Abstract
The generation and loading of dendritic cells (DC) ex-vivo for tumor vaccination purposes is laborious and costly. Direct intradermal (i.d.) administration of tumor-associated antigens could be an attractive alternative approach, provided that efficient uptake and cross-presentation by appropriately activated skin DCs can be achieved. Here, we compare the efficiency of i.d. delivery of relatively small apoptotic blebs (diameter ∼0.1–1 μm) derived from MART-1 transduced acute myeloid leukemia (AML) HL60 cells, to that of larger apoptotic cell remnants (ACR; 2–10 μm) in a physiologically highly relevant human skin explant model. Injection of either fluorescently-labelled ACRs or blebs alone did not affect the number or distribution of migrated DC subsets from skin biopsies after 48 hours, but resulted in a general up-regulation of the co-stimulatory molecules CD83 and CD86 on skin DCs that had ingested apoptotic material. We have previously shown that i.d. administration of GM-CSF and IL-4 resulted in preferential migration of a mature and highly T cell-stimulatory CD11hiCD1a+CD14− dermal DC subset. Here, we found that co-injection of GM-CSF and IL-4 together with either ACRs or blebs resulted in uptake efficiencies within this dermal DC subset of 7.6% (±6.1%) and 19.1% (±15.9%), respectively, thus revealing a significantly higher uptake frequency of blebs (P < 0.02). Intradermal delivery of tumor-derived blebs did not affect the T-cell priming and TH-skewing abilities of migratory skin DC. Nevertheless, in contrast to i.d. administration of ACR, the injection of blebs lead to effective cross-presentation of MART-1 to specific CD8+ effector T cells. We conclude that apoptotic bleb-based vaccines delivered through the skin may offer an attractive, and broadly applicable, cancer immunotherapy.
Abbreviations:
- 4/GM, IL-4 and GM-CSF
- ACR, apoptotic cell remnant
- AML, acute myeloid leukemia
- CFSE, carboxyfluorescein succinimidyl ester
- DC, dendritic cell
- DDC, dermal DC
- GM-CSF, granulocyte-macrophage colony-stimulating factor
- HLA, human leukocyte antigen
- HSCT, hematopoietic stem cell transplantation
- i.d., intradermal
- IFN, interferon
- Ig, immune globulin
- IL, interleukin
- LC, Langerhans cell
- LN, lymph node
- MART-1/melan-A, melanoma antigen recognized by T cell 1
- MLR, mixed leukocyte reaction
- MoDC, monocyte-derived dendritic cell
- TAA, tumor-associated antigen
- TH, T Helper
- TLR, Toll-like receptor
- TNFα, tumor necrosis factor α
Introduction
The culture and loading of dendritic cells (DCs) ex vivo for their preparation as an antitumor vaccine is both laborious and costly. Circumventing these caveats by direct administration and DC targeting of tumor-associated antigens (TAAs) in vivo is consequently of great interest. The skin is the most commonly used portal for the delivery of antitumor vaccines, since a dense network of DCs lining the skin is readily accessible and adjuvants can be more safely co-administered as compared to intravenous injection.Citation1 Migratory dermal DCs (DDC) and Langerhans cells (LCs) that reside in the dermis and epidermis respectively, are able to mediate both T- and B-cell responses in humans.Citation2,3 Therefore, targeting skin DCs via direct intradermal (i.d.) injection of whole tumor cell vaccines presents an attractive and clinically feasible cancer vaccination methodology. We, and others, have focused on characterizing human DC subsets present in the skin and draining lymph nodes (LN).Citation4-7 At least 4 DDC subsets and LCs have been identified in the skin, including both the epidermis and underlying dermal layer, to date, which can be discriminated based on their differential expression profiles of various markers, including but not limited to CD1a, CD11c and CD14.Citation4,8 It has been previously shown that intradermal injection of interleukin 4 (IL4) and granulocyte macrophage colony stimulating factor (GM-CSF), which are present in the skin under inflammatory conditions, are capable of inducing LC and DDC maturation, as well as LC differentiation,Citation9–11 Moreover, injection of IL-4 and GM-CSF in human skin results in the preferential migration of CD1a+CD14− DDCs from human skin explants, and subsequent ex vivo culture has demonstrated that this DDC subset is a potent inducer of TH and CD8+ effector T cells.Citation11-13 Indeed, under steady state conditions CD1a+ LCs and DDCs are the most mature DC subsets displaying a T-cell stimulatory phenotype.Citation12 From our prior observations, we have concluded that TAAs administered via i.d. injection should be selected to preferentially target CD1a+CD14− DDCs as these represent the most frequent skin DC subset with superior T-cell activation qualities, and as such, are thus likely to be the most potent in inducing tumor-specific T-cell responses.Citation1,12,13
Malignant cells have multiple ways of evading immune recognition, one of which is de-differentiation,Citation14 often accompanied by a loss of TAA expression, suggesting that broadening the tumor-directed immune response by increasing the array of presented TAAa is likely to enhance the effectiveness of antitumor vaccination strategies. Most immunotherapeutic strategies that target a single TAA, rely on the characterization of the selected TAA, have limited inter-patient antigenic overlap and are often HLA-restricted. Using apoptotic whole tumor cell preparations could be instrumental in circumventing these limitations, since it broadens the presented array of TAAs, can be patient-specific (provided that there is access to sufficient autologous tumor material) and overcomes HLA restriction. Moreover, novel T-cell epitopes (both HLA Class I and Class II), which are continually being mapped within known TAA antigens,Citation15,16 are included in whole apoptotic tumor cell preparations and, thus, further increase the number of targeted antigens and may enhance therapeutic efficacy. Finally, with the growing recognition of the immunogenicity of neo-epitopes specific to each tumor's individual mutantome,Citation17 autologous tumor-based vaccines deserve renewed attention as a means of effective personalized immunotherapy.
