Abstract
A diet enriched with citrulline (CIT) reduces white adipose tissue (WAT) mass. We recently showed that CIT stimulated β-oxidation in rat WAT explants from young (2–4 months) but not old (25 months) rats. Here we show that both in old rats and high-fat-diet-fed young rats, uncoupling protein one (UCP1) mRNA and protein expressions were weaker than those in young control rats. Selectively in WAT from young rats, a 24h CIT treatment up-regulated expressions of UCP1, peroxisome proliferator-activated receptor-α (PPARα), PPARγ-coactivator-1-α and mitochondrial-transcription-factor-A whereas it down-regulated PPARγ2 gene expression, whatever the diet. These results suggest that CIT induces a new metabolic status in WAT, with increased β-oxidation and uncoupling of respiratory chain, resulting in energy expenditure that favors fat mass reduction.
Abbreviations:
- ARG, arginine
- ASL, argininosuccinate lyase
- ASS, argininosuccinate synthase
- BSA, bovine serum albumin
- CD, control diet
- CIT, citrulline
- CPT1-b, carnitine palmitoyl transferase 1-b
- EPI, epididymal
- HFD, high-fat-diet
- KREBS, Krebs Ringer Buffer Saline
- NEFA, non-esterified fatty acids
- NO, nitric oxide
- NOS, nitric oxide synthase
- PEPCK-C, cytosolic phosphoenolpyruvate carboxykinase
- PGC-1α, peroxisome proliferator-activated receptor gamma co-activator 1α
- PKA, protein kinase A
- PPAR, peroxisome proliferator-activated receptor
- RET, retroperitoneal
- TFAM, mitochondrial transcription factor A
- VLCAD, very long chain acyl-CoA dehydrogenase
- WAT, white adipose tissue
Introduction
Excess energy storage in white adipose tissue (WAT) favors an increase in fat mass with pathophysiological consequences like insulin resistance and altered glucose tolerance leading to type-2 diabetes (T2DM). Aging and high-fat diet (HFD) often result in enlarged WAT mass, particularly intra-abdominal depots like the retroperitoneal (RET). One of the strategies to oppose fat mass gain would be to activate energy dissipation in brown adipose tissue (BAT). Brown adipocytes have the unique feature to express the uncoupling protein 1 (UCP1), responsible for the uncoupling between oxidative phosphorylation and ATP production, therefore dissipating energy as heat.Citation1,2 In 1992, Cousin et al. demonstrated the presence of UCP1-expressing adipocytes in well-defined WAT depots.Citation3 Such cells, resembling brown adipocytes in WAT, were further described and named BRITE for “brown-in-white” or beige adipocytes, then shown as having an intermediate signature between brown and white adipocytes.Citation4,5 Although the distinct origins of these cells remain a matter of debate, they both express UCP1.Citation6,7 Activators of UCP1 in BAT and inducers of WAT browning, i.e. the acquisition of a brown phenotype, have been determined. Among these agents, cold exposure, β-adrenergic agonists, retinoids, triiodothyronine, irisin, atrial natriuretic factor, prostaglandins, adenosine or lipolytic NEFA themselves have been shown to up-regulate UCP1.Citation8-11
An interesting observation of decreased WAT mass together with increased BAT mass and UCP1 arose from high-fat-diet-induced (HFD) overweight rats to which L-arginine (L-ARG) was given in drinking water.Citation12 L-ARG induced lipolysis and β-oxidation in WAT.Citation13 These studies were carried out either in vivo or in cultured cells. Studies on cultured explants from WAT are scarce. Using RET explants, we showed recently that citrulline (CIT) induced lipolysis and β-oxidation while it reduced glyceroneogenesis and NEFA re-esterification in control and HFD-fed young rats.Citation14,15 Similar data were obtained on RET explants from old rats, except that β-oxidation remained unaffected by CIT.Citation14,15 CIT is an amino acid that is not used for protein synthesis. Like L-ARG, CIT is an intermediary product in nitric oxide (NO) synthesis, provided that argininosuccinate synthase (ASS) and argininosuccinate lyase (ASL) are present, which is the case in macrophages but not in isolated adipocytes.Citation14–16 Unlike what occurs with L-ARG, CIT, administered as a food supplement, is not metabolized in the liver, therefore its biodisponibility is higher than that of L-ARG. CIT is thus proposed as a food supplement during aging and in sports to improve muscle mass and function.Citation17,18
In the present study we investigated whether CIT exerted a browning effect on epididymal (EPI) and RET WAT explants from control and HFD-fed young rats together with old rats. To this aim we analyzed variations in UCP1 expression and in its main transcriptional regulators.
