Abstract
With adipose-derived stem cells being in the focus of research in regenerative medicine, the need arises for fast reliable cultivation protocols. We have tested the cultivation of human adipose-derived stem cells in endothelial cell growth medium prior to induction and differentiation, against the long-established use of DMEM/F12 medium-based cultivation protocols. We found that cultivation in endothelial cell growth medium not only accelerates growth before induction and differentiation, but also allows shorter induction and differentiation times than those following precultivation with DMEM/F12 medium with regard to the formation of mature adipocytes and to the viability undifferentiated cells. These results were first observed morphologically but could be confirmed by performing adiponectin ELISA and cell proliferation assays.
Introduction
The clinical applications for tissue-engineered fat promise to be particularly wide-ranging with respect to the correction and reconstruction of soft tissue defects such as the deficit in volume and irregularity of contours. Indications include congenital defects, such as Romberg's disease and Poland syndrome, defects after trauma or tumor resection, particularly in the head and neck region, and the field of gynecology.Citation1,2
In the literature, the tissue engineering of fat has been described with adipocyte derived stem cells (ADSCs),Citation3 which are the adipose-tissue-residing progenitor cells of adipocytes.Citation4-7 A cornerstone for the successful implantation of cells in vivo for preclinical investigations will certainly be the development of consistent strategies of cell cultivation and seeding on scaffolds, which might serve as a template for the desired 3-dimensional shape of the tissue to be engineered. Various preadipocyte and stem cell culture conditions and expansion methods have been developed, all attempting to improve the quality of tissue-engineered adipose tissue.Citation8-10 The multipotency of preadipocytes,Citation5,6,11 which are fibroblast-like precursors of adipocytes, has previously been shown.Citation12 Differentiation takes place in the presence of fetal calf serum after inducing agents such as dexamethasone, isobutylmethylxantine, and insulin are added to the medium. The differentiation process is characterized by the formation of lipid droplets, which are a key characteristic of mature adipocytes.Citation13 However, no standardized and easily reproducible protocol for the isolation and cultivation of preadipocytes from fat tissue specimens is available to date. Besides the lack of standardization between research groups in defining the term ADSC, there is no consensus on the way of isolating those cells. This may cause significant limitations in the interpretation of clinical results. Therefore there is a strong need to optimize ADSC isolation for future clinical use.Citation14 Several culture conditions and diverse media for adipocyte culture have been studied. The most commonly used medium is based on a mixture containing Ham's F12 and Dulbecco´s MEM (DMEM)Citation8,9,11 supplemented with a series of growth factors and vitamins.
In our study, we tested the above mentioned medium in comparison with “endothelial cell growth medium” (ECGM) provided by Promocell, because of the ability of ECGM to serve as a basal medium prior to the initiation of the differentiation of preadipocytes. The differentiation of preadipocytes was assessed both by morphological criteria and by the determination of adiponectin secretion after stimulation under standardized adipogenic conditions.
We found that ECGM is a highly effective medium for the basic cultivation of preadipocytes. The cells grow faster and develop more rapidly, and the resulting adipocytes are of high quality, which is reflected by the quantity of adiponectin produced by mature adipocytes and the formation of fat cell clusters containing large lipid droplets that can be visualized by oil-red staining.
Results
Sample selection
Fat tissue was obtained from 4 female patients undergoing mamma reconstruction or abdominoplasty at the Clinic for Plastic Surgery at the Klinikum Rechts der Isar der TU München, Munich. In our experience, both mamma reduction and abdominoplastics achieved a comparable yield of preadipocytes.
Quantitative differences in the development of adipogenic cells cultured initially in 2 different basal media
Two differently formulated media were used to start adipocyte culture after the isolation of preadipocytes from surgically removed fat tissue biopsies. Our aim was to compare the competence of the 2 media as a basic medium for adipocyte derived stem cells prior to further induction and differentiation.
The isolated preadipocytes were cultured both in BM 1 as described above, and in a specialized endothelial cell growth medium (ECGM), BM2, which was supplied by Promocell and whose exact content will remain undisclosed because of the patent rights of the manufacturer.
