Depots within Depots
pp 236
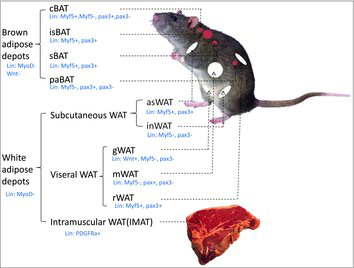
While once thought to be passive and dormant, advances in adipose research have shown that adipocytes are not only dynamic and highly influential but different with regards to how they influence and are influenced by nearby tissues. This includes differences from one adipose depot to another, leading to depots within depots with each one differing in cellularity. These differences may have a role in governing cellular development and regulation of fat deposition. This review by Dodson et al. presents an overview of current research in the field of adipocyte depot studies in order to greater understand the roles that adipose depots play in everything from diseases to athlete performance.
The Good, the Bad and the Marbled
pp 242-55
Highly desirable when it comes to flavor, the marbling of fat throughout meat is in reality intramuscular adipose tissue. Adipocytes that are found in different adipose depots have been found to differ with regards to their function and how they influence surrounding cells. Two of these depots, intermuscular fat (INTMF) and intramuscular (IM), are the focus of this review by Hausman et al. which studies INTMF vs IM adipose tissues in both humans and meat animals. Here the authors look for potential commonalities between the two species as well as the association of the two depots with physiological abnormalities such as metabolic syndrome and diabetes as well as gene regulation and expression.
Maternal Nutrition's Effect on Offspring
pp 256-62
Maternal undernutrition can lead to a low birth weight and an increased risk of adult-onset obesity, while maternal obesity with diabetes can lead to a high birth weight as well as a predisposition for fat accumulation. Recent research has also shown that adipose tissue may in fact be the root of these programmed events. Authors Lecoutre and Breton summarize adipose tissue's impact in the programming of offspring as manipulated by maternal nutrition, paying close attention to the last week of gestation and early postnatal life. Here they show that while the developmental windows may differ between rodents and larger mammals, the manipulation of maternal nutrition can result in increased adipogenesis and modified fat distribution and composition, predisposing the offspring to obesity and further fat accumulation.
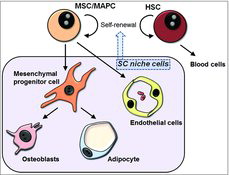
Marrow Adipogenesis in Diabetic Complications
pp 263-72
The continuing prevalence of diabetes in both America and the rest of the world has given rise to several other concerns, including the statistic that secondary complications caused by diabetes are found in three out of four diabetic patients. The vasculature disruptions of select organ systems caused by diabetes include both micro and macro angiopathies as well as bone marrow angiopathy. This review by Piccinin and Khan discusses the possible mechanistic relationship between increased marrow adipogenesis, changing of marrow cellular composition including disruption and depletion of reparative stem cells in the pathogenesis of secondary diabetic complications ().
Maximizing Fat Graft Retention
pp 273-9
Despite its rise in popularity with plastic surgeons as a soft tissue filler, the fat grafting of adipose tissue suffers from unpredictable resorption rates. While the ideal harvesting technique of adipose tissue remains unknown, it is thought that the various different harvesting procedures may play a role in the cellular stability of fat grafts. In an effort to obtain new insights on optimizing the procedure of fat grafting and harvesting, this review by Guase II et al. discusses the literature involving fat particle size and fat grafting outcomes. The evidence reviewed supports excisional methods and larger bore cannulas in order to both minimize cellular damage and maximize the amount of cells contained in the fat particles, thus preserving the native architecture of the adipose tissue.
Bringing Muscle to the Obesity Fight
pp 280-89
Due to over-nutrition, a lack of physical activity, and numerous genetic factors, early onset obesity is on the rise globally. Because of this epidemic, there has been a newfound interest in both adipose tissue as well as skeletal muscle derived factors (myokines) and their role in controlling inflammation status, adipose homeostasis and body energy balance. This review by authors Yang, Bi, and Kuang presents an overview of recent progress in the field of muscle-fat crosstalk with regards to obesity, diabetes, and energy metabolism. It is shown that skeletal muscle and brown fat share similar origins and several novel myokines may have roles in regulating fat mass and energy balance, supporting the idea that skeletal muscles may be a therapeutic target in the treatment of obesity and its metabolic complications ().