Results
Effect of age, diet, and CIT on the expression of UCP1 gene and protein
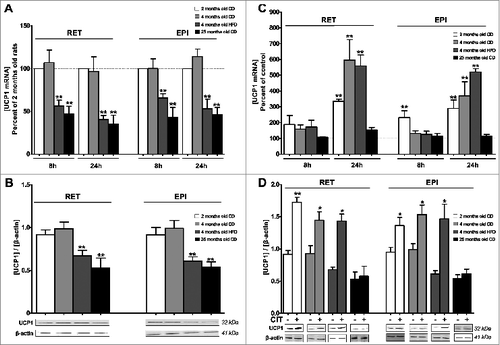
We first evaluated the effect of age or HFD on the relative UCP1 gene and protein expressions in RET and EPI WAT explants maintained in culture for either 8h or 24h. For CD in both depots, UCP1 mRNA level was similar in explants from 2-month-old and 4-month-old rats while for HFD, it presented a 45–55% decrease (). In both depots from 25-month-old rats, UCP1 mRNA was reduced to 50–60% when compared to 2-month-old rats (). A similar pattern of reduction of UCP1 protein occurred with 40–50% decrease at 24h of treatment (). We then assessed the effect of CIT treatment for 8h and 24h on UCP1 expression in RET and EPI WAT explants. At 24h CIT treatment, UCP1 mRNA was increased 3.5 to 6-fold, for both depots, in 2-month-old and 4-month-old rats, whether the latter were maintained or not on a HFD diet for 8 weeks, while it remained unaffected by CIT in 25-month-old rats (). A weaker (2.3-fold) increase in UCP1 mRNA was observed at 8h CIT treatment in EPI WAT from 2-month-old rats, although no other change occurred at this time (). At 24h CIT exposure, UCP1 protein was upregulated 1.5- to 2-fold in WAT from young rats for both depots, whatever the diet, while it remained unaffected in WAT from 25-month-old rats ().
Figure 1. Effect of age, diet and CIT on the expression of UCP1 gene and protein. RET or EPI WAT explants from 2 months old CD (white histogram), 4 months old CD (light gray histogram), 4 months old HFD (dark gray histogram) and 25 months old CD (black histogram) rats were incubated either in the absence of CIT (panels A and B) or with or without CIT (panels C and D) for 8h or 24h as described in materials and methods. Uncoupling protein 1 (UCP1) mRNA levels were evaluated by RT-qPCR. Results were normalized to RPL13 mRNA and expressed in % of control (panels A and C). UCP1 protein levels were estimated by western blots and results were normalized to β-actin (panels B and D). Results represent the mean ± SEM of independent experiments (n = 7) carried out in triplicate from different explants.*P < 0.05 vs. Control; ** P < 0.01 vs. Control. (A) Effects of diet or age on UCP1 gene expression. Histograms represent the ratio of the values obtained from 4-month-old CD rats, 4-month-old HFD rats or 25-month-old rats to the values obtained from 2-month-old CD rats, taken as controls. (B) Effects of diet or age on the expression of UCP1 protein. Histograms represent the relative UCP1 levels to ß-actin. A representative autoradiogram of protein gel blots of UCP1 and β-actin, is shown. (C) Effects of CIT on UCP1 mRNA levels. Histograms represent the ratio of the values obtained from CIT-treated explants from 2-month-old CD rats, 4-month-old CD rats, 4-month-old HFD rats or 25-month-old rats to the values obtained in untreated explants from rats of the corresponding age, taken as controls. (D) Effects of CIT on the expression of UCP1 protein. Histograms represent the relative UCP1 levels to ß-actin. A representative autoradiogram of western blots of UCP1 and β-actin, is shown.