In both basic media monolayer cultures, the freshly isolated cells readily adhered to the tissue culture plates and developed a spindle-shaped fibroblast-like morphology on day 2 of cultivation. However, preadipocytes cultured in BM 1 displayed a lower growth rate compared with cells cultured in BM 2.
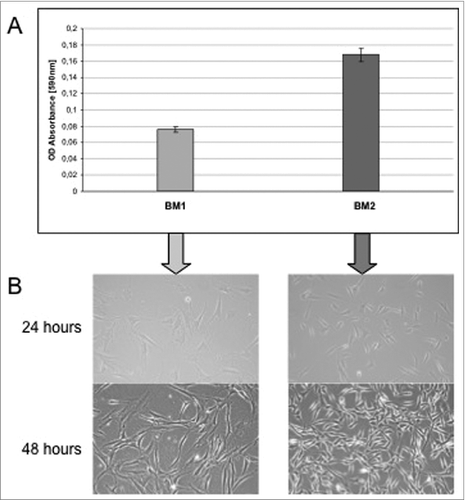
To monitor and verify the population doubling objectively, cells that were seeded in equal numbers on 10-cm tissue culture dishes and plates underwent daily monitoring of confluence. shows the sequential setup for the cultivation of isolated preadipocytes culture. In the cells cultured in BM 2 (right) show a higher proliferation rate during a 24-h cultivation period than cells cultivated in BM 1 (left). Each value represents the mean ± SD of triplicate values from 4 different ADSC-Preparations. Absorbance values obtained with medium alone (<0.02) were taken as the control and subsequently subtracted from all measurements.
Figure 2. (A) Cells cultured in BM 2 (right) show a higher proliferation rate during a 24-h cultivation period than cells cultivated in BM 1 (left). Each value represents the mean ± SD of triplicate values from 4 different ADSC-Preparations. Absorbance values obtained with medium alone (<0.02) were taken as the control and subsequently subtracted from all measurements. (B) Microscopic examination of ADSC cultured in 2 different culture media. 4 representative phase-contrast images are shown. left: ADSC were seeded at a density of 10.000 in DMEM/F12 medium (BM1) in 10 cm2 round tissue culture dishes and photographed 24 and 48 hours after seeding. Right: ADSC were seeded at a density of 10.000 in ECGM medium (BM2) in 10 cm2 round tissue culture dishes and photographed 24 and 48 hours after seeding.
Measurement of cell growth and viability
To further support these morphological findings, we tested the cells for their proliferative capacity. Growth of the differently cultured cells in BM1 and BM2 was determined by use of the colorimetric MTT cell viability/proliferation assay as described in the materials and methods section. As shown in , the proliferation rate of cells grown in BM 2 was significantly higher than that of cells grown in BM 1.
Induction of differentiation
During the process of differentiation, cells undergo a phase of growth arrest, which is invoked by components of the induction medium. During the growth phase, preadipocytes are morphologically similar to fibroblasts. After the induction of differentiation, the preadipocytes take on a spheric shape, accumulate lipid droplets, and progressively acquire the morphological and biochemical characteristics of mature white adipocytes.
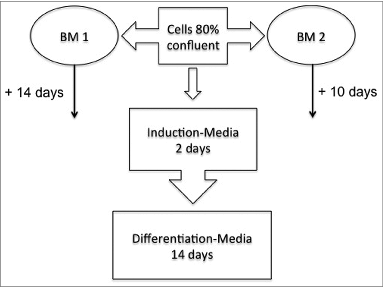
This change in cellular morphology and triglyceride storage was promoted by submitting both cultures to a standardized induction and differentiation scheme. As depicted in , after 10 to 14 days in culture and at a confluence of about 80%, the basic medium was removed, and an induction medium, containing insulin, hydrocortisone, and the phosphodiesterase inhibitor methyisobutylxanthine (IBMX), was added for 48 h. The induction medium was subsequently replaced by a differentiation medium based on standard α-MEM (a-MEM), supplemented with 5% FBS and insulin.
Subsequently, the cells were monitored for up to 14 days after administration of the differentiation medium. During the course of the histological examination, the mature adipocytes were stained with oil-red after 14 days of culture.
