Abstract
First-line chemotherapy to combat primary malignant brain cancer is often accompanied by lymphopenic immunologic deficiency. Although counterintuitive, chemotherapy-induced lymphopenia can provide excellent host conditioning that may actually be leveraged to potentiate antitumor immunotherapy. We discuss here our preclinical and clinical experiences applying immunotherapy against glioblastoma, the most common and lethal primary malignant brain tumor, as well as the use of immunotherapeutics in the setting of standard-of-care temozolomide chemotherapy.
Chemotherapy is standard-of-care (SOC) treatment for patients with most types of aggressive and advanced-stage cancers. The agent of choice for the treatment of glioblastoma (GBM), the most common and most lethal primary malignant brain tumor, is temozolomide (TMZ). TMZ is an oral alkylating agent that inactivates the DNA repair enzyme O6-methylguanine-DNA methyltransferase. In a recent Phase III randomized study for newly diagnosed GBM, TMZ combined with external beam irradiation prolonged median survival up to 15 months.Citation1 GBM, however, remains uniformly lethal, creating a desperate need for novel therapies that can be implemented safely alongside current SOC treatments.
To this end, immunotherapy has emerged as a leading strategy targeting malignant disease, especially for resident brain tumors. This approach aims to elicit cancer cell-specific cellular and humoral responses that recognize and eliminate neoplastic cells with exquisite precision. Although immune-retargeting can be accomplished in a number of ways, vaccination and adoptive cell therapy (ACT) represent 2 of the most promising techniques currently being explored in patient trials. The earliest attempts to translate these strategies into the clinic were met with a great deal of skepticism and produced largely unimpressive results. One of the hurdles to achieving ideal efficacy has been the a priori assumption that the implementation of optimal immunotherapy requires an intact immune system—a line of reasoning which has largely precluded patients with GBM due to the immune-compromising side-effects of SOC chemotherapy.
Our increasing knowledge of immune reconstitution following chemotherapy-induced lymphopenia has recently yielded critical information challenging this view. A wealth of evidence has now demonstrated that lymphopenia transiently leads to a reduction of endogenous lymphocytes, which creates a host environment with homeostatic elevations in several cytokines. These critical mediators, such as interleukin (IL) -2, IL-7, IL-15, and B lymphocyte stimulator (BLyS), provide positive signals that potentiate the functionality and clonal expansion of T and B cells, which in turn support reconstitution of the adaptive immune system.Citation2-4 Key signals aimed at restoring the leukocyte compartment also lead to an abundance of antigen presenting cells (APCs), and can trigger critical immunostimulatory pathways via Pattern Recognition Receptors (PRRs) that result in the activation of dendritic cells (DCs), which are professional APCs, as well as the induction of a subset of cytokines, such as IL-12 and type I interferons, which are known to promote strong cellular and humoral responses.Citation5 Lymphodepletion not only precipitates this surge in the availability of homeostatic cytokines, but may also eliminate regulatory cell subsets, which have been shown to directly limit or prevent the antitumor activity of tumor-reactive lymphocytes.Citation4
Remarkably, the processes underlying immune reconstitution can be readily leveraged with immunotherapy to potentiate antitumor responses (). Our experience with TMZ has demonstrated this proof-of-principle for both the cellular and humoral compartments.Citation2,3,6-8 Administration of TMZ depletes host T cells, increases levels of circulating pro-inflammatory cytokines, and reduces regulatory T-cell counts.Citation3,6 In preclinical studies, we have shown that this environment synergizes with immunotherapy by increasing the frequency of adoptively transferred tumor-specific T cells, significantly prolonging the median survival of mice with established tumors in the brain.Citation3,8 Interestingly, this effect was dose-dependent, with enhanced efficacy observed at the highest doses of TMZ, presumably due to a greater degree of host lymphodepletion. Importantly, our clinical data consistently recapitulate this phenomenon, as demonstrated by a Phase II study in which patients with GBM treated with high dose TMZ showed elevated levels of BLySCitation2 that was, in turn, associated with an increase in antigen-specific titers in patients undergoing B cell specific vaccination against the variant III tumor-specific mutation of the epidermal growth factor receptor (EGFRvIII).Citation2 Furthermore, patients conditioned with a higher degree of TMZ-induced lymphopenia displayed a significant increase in progression-free and overall survival,Citation6 exciting findings which have subsequently led to the initiation of an ongoing Phase III international multicenter study (NCT01480479).