Apoptosis is a tightly regulated process,Citation18-20 during which microvesicles are shed, referred to as blebs and typically ranging in size from 0.1–1 μm. Blebs have been shown to induce DC maturation in mice,Citation21 and importantly, in cross-priming experiments, we have shown that monocyte-derived DC (MoDC) loaded with blebs, more efficiently cross-present TAA to CD8+ T cells as compared to MoDC loaded with larger apoptotic cell remnants (ACRs) of sizes ranging from 2–10 μm.Citation22 Most reported studies assessing the pre-clinical efficacy of whole apoptotic tumor cell vaccines lack detailed descriptions of isolated apoptotic cells and their vesicle by-products, and since the isolation of these microvesicles requires an additional centrifugation and isolation step, the fraction enriched for the larger ACRs, also referred to as apoptotic bodies, have been used in most, if not all, previous studies. The loading of DCs with ACRs has been shown to induce tolerance and to impair DC function,Citation23-25 thus necessitating the use of powerful immunostimulatory adjuvants. These findings led us to investigate whether isolated apoptotic blebs could be a preferred source of TAAs over ACRs for the induction of tumor-directed immunity through dermal vaccination. To this end, we comparatively analyzed the efficacy of ACR versus bleb ingestion by human DDCs and LCs in situ, and their subsequent ability to activate (antigen-specific) T cells post-migration in vitro, using a near-physiological human skin explant model.Citation11,19
Results
Administration of apoptotic cell remnants or blebs does not affect the absolute number or distribution of migrated skin DCs
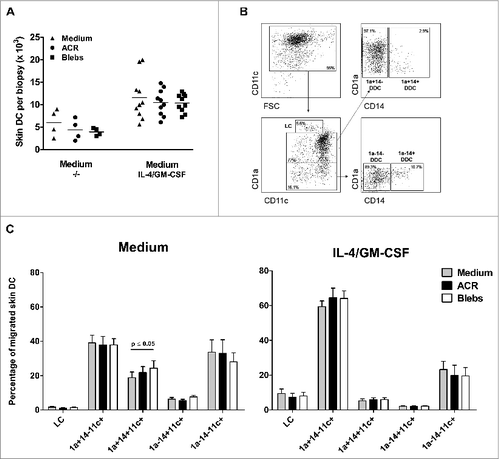
It has been previously demonstrated that i.d. co-administration of IL-4 and GM-CSF (referred to as 4/GM) stimulates the maturation and increased migration of LCs and DDCs from cultured skin explants.Citation11,27 Here, we comparatively analyzed the effects of administering ACR or apoptotic blebs on the absolute number of cells migrating from the skin explants, both in the absence and presence of 4/GM. As expected, co-administration of 4/GM led to an increase in the migration of the absolute number of DCs in all conditions (). Co-injecting either ACRs or blebs did not affect the absolute number of egressed DDC in absence or presence of 4/GM. Qualitative examination of skin-emigrated DC subsets was performed by the analysis of CD11c, CD1a and CD14 expression using flow cytometry () and compared the distribution of the 4/GM-induced migrated DC subsets after injection with ACRs or blebs (). Injecting blebs in plain medium induced a minor but significant increase in the relative migration of CD1a+CD14+ DDCs, compared to injection of plain medium or ACR respectively, but this effect was abrogated after co-administration of 4/GM (). The increased and preferential migration of CD1a+CD14− DDCs and LCs by i.d. administration of 4/GM, which was consistent with previous observations,Citation12 was not affected by co-injection of either ACRs or blebs ().
Figure 1. Intradermal administration of apoptotic blebs or apoptotic cell remnants does not affect the number and subset distribution of migratory DC. (A–C) Analysis of skin emigrated dendritic cells (DCs) following injection of medium, apoptotic cell remnants (ACR) or apoptotic blebs. Cultured human skin explants were intradermally injected with leukemic cell-derived blebs or ACR either alone (in plain medium) or co-administered with of IL-4/GM-CSF. A. DCs that egressed were harvested and counted after 48 hours post injection: Medium (−/−), n = 4; IL-4/GM-CSF, n = 9. (B) Egressed DCs were harvested and immunostained for CD11c, CD1a and CD14 and analyzed via fluorescence cytometry in order to discriminate LCs and the 4 dermal DC subsets, as indicated. (C) Skin DC subset distribution was analyzed after injecting either medium alone (grey bars), ACR (black bars), or blebs (white bars) in plain medium (left graph; n = 5), or supplemented with IL-4/GM-CSF (right graph; n = 5). Shown are the mean values ± SEM. Statistical significance was determined by one-way Anova.