Using BM 2 as an initial medium to promote the growth phase of the preadipocytes, we could demonstrate that, within our experimental setup, cells differentiated into clusters of cells having the classical characteristics of mature fat cells.
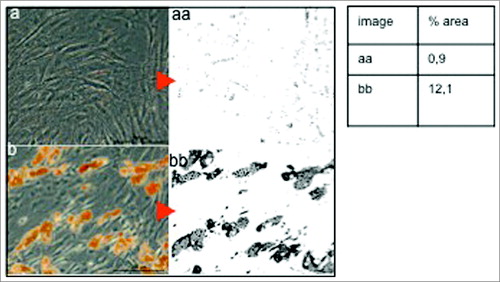
As shown in , cells cultured initially with BM 1 (left) and further subjected to the same differentiation regime as cells grown in BM 2 (right) failed to differentiate into fat cell clusters, with only insignificant droplets being identified after the 10-day differentiation period. The area covered by droplets in Figure 3aa is 0.9% in comparison to 12.1% in Figure 3bb with BM 2.
Figure 3. Quantification of Oil-red O positive surface areas representing the amount of mature Adipocytes. One representative example of ADCS submitted to either BM1 (A) or BM2 medium (B) simultaneously and subsequently differentiated using induction and differentiation regimen is shown. BM1 shows a coverage of 0.9 % in comparison to 12.1% with BM 2.
The effect of cultivation strategies on the secretory function of adipocyte culture
Adiponectin ELISA
Whereas white adipose tissue exhibits a large secretome, leptin and adiponectin are exclusively secreted by mature adipocytes. They are regarded as metabolic regulators. The specific role of adiponectin is the regulation of insulin sensitivity. In contrast to leptin, adiponectin levels decrease in obesity and diabetes.
To assess the secretory function of maturing adipocytes, we investigated the level of adiponectin secreted by preadipocytes during the process of differentiation. Adiponectin secretion was measured in a standard sandwich enzyme-linked immunosorbent assay (ELISA) with supernatants collected from both groups of preadipocyte cultures ( and ).
Figure 4. Adiponectin secretion (pg/ml) of preadipocytes cultured in BM 1 after stimulation with induction medium for 48 h and following cultivation in differentiation medium. Each value represents the mean ± SD of 4 independent experiments.
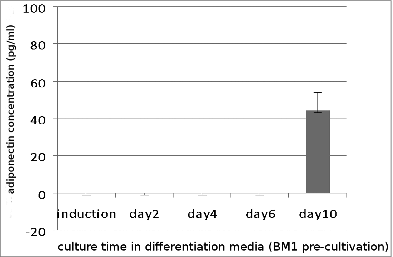
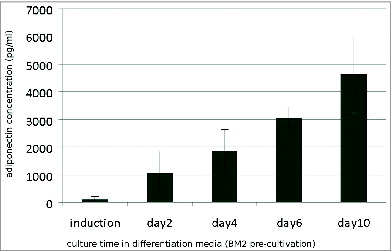
Figure 5. Adiponectin secretion (pg/ml) of preadipocytes cultured in BM 2 after stimulation with induction medium for 48 h and following culture in differentiation medium. Values represent the mean and standard deviation of 4 independent experiments.
As shown in , cells grown in BM1 secreted only minimal amounts of adiponectin on day 10 of culture in the differentiation medium while no adiponectin was detectable in earlier supernatants.
In contrast, preadipocytes grown in BM 2 () responded to the differentiation schedule with a high secretion of adiponectin. Cultures in differentiation medium exhibited a linear increase of adiponectin secretion over the cultivation period.
Adiponectin production critically depends on the differentiation status of the cells. Secretion of adiponectin is dose- and time-dependent. These findings correlated with the histological evaluation of the parallel cultures.