Figure 1. Potential mechanisms for enhanced immunotherapeutic outcome during the immune reconstitution phase that follows chemotherapy-induced lymphopenia. (A, B) Systemic chemotherapy may modify the immune milieu of a patient by (B) depleting regulatory cells (e.g., CD4+ CD25+ T cells and myeloid-derived suppressor cells) and endogenous cells that compete for activating cytokines. The removal of these immunosuppressive mechanisms and so called “cytokine sinks” is coupled with the induction of key cytokines geared toward reconstituting the host immune system. (C, D) Taken together, these factors can expand tumor-specific T cells, increase the function and availability of dendritic cells (DCs), stimulate maturation of immature (IM) DCs, and activate B cells to (D) mount an effective antitumor immune response. IL-7 = interleukin-7; IL-15 = interleukin-15; IL-2 = interleukin-2; BLyS = B lymphocyte stimulator; PRRs = pattern recognition receptors; TNFα = tumor necrosis factor α; IFNγ = interferon γ; REG cell = regulatory cell; IM DC = immature dendritic cell; DC = dendritic cell.
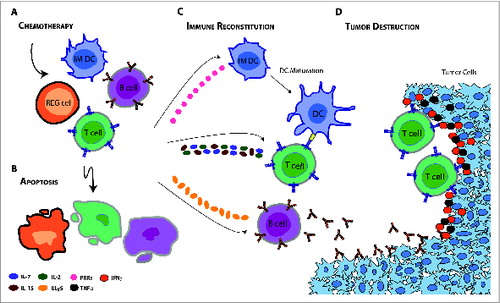
Indeed, there is now considerable evidence to support the finding that lymphopenia—a previously undesired consequence of chemotherapy—may actually synergize, rather than hinder, antitumor immunotherapeutic strategies (). This interplay is being explored for patients with several cancer types, including non-small cell lung cancer (NSCLC) and melanoma. In a recent Phase IIB study,Citation9 patients with NSCLC treated with an experimental vaccine targeting mucin-1 (MUC-1) achieved significantly prolonged progression-free survival in the setting of first-line cisplatin and gemcitabine chemotherapy—results that have propelled ongoing Phase III multicenter studies (NCT01383148). In a separate Phase I/II study of patients with melanoma, first-line chemotherapy dacarbazine has been shown to enhance T-cell responses in patients undergoing peptide vaccination.Citation10 While this vaccine regimen is currently being evaluated in the context of SOC chemotherapy, other immunotherapies are being coupled with experimental chemotherapeutic regimens in the adjuvant setting. Several of these agents, such as cyclophosphamide, paclitaxel, docetaxel, doxorubicin, and 5-fluorouracil, have demonstrated immunomodulatory properties in early Phase I/II studies that have enhanced responses and improved outcomes in patients undergoing vaccination or ACT.Citation4,5 Moreover, host conditioning with high-dose chemotherapy fludarabine and cyclophosphamide has been routinely employed in Phase I/II melanoma trials that evaluate the safety and efficacy of adoptively transferred ex vivo cultured T cells. These studies have been largely based on pioneering work by Rosenberg and colleagues, who have shown that prior lymphodepletive host conditioning may result in prolonged persistence of clonal adoptively-transferred, tumor-reactive lymphocytes, which, in theory, ultimately traffic to tumors and mediate regression.Citation4 Adoptive transfer of tumor-directed T cells has since been extended to a myriad of blood-borne and solid tumors in this context, lending greater credence to its claim as a global principle.