Effect of CIT on the expression of genes encoding transcription factors and regulators involved in mitochondria metabolism
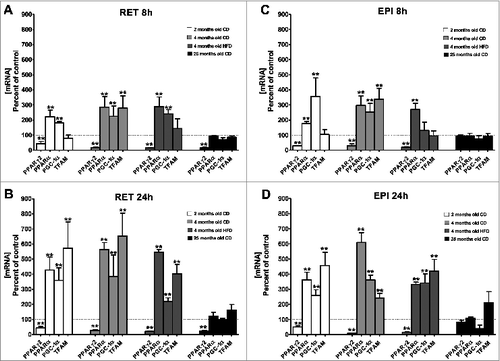
In agreement with our previously published results, peroxisome proliferator-activated receptor α (PPARα) gene expression was stimulated by CIT in RET explants from 2-month-old young rats whereas it remained unaffected in RET explants from 25-month-old rats ().Citation14 In contrast, PPARγ2 gene expression was downregulated (58–86%) by CIT in RET explants whatever the age of the animals ().Citation14 This reduced level was observed at 8h of exposure to CIT and remained stable at 24h. These previously described observations were extended to RET explants from CD-fed and HFD-fed 4-month-old rats that responded in a manner similar to those from 2-month-old rats (). In addition, the response of EPI explants to either 8h or 24h of CIT exposure was similar to that of RET, except for 25-month-old rats in which the PPARγ2 gene remained unaffected (). CIT treatment augmented PPARγ co-activator 1α (PGC-1α) and mitochondrial transcription factor A (TFAM) mRNA in both depots from young rats whatever the diet (). These increases were more pronounced at 24h than at 8h of treatment. In explants from 25-month-old rats, PGC-1α and TFAM genes remained unresponsive to CIT whatever the WAT depot and the time of treatment ().
Figure 2. Effect of CIT on the expression of genes coding for transcription factors and regulators involved in mitochondria metabolism. RET (panels A and B) or EPI (panels C and D) WAT explants from 2-month-old CD (white histogram), 4-month-old CD (light gray histogram), 4-month-old HFD (dark gray histogram) and 25-month-old CD (black histogram) rats were incubated with or without CIT for 8h (panels A and C) or 24h (panels B and D), as described in materials and methods. Peroxisome proliferator-activated receptor gamma2 (PPARγ2), peroxisome proliferator-activated receptor α (PPARα), peroxisome proliferator-activated receptor gamma coactivator 1α (PGC-1 α) and mitochondrial transcription factor A (TFAM) mRNA levels were evaluated by RT-qPCR. Histograms represent the ratio of the values obtained from CIT-treated explants from 2-month-old CD rats, 4-month-old CD rats, 4-month-old HFD rats or 25-month-old rats to the values obtained in untreated explants from rats of the corresponding age, taken as controls. Results represent the mean ± SEM of independent experiments (n = 7) carried out in triplicate from different explants, are normalized to RPL13 mRNA and expressed in % of control.*P < 0.05 vs. Control; ** P < 0.01 vs. Control
Discussion
We show that UCP1 mRNA and protein are expressed in RET and EPI WAT from rats, in accordance with previously published studies.Citation3 This expression is reduced with either aging or HFD. Our previous results established that CIT induced direct, rapid and deep modifications of NEFA metabolism in WAT explants from rats.Citation14,15 Notably, CIT increased β-oxidation capacity, together with the expression of the PPARα gene, in RET explants from young rats whether they were fed a CD or a HFD. Here, we observe that CIT was also able to induce UCP1 in WAT from young animals. However, no change in β-oxidation,Citation14,15 UCP1 expression and its transcriptional regulators (the present study) occurred in explants from old animals in response to this amino acid. The mechanism that underlines this young versus old difference in β-oxidation and UCP1 expression remains an open issue.
Therefore, in old animals, part of lipolytic NEFA are released from WAT and can be used as energetic substrates for demanding tissues like muscle.Citation14 Such observations are in line with the increase in muscle mass from old rats fed with either a L-ARG- or a CIT-supplemented diet.Citation19,20 We observed previously that in young animals, whether HFD-fed or not, NEFA output remained unaffected by CIT but NEFA oxidation was stimulated.Citation15 In the present study, we show that CIT up-regulates UCP1 expression in WAT explant from young rats. This effect is rapid since a 3-fold to 6-fold increase in UCP1 mRNA occurred at 24h of treatment, together with a 1.5- to 2-fold rise in the protein. Such a new metabolic feature is the sign of a situation of energetic expenditure in WAT.