Discussion
In our study, we extracted preadipocytes from fat tissue obtained from surgically removed abdominal fat flaps or from cosmetic breast reductions. Our aim was to study the effect of 2 different media with regard to their capacity to serve as a basic medium for preadipocytes before further differentiation into mature adipose tissue. One of the media used, BM 1, has been extensively described in the literature,Citation7,8,10 whereas the use of BM 2, which is the endothelial cell growth medium purchased from Promocell, has not yet been reported in preadipocyte cultivation protocols so far. By comparing both media before submitting the cells to identical culture conditions and differentiation schemes, we found a number of differences in the development of the 2 experimental groups. Because of the high amount of contaminating erythrocytes before the first cultivation, we could not determine the exact amount of cells derived from collagen-digested fat tissue in relation to cell number per gram fresh fat tissue. However, differences could be documented monitoring the cells optically by using an inverted microscope over time and, functionally, by performing a standard overnight MTT proliferation assay. Secondly and most prominently, we found that cells grown in BM 2 to a confluence of 80% responded more rapidly and more effectively to the following induction and differentiation stimuli than cells grown in BM 1. Cells grown in BM 2 had the capacity to develop into mature adipocytes after an induction period of only 48 h followed by a differentiation time of less than 1 week. In the literature, the use of culture medium similar to BM 1 for a much longer induction and differentiation period is described. The rationale of our study has been to develop a feasible methodology to expand ADSCs in reasonable numbers and simultaneously to maintain the high quality of the cells. The results obtained from this study provide the foundation for tissue engineering strategies of adipose derived stem cells (ADSCs) in the ongoing work in our group.
The quantification of the adiponectin production of maturing ADSCs and the histological evaluation with oil-red staining validated the differentiation of ADSCs into mature adipocytes. In addition, we aimed to establish a fast protocol, as the minimization of the pre-cultivation time can only be advantageous for the potential (pre-)clinical applications of these cells. The comparison of cells grown in either DMEM/F12 medium supplemented with a cocktail of growth factors and hormones and 10% fetal bovine serum with the Promocell endothelial cell growth medium supplemented with factors that are not exactly known because of the patent rights of the manufacturer, but contain only a minimum as 2% of fetal bovine serum, has revealed the clear advantage of the latter medium.
Since adipose tissue is increasingly being considered as an endocrinologically active organ that strongly participates in the regulation of both physiological and pathological processes,Citation18,19 we also investigated the amount of adiponectin secretion of the ADSCs cultures. Also, adiponectin has been established as a valid marker of adipogenic differentiation and thus further served as an indicator of the level of adipogenic differentiation in-vivo. Adiponectin, together with leptin, is the most important adipokine secreted by mature adipocytes and is critically involved in the regulation of insulin sensitivity and the development of obesity and autoimmune diseases as diabetes mellitus type II.Citation18,19 Adiponectin secretion decreases in obesity and diabetes, and the detection of its secretion level might therefore serve as a quality marker. We were able to show that adiponectin secretion occurred in a time-dependent manner only in cultures that were initially grown in BM 2. Small amounts of adiponectin production could also be detected in the cells cultured in BM 1, but only late in the process of differentiation. Since it has been documented that no standardized isolation protocol has been established between various research groups there is no consensus way in isolating those cells.Citation14 ADSCs are almost perfect candidates for use in regenerative medicine because of their ability to self-renew and to commit to multiple lineages.Citation6,20,21 In order to develop a robust, clinically applicable model for the tissue engineering of fat with the ultimate goal of the prefabrication of axial perfused soft tissue flaps to compensate for defects, we consider it to be of major importance to provide a strong and reproducible in vitro model that meets all the requirements of profound in vivo studies. In order to use stem cells for clinical treatments, there have to be further considerations in respect to the production of large quantities and to employ good manufacturing practices (GMP).Citation20 The abandonment of fetal calf serum as additive to the media is one of the major guidelines. From the cell culturing point of view supplementation of culture media with fetal calf serum is very practical since it provides the cells with essential nutrients and growth factors. In our protocol a minimum of only 2% of fetal calf serum is used. A replacement with human serum derivatives has been reported previously and shown to support equal proliferation rates and multilineage differentiation capacity.Citation20,23 In our further studies we plan to approach those findings.
