Abstract
Lenalidomide and its analogs, thalidomide and pomalidomide, specifically inhibit growth of mature B-cell lymphomas, including multiple myeloma, and induce interleukin-2 (IL-2) release from T cells. We recently found that this results from activation of the CRBN-CRL4 E3 ubiquitin ligase to degrade the lymphoid transcription factors IKZF1 (Ikaros) and IKZF3 (Aiolos).
Keywords::
Thalidomide was developed in Germany in the 1950s and 60s as a sedative and was broadly used in Europe as an antiemetic in pregnant women. Tragically, the drug caused severe teratogenicity, primarily limb deformations, that affected more than 10,000 children, and it was removed from the market a few years later. In the 1990s, additional properties of thalidomide were described including selective growth inhibition of multiple myeloma cells and modulation of immune cells. Thalidomide stimulates IL-2 production by co-activated T-cells and inhibits tumor necrosis factor (TNF) release from monocytes. Lenalidomide and pomalidomide were developed as more potent so-called immunomodulatory drugs (IMiDs). More than 50 y after the teratogenicity of thalidomide was first described, and 15 y following the first trials of thalidomide in cancer, the molecular basis of the activity of this class of drugs remained unknown until recently.
Using a lenalidomide derivative immobilized to a bead, we found that lenalidomide binds the CRBN-DDB1-CUL4A-ROC1 E3 ubiquitin ligase (CRBN-CRL4).Citation1 Ito et al. identified the same complex as the target of thalidomide,Citation2 demonstrated that binding of thalidomide inhibited CRBN auto-ubiquitination, and found that the CRBN-CRL4 E3 ligase is responsible for the teratogenicity of thalidomide.Citation2
To identify substrates of the CRBN-CRL4 E3 ligase that mediate the activity of lenalidomide, we applied SILAC (stable isotope labeling of amino acids in cell culture) based quantitative mass spectrometry studies to assess global changes in ubiquitination and protein levels in multiple myeloma cells. Lenalidomide increased the ubiquitination and decreased the protein levels of 2 members of the Ikaros family zinc finger transcription factors, IKZF1 (Ikaros) and IKZF3 (Aiolos), with striking specificity.Citation1 In addition, we found that both IKZF1 and IKZF3 bind the substrate adaptor, CRBN, and that this interaction is increased in the presence of lenalidomide, suggesting that lenalidomide may cause the CRBN-CRL4 E3 ligase to increase ubiquitination of these transcription factors. Subsequent validation experiments in different cell lines and patient samples demonstrated that lenalidomide, thalidomide, and pomalidomide, all cause a decrease in the protein levels of both endogenous and ectopically expressed IKZF1 and IKZF3 but not of the other Ikaros family members IKZF2, IKZF4 and IKZF5. In vitro ubiquitination reactions of IKZF3 co-immunoprecipitated with CRBN revealed that IKZF1 and IKZF3 are direct substrates of the CRBN-CLR4 E3 ligase.Citation1 Two other groups independently found that lenalidomide induces ubiquitination of IKZF1 and IKZF3 by using a near genome-wide library of luciferase-fusion proteins to measure protein stability in 293T cellsCitation3 and ubiquitination profiling in primary human T cells.Citation4
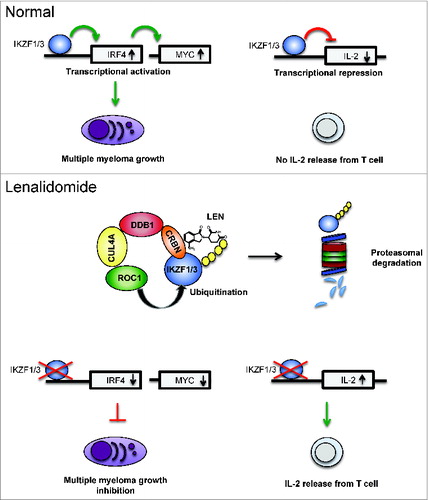
IKZF1 and IKZF3 are central transcriptional regulators of lymphopoiesis.Citation5 We found that IKZF1 and IKZF3 specific shRNAs or expression of an IKZF3 dominant negative mutant inhibited the growth and survival of multiple myeloma cell lines. One of the direct transcriptional targets of IKZF1 and IKZF3 in multiple myeloma is interferon regulatory factor 4 (IRF4), a gene that has recently been implicated in the activity of lenalidomide through transcriptional repression.Citation6 Overexpression of IRF4 conferred partial lenalidomide-resistance to multiple myeloma cell lines, suggesting that it is one of the relevant transcriptional targets of IKZF1 and IKZF3 in this disease. In T-cells, IKZF3 is a transcriptional repressor of IL-2,Citation7 and degradation of IKZF3 by lenalidomide leads to an increase of IL-2 expression and release. ()
Figure 1. Lenalidomide mode of action. The transcription factors IKZF1 and IKZF3 activate transcription of IRF4 which is a transcriptional activator of c-MYC. IKZF1, IKZF3, IRF4 and MYC form a transcriptional network that is essential for multiple myeloma survival and growth. On the IL-2 gene locus IKZF1 and IKZF3 are transcriptional repressors. Lenalidomide promotes ubiquitination of IKZF1 and IKZF3 by the CRBN-CRL4 E3 ligase resulting in their proteasomal degradation. As a consequence of IKZF1 and IKZF3 degradation, IRF4 and MYC transcription decrease resulting in growth inhibition of multiple myeloma cells and de-repression of IL-2 in T cells. Abbreviations: IKZF1 and 3, IKAROS family zinc finger 1 and 3; IL-2, interleukin-2; IRF4, interferon regulatory factor 4.