Blebs are taken up more efficiently by DDCs after co-administration of IL-4 and GM-CSF
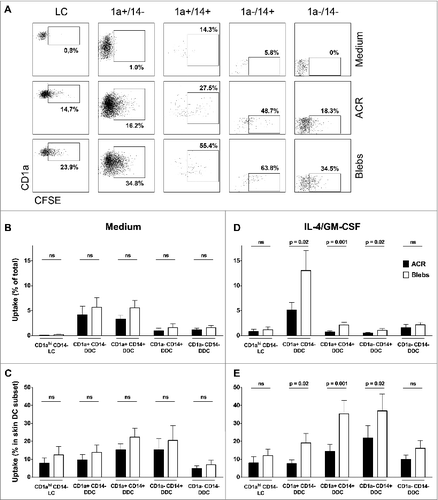
In order to analyze the ability of skin-emigrated DCs to ingest apoptotic cell material in situ, we injected carboxyfluorescein succinimidyl ester (CFSE)-labeled ACRs or blebs and analyzed the egressed DC subsets by flow cytometry for CFSE positivity, as a measure of uptake (). Next, we quantified the CFSE-positive cells per subset as a percentage of the total fraction of migrated CD11c-positive DCs, as well as the percentage within a given subset ( and , respectively). When injected in plain medium, both apoptotic cell fractions were taken up by all DC subsets to some extent (mean 12.9% ± 11.6% see ). Interestingly, co-administration of 4/GM led to an increase in the uptake of apoptotic blebs over the entire range of DC subsets (). Most notably, despite representing a minor fraction of migrated CD11c-positive DCs under these conditions, co-administration of 4/GM led to a significant increase in the CD14+ DDC subsets uptake of CFSE-labeled blebs over ACRs by the (, respectively), indicative of their superior endocytic capacity. Most importantly, blebs were also ingested by a significantly higher percentage of the predominant migratory CD1a+CD14− DDC subset (mean 19.1% ± 15.9%) as compared to ACRs (mean 7.6% ± 6.1%; ). Indeed, CFSE-positive CD1a+CD14− DDC constituted on average 13.0% (±12.1%) of the total skin-egressed DC after i.d. injection of blebs, as compared to 5.1% (±4.5%) after injection of ACRs ().
Figure 2. Quantification of the in situ uptake of apoptotic material by skin-emigrated dendritic cells. (A–E) Carboxyfluorescein succinimidyl ester (CFSE)-labeled apoptotic cell remnants (ACR) or blebs were injected into human skin explants, and the dendritic cell (DC) uptake was quantified using immunostaining and fluorescence cytometry. (A) Viable CD11c positive DCs were plotted against CD1a to discriminate Langerhans cells (LCs) as CD1ahi/CD11cdim. CD1a+ and CD1a− DCs were plotted against CD14 for the discrimination of the various dermal dendritic cell (DDC) subsets –as indicated. (B–E) For the analysis of the in situ uptake of CFSE-labeled apoptotic material, CFSE positivity of the egressed skin DC was determined by flow cytometry (indicated by percentages positive cells per subset; representative example from n = 9 shown). Quantification of the ingestion of apoptotic cell remnants (ACR, black bars) or blebs (white bars) in situ, is either displayed as the CFSE positive skin DC as a percentage of the total CD11c+ egressed skin DC (B and D) or as a percentage of CFSE positive cells within a subset (C and E). The apoptotic cell fractions were administered either in plain medium (B and C, n = 6) or with IL-4 and GM-CSF (D and E, n = 9). Shown are the mean values ±SEM. Statistical significance was determined by 2-tailed Student's t-test.
Increased maturation of DDCs after ingestion of ACRs or blebs
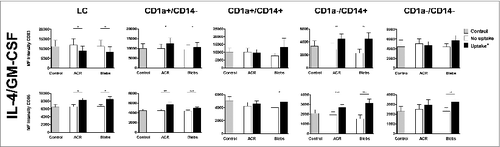
To determine whether the ingestion of apoptotic material (in the presence of 4/GM) by skin-emigrated DCs led to alteration in the expression of maturation markers, we analyzed egressed DCs for the expression of CD83 and CD86. Surprisingly, ingestion of apoptotic material by LCs significantly increased CD86 expression, but coincided with a significant down-regulation of CD83. In contrast, the expression of both CD86 and CD83 on the surface of CD1a+CD14− DDCs was increased when this subset had taken up either ACRs or blebs. (). Ingestion of apoptotic material by CD1a−CD14− led to minor changes in the expression of co-stimulatory molecules and only CD86 expression was significantly increased after ingestion of blebs (). Encouragingly, uptake of both ACR and blebs by CD1a−CD14+ DDC also induced a significant increase in both CD83 and CD86 expression on the cell surface of this otherwise immature and potentially immunosuppressive subset.
Figure 3. Effect of intradermally injected apoptotic remnants or blebs on maturation state of DC subsets subsequently emigrated from skin. Expression of the maturation markers CD83 and CD86 on the different DC subsets, that egressed from the skin explants after intradermal injection of carboxyfluorescein succinimidyl ester (CFSE)-labeled apoptotic cell remnants (ACR) or blebs co-injected with IL-4/GM-CSF versus media. Expression levels of the differentiation markers were determined by immunostaining and fluorescence cytometry. CD83 (top row) and CD86 expression is shown on DC which had ingested apoptotic material (black bars), and DC that had not taken up either ACR or blebs (white bars), as compared to background levels after injecting medium alone (grey bars). Shown are the mean values ± SEM (n = 9). Statistical significance was determined by 2-tailed Student's t-test; * P-value < 0.05; ** P-value < 0.01; *** P-value < 0.001.
Intradermal bleb or ACR administration does not interfere with T-cell induction and cytokine expression profiles
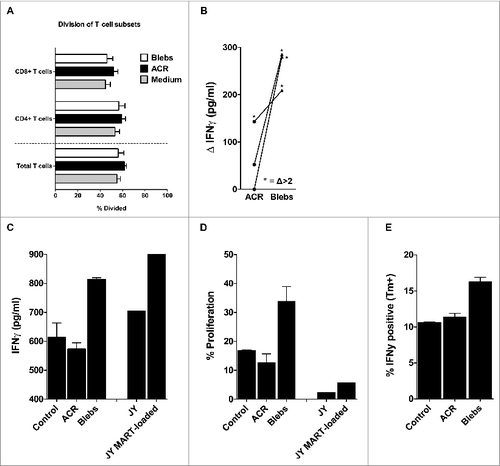
The effect on the cytokine balance in the dermal milieu after i.d. injection of ACRs or blebs was determined by analyzing the skin explant culture medium for the presence of the inflammation-related cytokines IL1β, IL-6, IL-8, IL-10, IL12p70 and tumor necrosis factor α (TNFα), 48 to 72 hours after injection. Although we were able to detect all measured cytokines to a larger or lesser extent (with the exception of TNFα), we observed no significant differences after intradermal delivery of ACR or blebs, either in plain medium or supplemented with 4/GM (data not shown). After i.d. injection of either ACR or blebs in 4/GM-supplemented medium, skin DC that egressed from the biopsies were co-cultured for 6 days with CFSE-labeled allogeneic peripheral blood lymphocytes. In order to analyze their capacity to prime T-cell responses, apoptotic fragment-induced T-cell proliferation was determined by CFSE dilution. Our results showed that the ability of the migrated DC to induce either CD4+ or CD8+ T cells was unaffected by i.d. injection of either blebs or ACRs (), nor was their T helper (TH) skewing ability, as determined by the analysis of T-cell cytokine release (IL-2, IL-4, Il-6, IL-10, IL-17a, IFNγ and TNFα) after 6 days of co-culture (data not shown).