What could be the mechanism of CIT action? Since cAMP, through protein kinase A (PKA) activation, is one of the major inducers of UCP1 gene expression in brown adipocytes, it could be the mediator of CIT action, although there is no report of the effect of this amino acid on PKA.Citation21 Such a mechanism is likely not occurring here. One of the best-known cAMP-responsive gene in adipocytes is pck1 (the cytosolic phosphoenolpyruvate carboxykinase—PEPCK-C—gene).Citation22,23 Therefore, any stimulation by CIT of cAMP production should induce pck1 but the inverse occurs here. However, CIT stimulates HSL phosphorylation in RET WAT and induces NEFA release when explants are isolated from HFD-fed young rats or from old rats.Citation14,15 Among the other agents that were demonstrated as capable of inducing HSL phosphorylation and lipolysis, the mitogen-activated protein kinase pathway and extracellular signal-regulated kinase (ERK) could play a role.Citation24 Alternatively, lipolysis could be activated because of the reduction of an inhibitory pathway, like those mediated by the AMP-activated protein kinase (AMPK), calcium/calmodulin-dependent protein kinase II or glycogen synthase kinase IV.Citation25 Interestingly, L-ARG was reported as being both lipolytic and capable of inducing AMPK, 2 potentially opposite phenomena in adipocytes, without clear explanations for this apparent contradiction.Citation13
One of the best candidates for mediating CIT action could be NO. As mentioned above, ASS and ASL are not expressed in WAT adipocytes. Hence, any NO production from CIT would primarily occur in macrophages present in WAT and would diffuse from macrophages to adipocytes where it would act on UCP1 expression. Previous reports showed the lipolytic and anti-glyceroneogenic nature of NO in WAT.Citation15,26–28 Besides, NO induces mitochondrial biogenesis via guanosine 3‘,5’ -monophosphate (cGMP) and PGC-1α, the central transcriptional activator in thermogenic adipocytes.Citation29,30 Both PPARα and PPARγ are transcription factors that interact with PGC-1α Citation31. CIT strongly induced PGC1α and PPARα gene expressions in both RET and EPI WAT from young rats, whereas PPARγ2 mRNA was down-regulated in the same animals, in agreement with our previous results.Citation14 The reduction in PPARγ2 reflects the previously observed strong decrease in PEPCK-C expression and in glyceroneogenesis.Citation14,15 Pck1 is indeed the major PPARγ2 target gene in adipocytes.Citation32–34
The simultaneous induction by CIT of PGC1α and PPARα is in line with the observed concomitant up-regulation of UCP1 in WAT, probably leading to a rise in the thermogenic status of the cells. CIT effect on UCP1 gene expression is supposedly transcriptional through a PPARα/PGC1α-process, since this couple was clearly demonstrated as acting as an inducer of the UCP1 gene in rodents and in humans.Citation35,36 PPARα and PGC1α were also shown to stimulate FA oxidation, notably in brown adipocytes, via the induction of expression of CPT 1b and VLCAD.Citation37–39 These observations are in agreement with our previous results showing that selectively in WAT explants from young rats, CIT induced an increase in β-oxidation capacity, together with an upregulation of CPT 1b and VLCAD.Citation14,15
Another interesting observation of the present study is the CIT effect on TFAM expression because TFAM is a PGC1-α target and a major transcription factor for mitochondrial DNA replication.Citation31,40,41 The possible CIT action as a factor favoring mitochondriogenesis during WAT browning remains an open issue with potential physiological consequences. Such a question also occurs with L-ARG-fed rats for which PGC 1α and mitochondrial biogenesis are enhanced, together with BAT and whole-body energy metabolism.Citation13,42
Altogether, our data identify a novel mechanism of CIT health effects and highlight the induction of UCP1 as a mediator of decreased WAT mass, browning of WAT and increased energy expenditure. The data open new and simple avenues of therapeutic intervention in overweight and obesity.