As immunotherapy continues to grow in relevance for clinical use, what was once believed to be detrimental to the efficacy of this strategy may prove not only to be beneficial, but rather optimal for the elicitation of both cellular and humoral responses. We believe that this synergy will allow clinicians to exploit SOC-induced lymphopenia for an enhanced immunotherapeutic approach to eradicate malignant disease.
Work was completed in Durham, NC, USA.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. New Engl J Med 2005; 352:987-96; PMID: 15758009; http://dx.doi.org/10.1056/NEJMoa043330
- Sanchez-Perez L, Choi BD, Reap EA, Sayour EJ, Norberg P, Schmittling RJ, Archer GE, Herndon II JE, Mitchell DA, Heimberger AB, et al. BLyS levels correlate with vaccine-induced antibody titers in patients with glioblastoma lymphodepleted by therapeutic temozolomide. Cancer Immunol Immun 2013; 62:983-7; http://dx.doi.org/10.1007/s00262-013-1405-y
- Sanchez-Perez LA, Choi BD, Archer GE, Cui X, Flores C, Johnson LA, Schmittling RJ, Snyder D, Herndon II JE, Bigner DD, et al. Myeloablative temozolomide enhances CD8(+) T-cell responses to vaccine and is required for efficacy against brain tumors in mice. PloS One 2013; 8:e59082; PMID: 23527092; http://dx.doi.org/10.1371/journal.pone.0059082
- Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer 2008; 8:299-308; PMID: 18354418; http://dx.doi.org/10.1038/nrc2355
- Zitvogel L, Galluzzi L, Smyth MJ, Kroemer G. Mechanism of action of conventional and targeted anticancer therapies: reinstating immunosurveillance. Immunity 2013; 39:74-88; PMID: 23890065; http://dx.doi.org/10.1016/j.immuni.2013.06.014
- Sampson JH, Aldape KD, Archer GE, Coan A, Desjardins A, Friedman AH, Friedman HS, Gilbert MR, Herndon JE, McLendon RE, et al. Greater chemotherapy-induced lymphopenia enhances tumor-specific immune responses that eliminate EGFRvIII-expressing tumor cells in patients with glioblastoma. Neuro-Oncol 2011; 13:324-33; PMID: 21149254; http://dx.doi.org/10.1093/neuonc/noq157
- Sampson JH, Schmittling RJ, Archer GE, Congdon KL, Nair SK, Reap EA, Desjardins A, Friedman AH, Friedman HS, Herndon II JE, et al. A pilot study of IL-2Ralpha blockade during lymphopenia depletes regulatory T-cells and correlates with enhanced immunity in patients with glioblastoma. PloS One 2012; 7:e31046; PMID: 22383993; http://dx.doi.org/10.1371/journal.pone.0031046
- Mitchell DA, Cui X, Schmittling RJ, Sanchez-Perez L, Snyder DJ, Congdon KL, Archer GE, Desjardins A, Friedman AH, Friedman HS, et al. Monoclonal antibody blockade of IL-2 receptor alpha during lymphopenia selectively depletes regulatory T cells in mice and humans. Blood 2011; 118:3003-12; PMID: 21768296; http://dx.doi.org/10.1182/blood-2011-02-334565
- Quoix E, Ramlau R, Westeel V, Papai Z, Madroszyk A, Riviere A, Koralewski P, Breton JL, Stoelben E, Braun D, et al. Therapeutic vaccination with TG4010 and first-line chemotherapy in advanced non-small-cell lung cancer: a controlled phase 2B trial. Lancet Oncol 2011; 12:1125-33; PMID: 22019520; http://dx.doi.org/10.1016/S1470-2045(11)70259-5
- Nistico P, Capone I, Palermo B, Del Bello D, Ferraresi V, Moschella F, Arico E, Valentini M, Bracci L, Cognetti F, et al. Chemotherapy enhances vaccine-induced antitumor immunity in melanoma patients. Int J Cancer J Inter du Cancer 2009; 124:130-9; PMID: 18839429; http://dx.doi.org/10.1002/ijc.23886