Figure 4. Mixed leukocyte reaction and antigen cross-presentation by egressed skin DCs after intradermal delivery of apoptotic cell remnants or blebs. (A) After 48–72 hours following intradermal injection of the indicated apoptotic cell fractions, the egressed dendritic cells (DCs) were harvested and co-cultured for 6 days with carboxyfluorescein succinimidyl ester (CFSE)-labeled allogeneic peripheral blood lymphocytes (PBLs) from healthy donors, in an allogeneic mixed leukocyte reaction (MLR) at a 1:10 ratio of DCs:PBLs. Shown are the mean percentages of divided PBLs after 6 days of co-culture, with the percentage of CFSE dilution utilized as a measure of CD4+ and CD8+ T-cell proliferation (n = 7). (B) DCs that egressed from the skin biopsies after injecting either wild-type or MART-1 expressing apoptotic cell remnants (ACRs) or blebs, were co-cultured with a MART-126-35 recognizing and HLA-A2 restricted cytotoxic T lymphocyte (CTL) line (> 95% pure) for 24 hours. Antigen-specific activation was determined by deducting the levels of interferon γ (IFNγ) produced following the injection of wild-type ACRs or blebs from the levels produced after injecting MART-1 expressing apoptotic fractions (n = 3). Antigen-specific induction of IFNγ levels exceeding those achieved by cross-presentation of wild-type ACR or blebs by more than 2-fold are indicated by asterisks. (C–D) Emigrated skin DCs stimulated by injecting either ACR or blebs derived from MART-1 expressing HL60 were co-cultured for 24 hours with a CFSE-labeled MART-1 specific cytotoxic T lymphocyte (MART-1 CTL) clone generated by stimulation with MART-1 loaded JY cells. IFNγ production was analyzed in the supernatant by cytokine bead array after 24 hours (C; n = 1) and proliferation, as measured by CFSE dilution (as in A, above), after 6 days (D; n = 1). (E) IFNγ production by a MART-1 CTL line (> 95% pure) as assessed by intracellular immunostaining and cytofluorimetric analysis, after 5 hours of co-culture with skin DCs, that had migrated from ACR- or bleb- (derived from MART-1 expressing HL60) injected skin biopsies (n = 1). Shown are the mean values ± SEM.
Dermal delivery of blebs leads to effective cross-presentation by migratory DC
Next, we set out to assess the ability of skin-emigrated DC to cross-present antigens derived from blebs or ACR. To this end, either MART-1-transduced or wild type HL60- or K562-derived ACR or blebs were i.d. injected in skin explants from HLA-A2 positive donors in the presence of 4/GM, and skin DC were harvested after 48-72 hours. After a subsequent 24 hours of co-culture with a MART-126-35 recognizing CTL line (HLA-A2 restricted; >95% pure), the supernatant was analyzed for the presence of IFNγ, as a measure of cross-presentation of the MART-126-35 epitope by egressed DC. The MART-126-35 specific IFNγ production was assessed by deducting the IFNγ production following a co-culture with DC from wild-type ACR or blebs injected skin, from that secreted after co-culturing with DC from MART-1-transduced ACR or blebs (Δ IFNγ, ). In 3 separate donors we found the IFNγ production after co-culture with skin DCs migrated from bleb-injected skin explants to be 2- to 3-fold elevated as compared to DCs egressing from ACR-injected skin (on average Δ 258 pg/mL versus. Δ 65 pg/mL, ). Moreover, in all donors the IFNγ production after injecting MART-1-containing blebs was at least 2-fold increased over the IFNγ production when injecting wild-type blebs (mean 13.2-fold increased), whereas this was the case in only one donor following ACR injection (mean 4.7 fold increased, ). We were able to confirm these findings in 2 additional HLA-A2 positive donors (). In brief, using a MART-1-reactive CTL clone Citation28 we detected elevated IFNγ production () particularly in response to MART-1 loaded blebs. Moreover, we could demonstrate expansion of the MART-1 CTL following a co-culture with skin DC that egressed from the biopsies injected with blebs, as compared to DC from medium or ACR-injected skin (). These findings were further validated by intracellular IFNγ staining of a MART-1 CTL line co-cultured with emigrated DCs, corroborating the enhanced cross-presentation of antigen from intradermal administration of apoptotic blebs versus ACRs or medium ().