Materials and Methods
Animals: housing and feeding
Two-month-old male Sprague Dawley rats from Center d'Elevage de Rats Janvier (Berthevin, France), were fed ad libitum with a standard (control) diet (CD) (n = 21). They were acclimatized for 2 weeks then randomized into 3 groups. The two-month-old CD rats (n = 7) were immediately used for the culture of AT explants. The two other groups were maintained for an additional 8 weeks under either a CD (4-month-old CD; n = 7) or a high-fat diet (4-month-old HFD; n = 7). Twenty-five-month-old male Sprague Dawley rats, fed ad libitum with CD, were a gift from Sainte Anne Hospital (Paris, France) (n = 7). All rats have free access to water and food ad libitum and were housed at constant room temperature (24°C) on a 12-h-light/dark cycle. CD rats were fed with a standard balanced diet (11.7 MJ/kg) (64% carbohydrates, 20% proteins, 3% lipids, 8% vitamins and minerals) from Safe (Augy, France). HFD rats were fed with a HFD (19.5 MJ/kg) (44.5% carbohydrates, 20% proteins, 27.5% lipids, 8% vitamins and minerals) from Safe (Augy, France). Rats were anaesthetized and after blood sampling from the jugular vein, they were killed by decapitation. Samples of WAT were removed and weighted. The protocol for the animal studies was conducted according to the French Guidelines for the care and use of experimental animals.
Rat adipose tissue explants
EPI and RET WAT from rats were sampled for analyses. About 200mg of tissue were cut in about 20mg fragments and cultured in Krebs Ringer buffer saline (KREBS) medium as previously described.Citation14,15 Explants were exposed to 2.5 mmol/L CIT for 8h or 24h then frozen before further studies.
RNA extraction, cDNA synthesis and real-time RT-PCR
Total RNA was extracted from RET WAT with Rneasy lipid tissue mini kit from Qiagen. RNA was quantified by spectrophotometric Nanodrop. Total RNA (500ng) was reverse transcribed using SuperScript® III First-Strand Synthesis SuperMix kit from Life Technologies. Samples of cDNA were diluted 1:40, amplified and used for RT-qPCR measurements using SYBR Green from Roche as previously described.Citation14 RT–qPCR was performed in the LightCycler1536 instrument from Roche as follows: 75°C for 2min, 95°C for 10min, 40 cycles of denaturation (95°C for 15s to the denaturation, 60°C for 30s to the annealing, 72°C for 30s to the extension). Results were analyzed with the LightCycler1536 software from Roche. Ribosomal RPL13 RNA was used to normalize cDNA. Quantification of mRNA was carried out by comparing the number of cycles required to reach reference and target threshold values (2^-(δ−δ Ct) method) as described previously.Citation43 Sequences of the rat sense and antisense nucleotides were: PGC1-α : 5′-tgcccctgccagtcacagga-3′ 5′-gctcagccgaggacacgagg-3′; PPARα : 5′-aagccatcttcacgatgctg-3′ 5′-tcagaggtccctgaacagtg-3′ ; PPARγ2 : 5′- ttatgctgttatgggtgaaa-3′ 5′- caaaggaatgggagtgtc-3′ ; TFAM : 5′-gttccggggaatgtggggcg-3′ 5′-gacaggcgagggtatgcggc-3′ ; UCP1 : 5′-tacagagttatagccaccaca-3′ 5′-tggaacgtcatcatgtttgtg-3′ ; RPL13 : 5′-tggcaggggcttcag-3′ 5′-tgggcatcacaggtcc-3′
Western blot
Protein fractions were prepared from WAT explants in a buffer containing 10mmol/L HEPES pH 7.9, then separated by SDS-PAGE and transferred to nitrocellulose filters. Blots were blocked with 3% BSA in PBS containing 0.05% Tween 20 and incubated with 1:1000 and 1:500 dilutions for the UCP1 and β-actin antisera, respectively. Rabbit UCP1 and mouse β-actin antisera were purchased from thermofisher and Abcam respectively.
The Odyssey method was used for detection, following the procedure described by the manufacturer (Li-COR). Quantitative results of Western blotting were obtained by densitometry in ImageJ software.
Statistical analysis
Data are presented as means ± SEM. Each independent experiment was carried out in triplicate. Statistical analysis was carried out using the nonparametric Mann & Whitney U test for pairwise comparisons, which was applied due to the small number of experiments (n < 10). A value of P < 0.05 was considered as significant.
*P < 0.05 vs. control; ** P < 0.01 vs. control
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgment
We thank Dr. Brigitte Potier for providing us with a series of old rats.
Funding
We acknowledge the Institut National de la Santé et de la Recherche Médicale and the Université Paris Descartes for their financial support.