Materials and Methods
Materials
Collagenase buffer (in house pharmacy preparation): 23.8 g/l Hepes (Merck, 391340), 7 g/l NaCl, 3.6 g/l KCl, 0.15 g/l CaCl2.2H2O, 1 g/l D-glucose in distilled water, supplemented with 1.5% BSA (Sigma, A9418)
Collagenase working solution: Collagenase NB4 (Serva, 17455) in Collagenase buffer to give a final concentration of 0.2 U/ml.
Dulbecco's modified Eagle's medium (DMEM) (Invitrogen, 61965-059)
Formalin 10% in deionized water (in house pharmacy preparation)
Isopropanol (isopropyl alcohol): 60% in deionized water (in house pharmacy preparation)
Oil-red O solution: 0.5% in isopropanol (isopropyl alcohol) (Sigma-Aldrich, O1391)
Phosphate-buffered saline (PBS) without calcium and magnesium (Biochrom, L1825)
Fetal bovine serum (FBS) (Invitrogen, 10270)
Trypan Blue (0.4%) (Sigma, T8154)
Trypsin / EDTA (0.05%) (Invitrogen, 25300054)
Basal Medium 1: DMEM/F12 (Invitrogen, 12634) supplemented with 15 mM Hepes (Merck, 391340), NaHCO3 (Sigma-Aldrich, 88975) , pyridoxine (Sigma-Aldrich, P9755) and 0.365 g/l L-Glutamine (Sigma-Aldrich, G6392), Insulin (8 μM/ (10 μg/ml) (Sigma-Aldrich, I3536), 1% Antibiotic-Antimycotic (Invitrogen, 15240-096), 10 μg/ml apo-transferrin (Sigma, T1428), 33 μM biotin (Sigma-Aldrich, B4639), 17 μM D-pantothenic acid (Sigma-Aldrich, P5155), and 10% FBS (Invitrogen, 10270)
Basal Medium 2: Endothelial Growth Medium (Promocell, C-22010) supplemented with Endothelial Growth Medium–Supplementmix (Promocell, C-39215) and 1% Antibiotic-Antimycotic (Invitrogen, 15240-096)
Induction Medium: a-MEM (Minimum Essential Medium Eagle, Sigma-Aldrich, M 2279) supplemented with 5% FBS (Invitrogen, 10270), 1% Antibiotic-Antimycotic (Invitrogen, 15240-096), Insulin (16 μM (10 μg/ml) (Sigma-Aldrich, I3536), 0.1 μM Hydrocortisone (Sigma-Aldrich, H0888), 0.5 mM IBMX (3-isobutyl-1-methylxanthine) (Sigma-Aldrich, I7018), 60 μM Indomethacin (Sigma-Aldich, I8280)
Differentiation Medium: a-MEM (Minimum Essential Medium Eagle, Sigma-Aldrich, M 2279) supplemented with 5% FBS (Invitrogen, 10270), 1% Antibiotic-Antimycotic (Invitrogen, 15240-096), Insulin 16 μM (10 μg/ml) (Sigma-Aldrich, I3536)
Methods
Isolation and cultivation of ADSCs
Fat tissue samples were obtained from 4 healthy female patients undergoing operations such as abdominoplasty, breast reduction, or liposuction at the Clinic for Plastic Surgery at the Klinikum Rechts der Isar der TU München, Munich, Germany. All research has have been approved by the authors' ethic committee of the Technical University of Munich, and all clinical investigations have been conducted according to the principles expressed in the Declaration of Helsinki. All patients gave their written consent, which was approved by the ethic committee of the Technical University of Munich (Permit Number: 5623/12). ADSCs were isolated according to a previously described protocol with slight modifications, which will be described here briefly. After removal of blood vessels and soft tissue, the fat tissue was minced into a paste-like texture and subsequently digested by using a Hepes-buffered collagenase solution at a final concentration of 0.2 U/ml. per gram fat suspension, 1 ml collagenase solution was used. The samples were then incubated in a waterbath with constant gentle agitation for 2 hours at 37°C. The digestion was stopped by adding 2 volumes of FBS-supplemented medium consisting of DMEM with added 10% FBS.