Discussion
The involvement of the immune system in controlling leukemia, as well as in constraining solid tumor development, has been well established. Thus, efforts to shape immune responses to target (residual) tumor cells have been a therapeutic goal pursued by researchers and clinicians for many decades. The induction of tumor-directed immunity by means of DC vaccination has been intensely studied. Unfortunately, the ex vivo generation of clinical grade DCs and the subsequent loading with tumor-associated antigens is costly and laborious, hindering wide spread, clinical applications of DC-based vaccination strategies. Moreover, there is growing evidence that, in order to eradicate tumors and prevent relapse, a broad spectrum of TAAs should be targeted.Citation29 Previously, we reported on apoptotic cell-derived blebs as a potent source of TAAs for ex vivo generated DC-based vaccines.Citation22 Here, we used a human skin explant model to explore the relative efficiency of loading skin resident DCs with either ACRs or blebs in situ as a tumor cell-based vaccine delivery method. We found i.d. injected blebs to be more efficiently taken up by cytokine-activated skin-resident and migratory DCs. Moreover, upon migration of these DCs from the skin, TAAs derived from blebs were more efficiently cross-presented to CD8+ effector T cells than those derived from i.d. injected ACRs. These observations support the dermal delivery of bleb-based vaccines as a means of inducing a broad tumor-reactive T-cell response.
In order for antigen-specific T-cell responses to be induced after i.d. administration of an apoptotic cell-based vaccine, it is essential that antigen is taken up by DDCs or LCs (both able to induce T- and B-cell responsesCitation2,3). The capacity of human DDCs to ingest apoptotic material after ex vivo culture has been demonstrated previously,Citation30 and furthermore LCs have been demonstrated to engage apoptotic keratinocytes.Citation31 Studies administering apoptotic cells for vaccination purposes have suggested apoptotic cells to be taken up by skin resident DCs, but to our knowledge this is the first demonstration that i.d. injected apoptotic material is taken up by migratory human DDCs and LCs. Interestingly, and in contrast to the dogma of reduced exogenous antigen uptake after inducing DC maturation, we observed enhanced uptake of blebs by all migratory DC subsets after co-administration of 4/GM, whereas the uptake was unaffected or even decreased by this cytokine cocktail when ACRs were injected. As CD1a+CD14− DDCs constitute the predominant subset migrating from the skin after administration of 4/GM and have been previously identified as the most potent in terms of effector T-cell activation,Citation11,12 preferential uptake of blebs by this subset may prove favorable in terms of vaccination efficacy. It has recently been described that CD14+ DDCs are far less potent in effectively priming CD8+ T cells to differentiate into cytotoxic effector cells, as compared to CD1a+ DDCs and LCs, and moreover induce regulatory T cells, a process driven by the production of IL-10 and the expression of Ig-like transcript inhibitory receptors.Citation32-34 Interestingly, next to the predominant migration of CD1a+ DDCs and a significant increase in uptake by this potent DDC subset, injecting 4/GM greatly diminished the migration of CD14+ DDCs as compared to injecting plain medium (7.4% vs. 25.1% respectively; P = 0.005), confirming our previous observations.Citation11,12
Effective T-cell priming requires skin DCs to migrate from the skin to the draining lymph nodes (LNs) after the ingestion of exogenous antigen. Whether the observed migration from the skin explants represents migration towards the LN is unclear, but we and others have shown that CD1a+ skin DCs express the LN-homing chemokine receptor CCR7 and that migrated LC and CD1a+ DDC are potent activators of T cells in vitro.Citation11,13,32 Moreover, both of these skin-derived DC subsets have been identified in skin-draining lymph nodes.Citation4 Injection of either ACRs or blebs (and subsequent ingestion) did not impair skin DC migration from the skin explants, nor their ability to subsequently activate T cells in vitro. Importantly, we demonstrated that MART-1 from blebs was efficiently cross-presented to MART-1 specific CD8+ cytotoxic T cells, whereas MART-1 from ACRs was cross-presented to a far lesser extent. Moreover, we showed a clear expansion of the MART-1 CTL following induction with DCs that had egressed from bleb-injected skin. Clearly, this is a strong argument for the preferred use of blebs over ACR as tumor vaccine.
The modest increased uptake of blebs compared to ACR (14.8% versus 9.2%, respectively), and thereby the higher number of MART-126–35 presenting skin DCs, is unlikely to be solely responsible for the selective activation of the TAA-specific CD8+ CTLs. It is known that heat shock proteins (HSPs), previously described to be present at high levels in apoptotic blebs,Citation35 greatly facilitate cross-presentation.Citation36 In our system, HSPs could, therefore, have been responsible for the observed enhancing effect on cross-presentation mediated by bleb-administered DCs. An alternative mechanism underlying the increased cross-presentation potential of blebs is the presence of defective ribosomal products, which are present in the endoplasmic reticulum (an organelle actively translocated into blebsCitation20), potential TAAs that have been described to be efficiently cross-presented.Citation37,38 However, this concept is speculation, as we currently have no data to support this hypothesis. In any case, bleb-derived antigen is clearly efficiently ingested and cross-presented by skin DC and direct i.d. administration of tumor-derived blebs can thus be regarded as a potent immunotherapeutic strategy.Tumors create immunosuppressive (micro)environments, which is evident by the preferential migration of CD14+ DDCs from explants taken from the skin proximate to the tumor of breast cancer patients.Citation12 This effect is most likely mediated by IL-10, as co-culture of IL-10-conditioned skin emigrated DC with T cells leads to a diminished T-cell proliferation, TH2 skewing, an increase in regulatory T cells, and a reduced priming of functional cytotoxic T cells.Citation12,32-34 The negative modulation of the skin surrounding the tumor area, or even systemically under advanced metastatic conditions, can thereby hamper i.d. vaccination strategies.39Counteracting this negative regulation is likely to be crucial, and can be accomplished by co-administering GM-CSF alone,Citation40 or combined with IL-4.Citation11 Moreover, treatment with CpG and/or Toll-like receptor (TLR) ligands (especially peptidoglycan, a TLR2 ligand) have been shown to greatly enhance the human skin DC maturation state and T-cell activating properties.Citation27,41 Next to creating a beneficial milieu for inducing tumor-directed immunity, specific or enhanced targeting of TAAs to DCs has shown beneficial effects, for example by conjugating TAA to TLR ligands,Citation42 or generating antibody-antigen complexes.Citation43 These strategies may also be applied to apoptotic bleb-based vaccines.