References
- Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev 2004; 84:277-359; PMID:14715917; http://dx.doi.org/10.1152/physrev.00015.2003
- Ricquier D, Bouillaud F. Mitochondrial uncoupling proteins: from mitochondria to the regulation of energy balance. J Physiol 2000; 529: 3-10; PMID:11080246; http://dx.doi.org/10.1111/j.1469-7793.2000.00003.x
- Cousin B, Cinti S, Morroni M, Raimbault S, Ricquier D, Pénicaud L, Casteilla L. Occurrence of brown adipocytes in rat white adipose tissue: molecular and morphological characterization. J Cell Sci 1992; 103:931-42; PMID:1362571.
- Petrovic N, Walden TB, Shabalina IG, Timmons JA, Cannon B, Nedergaard J. Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J Biol Chem 2010; 285:7153-64; PMID:20028987; http://dx.doi.org/10.1074/jbc.M109.053942
- Wu J, Boström P, Sparks LM, Ye L, Choi JH, Giang A-H, Khandekar M, Virtanen KA, Nuutila P, Schaart G, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 2012; 150:366-76; PMID:22796012; http://dx.doi.org/10.1016/j.cell.2012.05.016
- Tran K-V, Gealekman O, Frontini A, Zingaretti MC, Morroni M, Giordano A, Smorlesi A, Perugini J, De Matteis R, Sbarbati A, et al. The vascular endothelium of the adipose tissue gives rise to both white and brown fat cells. Cell Metab 2012; 15:222-9; PMID:22326223; http://dx.doi.org/10.1016/j.cmet.2012.01.008
- Lee Y-H, Petkova AP, Mottillo EP, Granneman JG. In vivo identification of bipotential adipocyte progenitors recruited by β3-adrenoceptor activation and high-fat feeding. Cell Metab 2012; 15:480-91; PMID:22482730; http://dx.doi.org/10.1016/j.cmet.2012.03.009
- Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nat Med 2013; 19:1252-63; PMID:24100998; http://dx.doi.org/10.1038/nm.3361
- Carrière A, Jeanson Y, Cousin B, Arnaud E, Casteilla L. Le recrutement et l‘activation d’adipocytes bruns et/ou BRITE: une perspective réelle pour le traitement des maladies métaboliques ? Méd Sci 2013; 29:729-35; PMID:24005627; http://dx.doi.org/10.1051/medsci/2013298011
- Carrière A, Jeanson Y, Berger-Müller S, André M, Chenouard V, Arnaud E, Barreau C, Walther R, Galinier A, Wdziekonski B, et al. Browning of white adipose cells by intermediate metabolites: an adaptive mechanism to alleviate redox pressure. Diabetes 2014; 63:3253-65; PMID:24789919; http://dx.doi.org/10.2337/db13-1885
- Gnad T, Scheibler S, von Kügelgen I, Scheele C, Kilić A, Glöde A, Hoffmann LS, Reverte-Salisa L, Horn P, Mutlu S, et al. Adenosine activates brown adipose tissue and recruits beige adipocytes via A2A receptors. Nature 2014; 516:395-9; PMID:25317558; http://dx.doi.org/10.1038/nature13816
- Jobgen W, Fu WJ, Gao H, Li P, Meininger CJ, Smith SB, Spencer TE, Wu G. High fat feeding and dietary L-arginine supplementation differentially regulate gene expression in rat white adipose tissue. Amino Acids 2009; 37:187-98; PMID:19212806; http://dx.doi.org/10.1007/s00726-009-0246-7
- Tan B, Li X, Yin Y, Wu Z, Liu C, Tekwe CD, Wu G. Regulatory roles for L-arginine in reducing white adipose tissue. Front Biosci J Virtual Libr 2012; 17:2237-46; PMID:22652774; http://dx.doi.org/10.2741/4047
- Joffin N, Jaubert A-M, Durant S, Bastin J, De Bandt J-P, Cynober L, Moinard C, Forest C, Noirez P. Citrulline induces fatty acid release selectively in visceral adipose tissue from old rats. Mol Nutr Food Res 2014; 58: 1765-75; PMID:24913603; http://dx.doi.org/10.1002/mnfr.201400053
- Joffin N, Jaubert A-M, Durant S, Bastin J, De Bandt J-P, Cynober L, Moinard C, Coumoul X, Forest C, Noirez P. Citrulline reduces glyceroneogenesis and induces fatty acid release in visceral adipose tissue from overweight rats. Mol Nutr Food Res 2014; 58:2320-30; PMID:25271764; http://dx.doi.org/10.1002/mnfr.201400507
- Haines RJ, Pendleton LC, Eichler DC. Argininosuccinate synthase: at the center of arginine metabolism. Int J Biochem Mol Biol 2011; 2:8-23; PMID:21494411.