The mixture was subsequently fractionated by sedimentation centrifugation at 2000 rpm for 1 min at 20°C. The upper layer was removed, and the cell pellet was aspirated with phosphate-buffered saline (PBS) and filtered through a 70-μm mesh cell strainer (BD Bioscience, Denmark) to remove debris. The filtrate was divided into 2 equal quantities in 50 ml Falcon tubes that were filled up with PBS prior to additional centrifugation. Following centrifugation, PBS was removed, and the pellets were resuspended in either basic medium 1 (BM 1) or BM2 and seeded onto 10 cm2 tissue culture dishes. Because of the high amount of contaminating erythrocytes, accurate counting of the cells was not possible. However, according to our experience, we obtained 4 10 cm2 cell culture plates from 10 g digested fat. After 24 hours, the medium was removed from the plate, which was carefully washed with pre-warmed PBS in order to remove the remaining erythrocytes and debris. Fresh medium was added and renewed every 3-4 days until a confluence rate of approximately 80% was achieved, which usually took place after 10-14 days in culture. Both groups of ADSCs then individually underwent a 2-step differentiation protocol. To induce adipocyte differentiation, basal medium was removed, and the cells were washed with pre-warmed PBS prior to the addition of induction medium (IM) whose contents are described above. After 48 hours, IM was replaced with a differentiation medium (DM) whose contents are also described above. No washing step of the cells was carried out between the removal of IM and the addition of DM. Differentiating cultures were kept for 14 days with DM being changed every 3 days. The cells were monitored by light microscopy for lipid droplet formation and finally stained with oil-red to confirm differentiation and to document the differences between the 2 culture strategies.
For conducting functional assays, the primary culture cells (P0) were harvested as follows.
At confluence, the cell culture medium was removed, and the cell monolayer was washed with 5 ml pre-warmed PBS. Subsequently, 2 ml trypsin-EDTA solution was added to cover the plate surface. The plate was then incubated at 37°C for 3 min. The trypsin reaction was terminated by the addition of 10 mL FBS-supplemented DMEM, and the detached cells were resuspended by repeatedly pipetting medium over the surface of the culture plate. The resuspended cells were transferred to a 50 ml Falcon tube and centrifuged at 2000 rpm for 10 minutes at 20°C. Then, the supernatant was discarded, and the cell pellet was resuspended in 10 ml medium. Cells were subsequently quantified by Trypan blue exclusion by using a hemacytometer and an inverted optical microscope.
Adiponectin ELISA
Primary culture cells were seeded at a density of 2.5 × 104 in 96-well culture plates. Culture conditions were either in BM1 or BM2 as described above. Supernatants were collected at day 3 and day 8 of culture in BM. After 48 h of culture in induction medium (IM), supernatant was also collected. After DM had been added to the culture, supernatants were collected at days 2, 4, 6, and 10, and every other day afterwards. All collected supernatant samples were immediately frozen at −20°C. For adiponectin ELISA, the human Adiponectin DuoSet ELISA Development System from R&D Systems (DY1065) was used following the manufacturer's protocol. Absorbance was read at 450 nm in a multiwell spectrophotometer (Thermo-Scientific Multiskan-Photometer). Calculation of results was performed by creating a standard curve as recommended by the manufacturer's protocol. Data were linearized by plotting the log of the adiponectin concentrations versus the log of the optical density (O.D.), and the best fit line was determined by regression analysis. Samples were used undiluted, and culture medium alone was used as the control.
Cell proliferation assay
To quantify cell proliferation, we used an MTT proliferation assay based on the Cell Proliferation Kit I (MTT; Roche Cat. No. 11 465 007 001) in which metabolically active cells cleave the tetrazolium salt MTT to a water-insoluble formazan that can be solubilized and quantified with a multiwell spectrophotometer (Thermo-Scientific Multiskan) at a wavelength of 550 nm and a reference wavelength of >650 nm.
Procedure: At confluence, cells from parallel cultures were harvested and counted. Cells were suspended in 1 ml of the appropriate medium and diluted to a density of 75,000 cells per ml. Using a 96-well flat bottom plate, 100 μl cells (i.e., 7500 cells in total) were seeded per well and incubated overnight. All measurements were conducted in triplicate.