Standard therapy for advanced leukemia patients, and those afflicted with other types of cancer, often consists of chemotherapeutic regimens. Since the skin can be affected by chemotherapy, there is a possibility that skin DCs are affected as well, in which case the vaccination efficacy of direct administration of blebs in the skin is likely to be hampered. Whether this occurs and the kinetics of DC reconstitution in the skin after chemotherapy or, in the case of leukemia, following hematopoietic stem cell transplantation (HSCT), remains largely unclear. However, a small number of studies has analyzed the presence of DCs in the skin after chemotherapeutic conditioning, demonstrating that DCs can be detected in the skin even at early time points (e.g., 18 d).Citation44,45 Moreover, we have reported improved migration of activated DCs and normalized distribution of migrating cutaneous DC subsets (i.e., predominant migration of mature CD1a+ DDC and reduced migration of immature macrophage-like CD14+ DDC) after neoadjuvant chemotherapy in patients with breast cancer.Citation12 These data suggest that it is indeed possible to target skin DCs within approximately 3 weeks following chemotherapy and HSCT. In an autologous setting, vaccination using blebs will be primarily applicable to the treatment of leukemias, as tumor cells are readily available in large numbers in the bone marrow and blood. However, in an allogeneic setting bleb-based vaccines could also be applied to solid tumors (e.g., by generating blebs from cell lines).
In conclusion, we have demonstrated that intradermally administered apoptotic cell-derived blebs are efficiently ingested by mature skin DC subsets, which subsequently retain their ability to migrate from the skin, efficaciously cross-present TAA, and support subsequent priming and expansion of effector T cells, without detrimental skewing of the cell-mediated response. By co-administering immunostimulatory agents (e.g., 4/GM, CpG, and TLR ligands) tumor-enforced immunosuppression may be counteracted and the T cell stimulatory capacity of skin DCs greatly enhanced.Citation11,12,27,46 The data presented here, combined with our previous data,Citation22 present a strong case for the use of blebs as potent source of TAAs for the induction of tumor-directed immunity through dermally applied vaccines.
Materials and methods
Apoptotic cell fractions
ACR and blebs were isolated as described previously.Citation22 MART-1 expressing HL60 (HLA-A2−, HLA-A3+), or K562 chronic myeloid leukemia (HLA class I negative) cell lines were generated by retroviral transduction of LZRS-MART-1-IRES-ΔNGFR, also as previously reported.Citation28 The MART-1 expressing cells were spun down at 500 g for 5 minutes, resuspended at 5 × 106 cells/mL in RPMI 1640 (Gibco, Paisley, UK), after which apoptosis was induced by incubating the cells for 2 hours at 42°C, as previously described.Citation47 Next, the cells were irradiated with 50 Gray, washed, and resuspended at 2 × 106 per mL in RPMI containing 10% FCS, 100 IU/mL penicillin and 100 μg/mL streptomycin (all Gibco, Paisley, UK). After 72 h of culture at 37°C and 5% CO2, ACR were pelleted by centrifugation at 600 g and 4°C for 10 min, after which the apoptotic blebs were isolated from the resulting supernatant by centrifugation at 4,000 g at 4°C for 10 min. ACR and blebs were washed twice with PBS, after which the protein concentration was determined; the fractions were lysed using 5 cycles of sonication (5 sec pulse, followed by 5 sec rest) at 18 micron (Sanyo MSE Soniprep 150, Amsterdam, The Netherlands) and the protein concentration was determined using a ND-1000 Nanodrop spectrophotometer (Thermo Fisher Scientific, Breda, The Netherlands). The apoptotic cell fractions were frozen for future use. Prior to usage, ACR and blebs were thawed and washed twice with PBS (with all centrifugation steps at 600g and 4000g for ACR and blebs respectively), after which the fractions were resuspended at an equivalent protein concentration and used directly, or fluorescently labeled with 1 μM carboxyfluorescein succinimidyl ester (CFSE; Invitrogen, Breda, The Netherlands) according to the manufacturer's protocol, and washed with Iscove's modified Dulbecco's medium (IMDM; Gibco) containing 10% FCS, and subsequently with plain IMDM. The preparations were resuspended and 40 μg protein equivalent was injected per skin explant (20 μL per explant), either in plain IMDM or IMDM supplemented with GM-CSF (100 ng/biopsy; Berlex Laboratories Montville, NJ), and IL-4 (10 ng/biopsy; R&D Systems, Minneapolis, MN).
Skin explants and dermal dendritic cell subset analysis
Human abdominal skin explants were obtained after informed consent from patients undergoing corrective breast or abdominal plastic surgery at the Tergooi hospital (Hilversum, The Netherlands) and used in an anonymous fashion in accordance with the “Code for Proper Use of Human Tissues” as formulated by the Dutch Federation of Medical Scientific Organizations (www.fmwv.nl) and following procedures approved by the institutional review board. Skin samples were used within 24 h after surgery. Per explant, a volume of 20 μL was injected i.d. using a 29G BD Micro-Fine insulin syringe (0.33-mm × 12.7-mm needle). 6 mm punch biopsies were taken from the 5 mm injection sites (20–25 explants were taken per condition). Explants were lifted from the skin specimen, cut from the hypodermis, and subsequently cultured in IMDM containing 50 U/mL penicillin-streptomycin, 1.6 mM L-glutamine, and 5% heat-inactivated normal human pooled serum (referred to as skin medium). After 48 to 72 h the spontaneously migrated cells were harvested, counted and used for subsequent experiments. The biopsy culture medium was frozen and stored for cytokine analysis. Cytokines in the biopsy culture medium were analyzed using an inflammation cytokine bead array (BD Biosciences, Breda, The Netherlands), following manufacturer's protocol. Cells that had egressed from the biopsies were immunostained as previously describedCitation11 using fluorophore-conjuged antibodies (all from BD Pharmingen) for CD11c (APC), CD1a (PE), CD14 (PerCP), CD83 (PC7), CD86 (V450). Cytofluorimetric analysis was performed using a BD LSRFortessa™ flow cytometer and BD FACSDiva™ software version 6.3 (BD Biosciences).