While IKZF1 and IKZF3 are essential for the survival of multiple myeloma and potentially other mature B-cell malignancies, these proteins are tumor suppressors in acute lymphocytic leukemia (ALL). Deletions, loss-of-function mutations, and dominant-negative mutations of IKZF1 and IKZF3 are common somatic genetic lesions in ALL.Citation8 Mice with a germline dominant negative IKZF1 mutation that also inactivates IKZF3 due to heterodimerization fail to generate mature lymphocytes and develop leukemia with a high penetrance.Citation5 While IKZF1 is expressed throughout lymphocytic maturation, including stem cells and early progenitors, IKZF3 expression is restricted to more differentiated cells. Remarkably, IKZF3 is essential for the generation of plasma cells, the physiologic counterparts of multiple myeloma.Citation9 The effects of IMiDs on lymphocytes are therefore highly consistent with observations made in IKZF1 and IKZF3 knockout mice.
In other cell types, IMiDs likely alter the abundance of different proteins through modulation of the activity of the CRBN-CRL4 ubiquitin ligase. Limb deformations, for example, do not occur with germline genetic inactivation of IKZF1 or IKZF3. It is also unlikely that depletion of IKZF1 and IKZF3 can cause the dramatic therapeutic efficacy of lenalidomide in myelodysplastic syndrome (MDS) patients with del(5q). Similarly, IKZF1 and IKZF3 have not been implicated in the cellular pathways that lead to TNF release in monocytes. Identification of the substrates responsible for each of these biological effects could enable the development of more specific drugs that modify ubiquitination of different sets of proteins with notably fewer side effects.
Insights into the molecular basis of lenalidomide activity raise the possibility of a novel class of therapeutic agents that target CRBN-CRL4 or other E3 ubiquitin ligases to modulate degradation of specific proteins. A biological precedent for this approach, in addition to the IMiDs, is the plant hormone auxin. Like the IMiDs, auxin binds to an E3 ubiquitin ligase, SCF-TIR1, and promotes binding and degradation of the transcription factor Aux/IAA.Citation10 Since both Auxin and IMiDs target transcription factors, a particularly intriguing possibility is that this approach could target other “undruggable” proteins, involved in human diseases. Insights into the structural basis of IMiD binding to CRBN will aid in the rational design of novel small molecule modulators of E3 ubiquitin ligases.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Krönke J, Udeshi ND, Narla A, Grauman P, Hurst SN, McConkey M, Svinkina T, Heckl D, Comer E, Li X, et al. Lenalidomide causes selective degradation of IKZF1 and IKZF3 in multiple myeloma cells. Science 2014; 343:301-5; PMID: 24292625; http://dx.doi.org/10.1126/science.1244851
- Ito T, Ando H, Suzuki T, Ogura T, Hotta K, Imamura Y, Yamaguchi Y, Handa H. Identification of a primary target of thalidomide teratogenicity. Science 2010; 327:1345-50; PMID: 20223979; http://dx.doi.org/10.1126/science.1177319
- Lu G, Middleton RE, Sun H, Naniong M, Ott CJ, Mitsiades CS, Wong KK, Bradner JE, Kaelin WG, Jr. The myeloma drug lenalidomide promotes the cereblon-dependent destruction of Ikaros proteins. Science 2014; 343:305-9; PMID: 24292623; http://dx.doi.org/10.1126/science.1244917
- Gandhi AK, Kang J, Havens CG, Conklin T, Ning Y, Wu L, Ito T, Ando H, Waldman MF, Thakurta A, et al. Immunomodulatory agents lenalidomide and pomalidomide co-stimulate T cells by inducing degradation of T cell repressors Ikaros and Aiolos via modulation of the E3 ubiquitin ligase complex CRL4(CRBN.). Br J Haematol 2014; 164:811-21; PMID: 24328678; http://dx.doi.org/10.1111/bjh.12708
- Winandy S, Wu P, Georgopoulos K. A dominant mutation in the Ikaros gene leads to rapid development of leukemia and lymphoma. Cell 1995; 83:289-99; PMID: 7585946; http://dx.doi.org/10.1016/0092-8674(95)90170-1
- Yang Y, Shaffer AL, 3rd, Emre NC, Ceribelli M, Zhang M, Wright G, Xiao W, Powell J, Platig J, Kohlhammer H, et al. Exploiting synthetic lethality for the therapy of ABC diffuse large B cell lymphoma. Cancer Cell 2012; 21:723-37; PMID: 22698399; http://dx.doi.org/10.1016/j.ccr.2012.05.024
- Gandhi R, Kumar D, Burns EJ, Nadeau M, Dake B, Laroni A, Kozoriz D, Weiner HL, Quintana FJ. Activation of the aryl hydrocarbon receptor induces human type 1 regulatory T cell-like and Foxp3(+) regulatory T cells. Nat Immunol 2010; 11:846-53; PMID: 20676092; http://dx.doi.org/10.1038/ni.1915
- Mullighan CG, Goorha S, Radtke I, Miller CB, Coustan-Smith E, Dalton JD, Girtman K, Mathew S, Ma J, Pounds SB, et al. Genome-wide analysis of genetic alterations in acute lymphoblastic leukaemia. Nature 2007; 446:758-64; PMID: 17344859; http://dx.doi.org/10.1038/nature05690
- Cortes M, Georgopoulos K. Aiolos is required for the generation of high affinity bone marrow plasma cells responsible for long-term immunity. J Exp Med 2004; 199:209-19; PMID: 14718515; http://dx.doi.org/10.1084/jem.20031571
- Tan X, Calderon-Villalobos LI, Sharon M, Zheng C, Robinson CV, Estelle M, Zheng N. Mechanism of auxin perception by the TIR1 ubiquitin ligase. Nature 2007; 446:640-5; PMID: 17410169; http://dx.doi.org/10.1038/nature05731