After 16 hours of incubation, 10 μl of 5 mg/ml MTT was added to each well, after which the cells were incubated at 37°C for another 4 h. Triplicate wells in which MTT and media were added in the absence of cells were used as controls. After the 4-h incubation period, 100 μl MTT solution was added and incubated overnight at 37°C. The absorbance was then read at 590 nm with a reference filter of 620 nm.
Oil-red O staining of adipose tissue in culture
Red O staining is an assay performed to stain induced adipogenic cultures in order to detect mature adipocytes. Tissue culture plates were removed from the incubator and placed in a fume hood. Medium was completely removed, and the plates were subsequently rinsed with PBS. After removal of PBS, 10 ml of 10% formalin solution was added, and the plates were incubated for 30 min at room temperature. Next, the formalin was removed from each plate and discarded according to general chemical waste disposal procedures. Subsequently, each plate was gently rinsed with 5 ml purified water followed by the addition of 5 ml 60% isopropanol. Isopropanol was allowed to remain in the tissue culture plates for 5 min before being discarded. Aliquots of 6 ml of oil-red O working solution was added sufficient to cover the entire monolayer. The plates were then incubated for 2 hours in the refrigerator at 4°C before the oil-red O solution was discarded. In a last step, the cultures were washed several times with PBS. Cells were finally monitored for lipid droplet formation by using an inverted optical microscope.
Quantification of Oil-red positive surface areas representing the amount of mature Adipocytes
The percentage of darker shaded areas (Oil-red positive) were quantified using ImageJ programFootnote with the Threshhold_color plugin. The whole area of the native image was selected and duplicated into a secondary image and, by applying the threshold color plugin tool (brightness threshold pass filter set to 125), a black and white picture was created from it and the to-be analyzed area turns black. Following a process of color inversion, an 8-bit format was converted. Then a threshold was applied and the percentage of image area corresponding to that threshold was analyzed and quantified by using the Analyze Particles function of Image J.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
We thank the Legerlotz Foundation for the financial support.
Notes
1 ImageJ Source: http://imagej.nih.gov/ij/, last accessed June 8, 2014, created by Wayne Rasband Rasband Rasband ([email protected]) at the Research Services Branch, National Institute of Mental Health Color Threshold Plugin source: www.dentistry.bham.ac.uk/landinig/software/software.html, last accessed June 8, 2014, created by Gabriel Landini ([email protected]), University of Birmingham
References
- Patrick CW Jr. Breast tissue engineering. Annu Rev Biomed Eng 2004; 6:109-130; PMID:15255764
- Von Heimburg D, Zachariah S, Heschel I, Kühling H, Schoof H, Hafemann B, Pallua N. Human ADSCs seeded on freeze-dried collagen scaffolds investigated in vitro and in vivo. Biomaterials 2001; 22:429-438; PMID:11214753; http://dx.doi.org/10.1016/S0142-9612(00)00186-1
- Cawthorn WP, Scheller EL, MacDougald OA. Adipose tissue stem cells meet preadipocyte commitment: going back to the future. J Lipid Res 2012; 53(2):227-46; PMID:22140268; http://dx.doi.org/10.1194/jlr.R021089
- Mizuno H. Adipose-derived Stem cells for tissue repair and regeneration: ten years of research and a literature review. J Nippon Med Sch 2009; 76(2):56-66; PMID:19443990; http://dx.doi.org/10.1272/jnms.76.56
- Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 2002; 13:4279-4295; PMID:12475952; http://dx.doi.org/10.1091/mbc.E02-02-0105
- Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 2001; 7:211-228; PMID:11304456; http://dx.doi.org/10.1089/107632701300062859
- Tanzi MC, Farè S. Adipose tissue engineering: state of the art, recent advances and innovative approaches. Expert Rev Med Devices 2009; 6(5):533-551; PMID:19751125; http://dx.doi.org/10.1586/erd.09.37
- Loeffler G, Hauner H. Adipose tissue development: the role of precursor cells and adipogenic factors. Part II: the regulation of the adipogenic conversion by hormones and serum factors. Klin Wochenschr 1987; 65:812-7; PMID:3309457; http://dx.doi.org/10.1007/BF01727475
- Hauner H, Skurk T, Wabitsch M. Cultures of adipose Precursor cells. Methods Mol Biol 2000; 155:747-9.