Mixed leukocyte reaction
Migrated DC were cultured with 1 μM CFSE-labeled allogeneic peripheral blood lymphocytes at stimulator to effector ratios of 1:10 for 6 days, at which time T-cell cytokines were analyzed in the supernatant using a TH1/TH2/TH17 cytokine bead array (BD Biosciences, Breda, The Netherlands), following the manufacturer's protocol. T-cell proliferation was analyzed by further labeling the isolated cells with fluorophore-conjugated antibodies against CD3, CD4, and CD8 (BD Biosciences), and determining the CFSE dilution in subsets of viable T cells by cytofluorimetric analysis on a BD LSRFortessa™ flow cytometer using BD FACSDiva™ software (BD Biosciences).
Antigen cross-presentation
We generated a CTL line recognizing MART-126-35 in an HLA-A2-restricted fashion (>95% pure, as tested by dextramer staining), by priming CD8+ T cells from healthy donors with MART-126-35(27L) peptide-loaded JY cells. After stimulation, the expanded MART-1-specific T cells were sorted using a MART-126-35 dextramer (Immudex). Skin explants were HLA-A2, typed by taking 5 biopsies on the day of surgery, were subsequently cultured in skin medium. After overnight culture, migrated cells were harvested, stained using a FITC-labeled anti-HLA-A2 antibody (MBL International, Des Plaines, Unites States), and subsequently analyzed using a BD LSRFortessa™ flow cytometer. Skin explant-emigrated HLA-A2-matched DCs were co-cultured with the CFSE-labeled MART-126-35 CTL line (resting). After 24 h the supernatant was isolated and analyzed for the production of IFNγ using a cytokine bead array (BD Biosciences). After 6 days of co-culture, proliferation was assessed by determining the CFSE dilution on a BD LSRFortessa™ flow cytometer using BD FACSDiva™ software (BD Biosciences).
Statistical analysis
Statistical analysis was performed using GraphPad Prism for Windows version 5 (Graphpad Software Inc.). Statistical significance of the ingestion of either ACR or blebs was determined using a paired 2-tailed Student's t-test. Multiple group analysis was performed using one-way ANOVA, with a Turkey's multiple comparison test. In all cases, P-values ≤ 0.05 were regarded as significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Yvonne Baggerman and the OR team of the Plastic Surgery Department, Tergooi hospital location Hilversum, The Netherlands, for providing the abdominoplasty skin specimens.
References
- Oosterhoff D, Sluijter BJR, Hangalapura BN, de Gruijl, TD. The dermis as a portal for dendritic cell-targeted immunotherapy of cutaneous melanoma. Curr Top Microbiol Immunol 2012 351:181-220; PMID:21681685
- Klechevsky E, Morita R, Liu M, Cao Y, Coquery S, Thompson-Snipes L, Briere F, Chaussabel D, Zurawski G, Palucka aK, Reiter Y, Banchereau J, Ueno H. Functional specializations of human epidermal Langerhans cells and CD14+ dermal dendritic cells. Immunity 2008 29:497-510; PMID:18789730; http://dx.doi.org/10.1016/j.immuni.2008.07.013
- Matthews K, Chung NPY, Klasse PJ, Moutaftsi M, Carter D, Salazar AM, Reed SG, Sanders RW, Moore JP. Clinical Adjuvant combinations stimulate potent B-cell responses in vitro by activating dermal dendritic cells. PLoS One 2013 8:e63785; PMID:23700434; http://dx.doi.org/10.1371/journal.pone.0063785
- Van de Ven R, van den Hout MFCM, Lindenberg JJ, Sluijter BJR, van Leeuwen PaM, Lougheed SM, Meijer S, van den Tol MP, Scheper RJ, de Gruijl TD. Characterization of four conventional dendritic cell subsets in human skin-draining lymph nodes in relation to T-cell activation. Blood 2011 118:2502-10; PMID:21750314; http://dx.doi.org/10.1182/blood-2011-03-344838
- Santegoets SJaM, Gibbs S, Kroeze K, van de Ven R, Scheper RJ, Borrebaeck Ca, de Gruijl TD, Lindstedt M. Transcriptional profiling of human skin-resident Langerhans cells and CD1a+ dermal dendritic cells: differential activation states suggest distinct functions. J Leukoc Biol 2008 84:143-51; PMID:18436579; http://dx.doi.org/10.1189/jlb.1107750
- Nestle FO, Zheng XG, Thompson CB, Turka LA, Nickoloff BJ. Characterization of dermal dendritic cells obtained from normal human skin reveals phenotypic and functionally distinctive subsets. J Immunol 1993 151:6535-45; PMID:7504023
- Turville SG, Cameron PU, Handley A, Lin G, Pöhlmann S, Doms RW, Cunningham AL. Diversity of receptors binding HIV on dendritic cell subsets. Nat Immunol 2002 3:975-83; PMID:12352970; http://dx.doi.org/10.1038/ni841
- Kaplan DH. In vivo function of Langerhans cells and dermal dendritic cells. Trends Immunol 2010 31:446-51; PMID:21035396; http://dx.doi.org/10.1016/j.it.2010.08.006