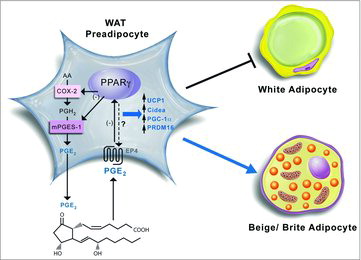
Brown-In-White gives Beige and Brite
pp 290-96
While white adipose tissue functions to store energy and contributes to metabolic dysfunction in obesity, brown adipose tissues dissipate energy in the form of heat and protects from obesity and associated metabolic disorders. Increasing data suggest that “browning” of white adipose tissues to produce beige/brite adipocytes induces a thermogenic program that enhances energy expenditure, promotes weight loss and protects from obesity related abnormalities including T2D and cardiovascular disease. Here, García-Alonso and Clària review data on the role of bioactive lipid mediators, specifically focusing on the role of prostaglandin E2 (PGE2) as a regulator of beige/bright adipogenesis in white adipose tissue. These studies point to the cyclooxygenase-2/microsomal prostaglandin E synthase 1/PGE2 axis as a key pathway of white to brown conversion in the fat; however caution is in order since this pathway is also involved in inflammatory responses ().
Adipose Gene Expression of the Fastest Growing Chicken
pp 297-303
The broiler chicken is known as the fastest, most efficiently growing species. However while the reduction of white adipose tissue in chickens seems desirable due to the benefits of decreased production costs and increased feed efficiency, the secretions of this adipose tissue have an impact on numerous metabolic and physiological processes. In this research paper, Hausman et al. examine the possible response between the rapid rate of broiler chicken growth and the expression of numerous factors in broiler adipose tissue due to inflammatory response. Using microarray and qPCR analysis the authors confirm numerous gene expression increases (such as TLR2, CCL19, LITAF), demonstrating for the first time age associated changes in immune related gene expression in chicken adipose tissue.
Donor Nutritional Milieu Influences in vitro Adipogenesis
pp 304-13
The diet given to grazing steers is known to alter their fat deposition, with adipose tissue deposition being greater for grain-finished steers when compared to their grass-finished counterparts. The corn grain supplementation given to steers is also known to alter fatty acid composition as well as lipogenic gene expression. Authors Kadegowda, Wright, and Duckett hypothesize that the nutritional environment of stromal vascular cell donor animals may have an effect on the proliferative, adipogenic, and lipogenic capacity of cultured preadipocytes and adipocytes. By comparing preadipocyte and adipocyte cultures from steers both supplemented with corn and without corn grain, the authors found that corn grain supplementation resulted in lower preadipocyte proliferation and decreased adipogenic genes, including AP2. This study underscores the importance of the donor nutritional background when using in vitro differentiated primary adipocyte cultures for optimum results.
Increased Insulin Sensitivity, both Big and Small Adipocytes
pp 314-21
While it was previously thought that there was a positive correlation between adipose cell size and insulin resistance, new research suggests that this relationship is in fact more complex. Whereas at the cellular levels, larger adipocytes are more insulin resistant, at the population level this relationship between adipocyte size and insulin sensitivity was dependent on the metabolic status of the subjects. This research paper by authors Eliasson et al. look at T2D patients and the hypothesis that insulin sensitization may result in an increase of fat-cell size as well as an increase in small adipocyte recruitment. Using the insulin-sensitizer rosiglitazone, the authors found that increased insulin sensitivity not only enlarges existing large adipocytes but also recruits new smaller adipose cells, thus increasing fat storage capacity.
The 2 Degree Difference in Pigs
pp 322-32
Due to its similar cardiovascular physiology and metabolic parameters, the study of metabolic disease and obesity in pigs may serve as a good model for humans. However, the incubation temperature of 37ᵒ C generally used for in vitro cultures of porcine adipocytes is in fact 2ᵒ lower than the body temperature of a pig, which raises several questions with regards to physiological relevance of results obtained from such studies. The research paper presented here by authors Bohan, Purvis, Bartosh and Brandebourg looks into the various effects that culture temperature (37ᵒC versus the normal pig body temperature of 39ᵒC) may have on primary pig preadipocyte proliferation and differentiation, and show that both are actually suppressed at 37ᵒC.
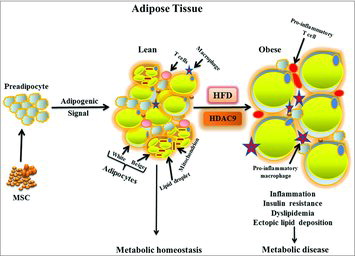
Histone Deacetylase 9 and Adipogenesis
pp 333-8
Impaired adipogenesis observed in diet induced obese (DIO) mice and in obese humans leads to hypertrophic obesity, inflammation and ectopic lipid accumulation in tissues such as the liver and muscle, which ultimately contributes to metabolic derangements including insulin resistance and T2D. In this commentary, Chatterjee et al. expands on their recent findings pointing to histone deacetylase 9 (HDAC9) as a novel signal that impairs adipogenesis during weight gain. Their studies show that HDAC9 expression is increased in adipose tissues of high fat DIO mice, and that genetic deletion of HDAC9 increases adipogenesis of both white and beige adipocytes and leads to improved metabolic homeostasis ().