- Fischbach C, Seufert J, Staiger H, Hacker M, Neubauer M, Göpferich A, Blunk T. Three-Dimensional in vitro Model of adipogenesis: comparison of culture conditions. Tissue Eng 2004; 10:215-229; PMID:15009947; http://dx.doi.org/10.1089/107632704322791862
- Fischbach C, Spruß T, Weiser B, Neubauer M, Becekr C, Hacker M, Göpferich A, Blunk T. Generation of mature fat pads in vitro an in vivo utilizing 3-D long- term culture of 3T3-L1 preadipozytes. Exp Cell Res 2004; 300:54-64; PMID:15383314
- Rodriguez A, Elabd C, Delteil F, Astier J, Vernochet C, Saint Marie P, Guesnet J, Guezennet J, Amri E. Adipocyte differentiation of multipotent cells established from human adipose tissue. Biochem Biophys Res Commun 2004; 315:255-263; PMID:14766202; http://dx.doi.org/10.1016/j.bbrc.2004.01.053
- Niemelä SM, Miettinen S, Konttinen Y, Waris T, Kellomäki M, Ashammakhi N, Ylikomi T. Fat Tissue: views on Reconstruction and exploitation. J Craniofac Surgery 2007; 18:325-35; PMID:17414282; http://dx.doi.org/10.1097/scs.0b013e3180333b6a
- Casadei A, Epis R, Ferroni L, Tocco I, Gardin C, Bressan Sivolella S, Vindigni V, Pinton P, Mucci G, Zavan B. Adipose tissue regeneration: state of the art. J Biomed Biotechnol 2012; 2012:462543; PMID:23193362; http://dx.doi.org/10.1155/2012/462543
- Hong L, Peptan IA, Colpan A, Daw JL. Adipose tissue engineering by human adipose-derived stromal cells. Cells Tissues Organs 2006; 183:133-40; PMID:17108684; http://dx.doi.org/10.1159/000095987
- Hauner H, Entenmann G, Wabitsch M, Gaillard D, Ailhaud G, Negrel R, Pfeiffer E. Promoting effect of glucocorticoids on the differentiation on human adipocyte precursor cells cultured in a chemically defined medium. J Clin Invest 1989; 84:1663-70; PMID:2681273; http://dx.doi.org/10.1172/JCI114345
- Ferris WF, Crother NJ. Once fat was fat and that was that: our changing perspective on adipose tissue. Cardiovasc J Af 2011; 22:147-53; PMID:21713306; http://dx.doi.org/10.5830/CVJA-2010-083
- Fantuzzi G. Adipose tissue, adipokines, and inflammation J Allergy Clin Immunol 2005; 115:911-9; PMID:15867843
- Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Medicine 2001; 7:941-6; PMID:11479627; http://dx.doi.org/10.1038/90984
- Lindroos B, Suuronen R, Miettinen S. The potential of adipose stem cells in regenerative medicine. Stem Cell Rev 2011; 7:269-91; PMID:20853072; http://dx.doi.org/10.1007/s12015-010-9193-7
- Gimble JM, Katz AJ, Brunell BA. Adipose derived stem cells fopr regenerative medicine. Circ Res 2007; 100:1249-60; PMID:17495232; http://dx.doi.org/10.1161/01.RES.0000265074.83288.09
- Wuchter P, Bieback K, Schrezenmeier H, Bornhäuser M, Müller LP, Bönig H, Wagner W, Meisel R, Pavel P, Tonn T, et al. Standardization of Good Manufacturing Practice-compliant production of bone marrow-derived human mesenchymal stromal cells for immunotherapeutic applications. Cytotherapy 2014; pii: S1465-3249(14):00558-1; PMID:24856898; http://dx.doi.org/10.1016/j.jcyt.2014.04.002
- Bieback K. Platelet lysate as replacement for fetal bovine serum in mesenchymal stromal cell cultures. Transfus Med Hemother 2013; 40:326-35; PMID:24273486; http://dx.doi.org/10.1159/000354061