrosis factor-alpha-induced insulin resistance in 3T3-L1 adipocytes is accompanied by a loss of insulin receptor substrate-1 and GLUT4 expression without a loss of insulin receptor-mediated signal transduction. J Biol Chem 1997; 272:971-6.
- Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature 1997; 389:610-4.
- Rotter V, Nagaev I, Smith U. Interleukin-6 (IL-6) induces insulin resistance in 3T3-L1 adipocytes and is, like IL-8 and tumor necrosis factor-alpha, overexpressed in human fat cells from insulin-resistant subjects. J Biol Chem 2003; 278:45777-84.
- van Asseldonk EJ, Stienstra R, Koenen TB, van Tits LJ, Joosten LA, Tack CJ, et al. The effect of the interleukin-1 cytokine family members IL-1F6 and IL-1F8 on adipocyte differentiation. Obesity (Silver Spring) 2010; 18:2234-6.
- Sims JE, Smith DE. The IL-1 family: regulators of immunity. Nat Rev Immunol 2010; 10:89-102.
- Stienstra R, Joosten LA, Koenen T, van Tits B, van Diepen JA, van den Berg SA, et al. The inflammasome-mediated caspase-1 activation controls adipocyte differentiation and insulin sensitivity. Cell Metab 2010; 12:593-605.
- Tack CJ, Stienstra R, Joosten LA, Netea MG. Inflammation links excess fat to insulin resistance: the role of the interleukin-1 family. Immunological reviews 2012; 249:239-52.
- Wen H, Gris D, Lei Y, Jha S, Zhang L, Huang MT, et al. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nature immunology 2011; 12:408-15.
- Fain JN. Release of interleukins and other inflammatory cytokines by human adipose tissue is enhanced in obesity and primarily due to the nonfat cells. Vitam Horm 2006; 74:443-77.
- Koenen TB, Stienstra R, van Tits LJ, Joosten LA, van Velzen JF, Hijmans A, et al. The inflammasome and caspase-1 activation: a new mechanism underlying increased inflammatory activity in human visceral adipose tissue. Endocrinology 2011; 152:3769-78.
- Lagathu C, Yvan-Charvet L, Bastard JP, Maachi M, Quignard-Boulange A, Capeau J, et al. Long-term treatment with interleukin-1beta induces insulin resistance in murine and human adipocytes. Diabetologia 2006; 49:2162-73.
- Jager J, Gremeaux T, Cormont M, Le Marchand-Brustel Y, Tanti JF. Interleukin-1beta-induced insulin resistance in adipocytes through down-regulation of insulin receptor substrate-1 expression. Endocrinology 2007; 148:241-51.
- Gao D, Bing C. Macrophage-induced expression and release of matrix metalloproteinase 1 and 3 by human preadipocytes is mediated by IL-1beta via activation of MAPK signaling. J Cell Physiol 2011; 226:2869-80.
- Gao D, Trayhurn P, Bing C. 1,25-Dihydroxyvitamin D3 inhibits the cytokine-induced secretion of MCP-1 and reduces monocyte recruitment by human preadipocytes. Int J Obes (Lond) 2013; 37:357-65.
- Keophiphath M, Rouault C, Divoux A, Clement K, Lacasa D. CCL5 promotes macrophage recruitment and survival in human adipose tissue. Arterioscler Thromb Vasc Biol 2010; 30:39-45.
- Kitade H, Sawamoto K, Nagashimada M, Inoue H, Yamamoto Y, Sai Y, et al. CCR5 plays a critical role in obesity-induced adipose tissue inflammation and insulin resistance by regulating both macrophage recruitment and M1/M2 status. Diabetes 2012; 61:1680-90.
- Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest 2006; 116:1494-505.
- Tsou CL, Peters W, Si Y, Slaymaker S, Aslanian AM, Weisberg SP, et al. Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J Clin Invest 2007; 117:902-9.
- Owyang AM, Maedler K, Gross L, Yin J, Esposito L, Shu L, et al. XOMA 052, an anti-IL-1{beta} monoclonal antibody, improves glucose control and {beta}-cell function in the diet-induced obesity mouse model. Endocrinology 2010; 151:2515-27.
- McGillicuddy FC, Harford KA, Reynolds CM, Oliver E, Claessens M, Mills KH, et al. Lack of interleukin-1 receptor I (IL-1RI) protects mice from high-fat diet-induced adipose tissue inflammation coincident with improved glucose homeostasis. Diabetes 2011; 60:1688-98.
- Ehses JA, Lacraz G, Giroix MH, Schmidlin F, Coulaud J, Kassis N, et al. IL-1 antagonism reduces hyperglycemia and tissue inflammation in the type 2 diabetic GK rat. Proc Natl Acad Sci U S A 2009; 106:13998-4003.
- Sauter NS, Schulthess FT, Galasso R, Castellani LW, Maedler K. The antiinflammatory cytokine interleukin-1 receptor antagonist protects from high-fat diet-induced hyperglycemia. Endocrinology 2008; 149:2208-18.
- Larsen CM, Faulenbach M, Vaag A, Volund A, Ehses JA, Seifert B, et al. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med 2007; 356:1517-26.
- Cavelti-Weder C, Babians-Brunner A, Keller C, Stahel MA, Kurz-Levin M, Zayed H, et al. Effects of gevokizumab on glycemia and inflammatory markers in type 2 diabetes. Diabetes Care 2012; 35:1654-62.
- Sloan-Lancaster J, Abu-Raddad E, Polzer J, Miller JW, Scherer JC, De Gaetano A, et al. Double-blind, randomized study evaluating the glycemic and anti-inflammatory effects of subcutaneous LY2189102, a neutralizing IL-1beta antibody, in patients with type 2 diabetes. Diabetes Care 2013; 36:2239-46.