- Osowska S, Duchemann T, Walrand S, Paillard A, Boirie Y, Cynober L, Moinard C. Citrulline modulates muscle protein metabolism in old malnourished rats. Am J Physiol Endocrinol Metab 2006; 29:582-6; PMID:16608884; http://dx.doi.org/10.1152/ajpendo.00398.2005
- Sureda A, Pons A. Arginine and citrulline supplementation in sports and exercise: ergogenic nutrients? Med Sport Sci 2012; 59:18-28; PMID:23075551; http://dx.doi.org/10.1159/000341937
- Jobgen W, Meininger CJ, Jobgen SC, Li P, Lee M-J, Smith SB, Spencer TE, Fried SK, Wu G. Dietary L-arginine supplementation reduces white fat gain and enhances skeletal muscle and brown fat masses in diet-induced obese rats. J Nutr 2009; 139:230-7; PMID:19106310; http://dx.doi.org/10.3945/jn.108.096362
- Moinard, C., Le Plénier, S., Cynober, L., Raynaud-Simon, A., Long-term effect of citrulline supplementation in healthy aged rats: effect on body composition. Clin. Nutr. 2009, 4, O025. Clin Nutr Suppl 4:12.
- Forest C, Doglio A, Casteilla L, Ricquier D, Ailhaud G. Expression of the mitochondrial uncoupling protein in brown adipocytes. absence in brown preadipocytes and BFC-1 cells. modulation by isoproterenol in adipocytes. Exp Cell Res 1987; 168:233-46; PMID:3023117; http://dx.doi.org/10.1016/0014-4827(87)90431-9
- Reshef L, Hanson RW. The interaction of catecholamines and adrenal corticosteroids in the induction of phosphopyruvate carboxylase in rat liver and adipose tissue. Biochem J 1972; 127:809-18; PMID:4342497.
- Franckhauser S, Antras-Ferry J, Robin P, Robin D, Granner DK, Forest C. Expression of the phosphoenolpyruvate carboxykinase gene in 3T3-F442A adipose cells: opposite effects of dexamethasone and isoprenaline on transcription. Biochem J 1995; 305:65-71; PMID:7826355.
- Greenberg AS. Stimulation of lipolysis and hormone-sensitive lipase via the extracellular signal-regulated kinase pathway. J Biol Chem 2001; 276:45456-61; PMID:11581251; http://dx.doi.org/10.1074/jbc.M104436200
- Watt MJ, Steinberg GR. Regulation and function of triacylglycerol lipases in cellular metabolism. Biochem J 2008; 414:313-25; PMID:18717647; http://dx.doi.org/10.1042/BJ20080305
- Gaudiot N, Ribière C, Jaubert AM, Giudicelli Y. Endogenous nitric oxide is implicated in the regulation of lipolysis through antioxidant-related effect. Am J Physiol Cell Physiol 2000; 279:C1603-10; PMID:11029308.
- Jobgen WS, Fried SK, Fu WJ, Meininger CJ, Wu G. Regulatory role for the arginine-nitric oxide pathway in metabolism of energy substrates. J Nutr Biochem 2006; 17:571-88; PMID:16524713; http://dx.doi.org/10.1016/j.jnutbio.2005.12.001
- Niang F, Benelli C, Ribière C, Collinet M, Mehebik-Mojaat N, Penot G, Forest C, Jaubert A-M. Leptin induces nitric oxide-mediated inhibition of lipolysis and glyceroneogenesis in rat white adipose tissue. J Nutr 2011; 141:4-9; PMID:21068181; http://dx.doi.org/10.3945/jn.110.125765
- Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 1998; 92:829-39; PMID:9529258; http://dx.doi.org/10.1016/S0092-8674(00)81410-5
- Nisoli E, Clementi E, Paolucci C, Cozzi V, Tonello C, Sciorati C, Bracale R, Valerio A, Francolini M, Moncada S, et al. Mitochondrial biogenesis in mammals: the role of endogenous nitric oxide. Science 2003; 299:896-9; PMID:12574632; http://dx.doi.org/10.1126/science.1079368
- Lehman JJ, Barger PM, Kovacs A, Saffitz JE, Medeiros DM, Kelly DP. Peroxisome proliferator-activated receptor gamma coactivator-1 promotes cardiac mitochondrial biogenesis. J Clin Invest 2000; 106:847-56; PMID:11018072; http://dx.doi.org/10.1172/JCI10268
- Glorian M, Duplus E, Beale EG, Scott DK, Granner DK, Forest C. A single element in the phosphoenolpyruvate carboxykinase gene mediates thiazolidinedione action specifically in adipocytes. Biochimie 2001; 83:933-43; PMID:11728630; http://dx.doi.org/10.1016/S0300-9084(01)01343-8
- Forest C, Tordjman J, Glorian M, Duplus E, Chauvet G, Quette J, Beale EG, Antoine B. Fatty acid recycling in adipocytes: a role for glyceroneogenesis and phosphoenolpyruvate carboxykinase. Biochem Soc Trans 2003; 31:1125-9; PMID:14641009; http://dx.doi.org/10.1042/BST0311125
- Cadoudal T, Blouin JM, Collinet M, Fouque F, Tan GD, Loizon E, Beale EG, Frayn KN, Karpe F, Vidal H, et al. Acute and selective regulation of glyceroneogenesis and cytosolic phosphoenolpyruvate carboxykinase in adipose tissue by thiazolidinediones in type 2 diabetes. Diabetologia 2007; 50:666-75; PMID:17242918; http://dx.doi.org/10.1007/s00125-006-0560-5
- Barbera MJ, Schluter A, Pedraza N, Iglesias R, Villarroya F, Giralt M. Peroxisome proliferator-activated receptor α activates transcription of the brown fat uncoupling protein-1 gene. A link between regulation of the thermogenic and lipid oxidation pathways in the brown fat cell. J Biol Chem 2001; 276:1486-93; PMID:11050084; http://dx.doi.org/10.1074/jbc.M006246200
- Tiraby C, Tavernier G, Lefort C, Larrouy D, Bouillaud F, Ricquier D, Langin D. Acquirement of brown fat cell features by human white adipocytes. J Biol Chem 2003; 278:33370-6; PMID:12807871; http://dx.doi.org/10.1074/jbc.M305235200
- Mandard S, Müller M, Kersten S. Peroxisome proliferator-activated receptor α target genes. Cell Mol Life Sci CMLS 2004; 61:393-416; PMID:14999402; http://dx.doi.org/10.1007/s00018-003-3216-3
- Lefebvre P, Chinetti G, Fruchart J-C, Staels B. Sorting out the roles of PPAR α in energy metabolism and vascular homeostasis. J Clin Invest 2006; 116:571-80; PMID:16511589; http://dx.doi.org/10.1172/JCI27989
- Finck BN, Kelly DP. Peroxisome proliferator–activated receptor γ coactivator-1 (PGC-1) regulatory cascade in cardiac physiology and disease. Circulation 2007; 115:2540-8; PMID:17502589; http://dx.doi.org/10.1161/CIRCULATIONAHA.107.670588
- Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 1999; 98:115-24; PMID:10412986; http://dx.doi.org/10.1016/S0092-8674(00)80611-X
- Kelly DP, Scarpulla RC. Transcriptional regulatory circuits controlling mitochondrial biogenesis and function. Genes Dev 2004; 18:357-68; PMID:15004004; http://dx.doi.org/10.1101/gad.1177604
- Fu WJ, Haynes TE, Kohli R, Hu J, Shi W, Spencer TE, Carroll RJ, Meininger CJ, Wu G. Dietary L-arginine supplementation reduces fat mass in zucker diabetic fatty rats. J Nutr 2005; 135:714-21; PMID:15795423.
- Livak KJ, Schmittgen TD. Analysis of elative gene expression data using real-time quantitative PCR and the 2-; [delta]; [delta]CT method. Methods 2001; 25:402-8; PMID:11846609; http://dx.doi.org/10.1006/meth.2001.1262