- Larregina AT, Morelli AE, Spencer LA, Logar AJ, Watkins SC, Thomson AW, Falo LD. Dermal-resident CD14+ cells differentiate into Langerhans cells. Nat Immunol 2001 2:1151-8; PMID:1170206-5; http://dx.doi.org/10.1038/ni731
- Geissmann F, Prost C, Monnet J.-P, Dy M, Brousse N, Hermine O. Transforming growth factor 1, in the presence of granulocyte/macrophage colony-stimulating factor and interleukin 4, induces differentiation of human peripheral blood monocytes into dendritic langerhans cells. J Exp Med 1998 187:961-6; PMID:9500798; http://dx.doi.org/10.1084/jem.187.6.961
- De Gruijl TD, Sombroek CC, Lougheed SM, Oosterhoff D, Buter J, van den Eertwegh AJM, Scheper RJ, Pinedo HM. A postmigrational switch among skin-derived dendritic cells to a macrophage-like phenotype is predetermined by the intracutaneous cytokine balance. J Immunol 2006 176:7232-42; PMID:16751366; http://dx.doi.org/10.4049/jimmunol.176.12.7232
- Lindenberg JJ, Oosterhoff D, Sombroek CC, Lougheed SM, Hooijberg E, Stam AGM, Santegoets SJAM, Tijssen HJ, Buter J, Pinedo HM, et al. IL-10 conditioning of human skin affects the distribution of migratory dendritic cell subsets and functional T cell differentiation. PLoS One 2013 8:e70237; PMID:23875023; http://dx.doi.org/10.1371/journal.pone.0070237
- Santegoets SJaM, Bontkes HJ, Stam AGM, Bhoelan F, Ruizendaal JJ, van den Eertwegh AJM, Hooijberg E, Scheper RJ, de Gruijl TD. Inducing antitumor T cell immunity: comparative functional analysis of interstitial versus Langerhans dendritic cells in a human cell line model. J Immunol 2008 180:4540-9; PMID:18354176; http://dx.doi.org/10.4049/jimmunol.180.7.4540
- Landsberg J, Kohlmeyer J, Renn M, Bald T, Rogava M, Cron M, Fatho M, Lennerz V, Wölfel T, Hölzel M, et al. Melanomas resist T-cell therapy through inflammation-induced reversible dedifferentiation. Nature 2012 490:412-6; PMID:23051752; http://dx.doi.org/10.1038/nature11538
- Doubrovina E, Carpenter T, Pankov D, Selvakumar A, Hasan A, O’Reilly RJ. Mapping of novel peptides of WT-1 and presenting HLA alleles that induce epitope-specific HLA-restricted T cells with cytotoxic activity against WT-1(+) leukemias. Blood 2012 120:1633-46; PMID:22623625; http://dx.doi.org/10.1182/blood-2011-11-394619
- Stevanovic S. Identification of tumour-associated T-cell epitopes for vaccine development. Nat Rev Cancer 2002 2:514-20; PMID:12094237; http://dx.doi.org/10.1038/nrc841
- Van Rooij N, van Buuren MM, Philips D, Velds A, Toebes M, Heemskerk B, van Dijk LJA, Behjati S, Hilkmann H, El Atmioui D, et al. Tumor exome analysis reveals neoantigen-specific T-cell reactivity in an ipilimumab-responsive melanoma. J Clin Oncol 2013 31:e439-42; PMID:24043743; http://dx.doi.org/10.1200/JCO.2012.47.7521
- Lomonosova E, Chinnadurai G. BH3-only proteins in apoptosis and beyond: an overview. Oncogene 2008 27 Suppl 1: S2-S19; PMID:19641503; http://dx.doi.org/10.1038/onc.2009.39
- Franz S, Herrmann K, Fürnrohr BG, Führnrohr B, Sheriff A, Frey B, Gaipl US, Voll RE, Kalden JR, Jäck H-M, et al. After shrinkage apoptotic cells expose internal membrane-derived epitopes on their plasma membranes. Cell Death Differ 2007 14:733-42; PMID:17170754; http://dx.doi.org/10.1038/sj.cdd.4402066
- Lane JD, Allan VJ, Woodman PG. Active relocation of chromatin and endoplasmic reticulum into blebs in late apoptotic cells. J Cell Sci 2005 118:4059-71; PMID:16129889; http://dx.doi.org/10.1242/jcs.02529
- Fransen JH, Hilbrands LB, Ruben J, Stoffels M, Adema GJ, van der Vlag J, Berden JH. Mouse dendritic cells matured by ingestion of apoptotic blebs induce T cells to produce interleukin-17. Arthritis Rheum 2009 60:2304-13; PMID:19644874; http://dx.doi.org/10.1002/art.24719
- Ruben JM, van den Ancker W, Bontkes HJ, Westers TM, Hooijberg E, Ossenkoppele GJ, de Gruijl TD, van de Loosdrecht AA. Apoptotic blebs from leukemic cells as a preferred source of tumor-associated antigen for dendritic cell-based vaccines. Cancer Immunol Immunother 2014 63:335-45; PMID:24384837; http://dx.doi.org/10.1007/s00262-013-1515-6
- Liu K, Iyoda T, Saternus M, Kimura Y, Inaba K., Steinman RM. Immune tolerance after delivery of dying cells to dendritic cells in situ. J Exp Med 2002 196:1091-7; PMID:12391020; http://dx.doi.org/10.1084/jem.20021215
- Morelli aE. The immune regulatory effect of apoptotic cells and exosomes on dendritic cells: its impact on transplantation. Am J Transplant 2006 6:254-61; PMID:16426309; http://dx.doi.org/10.1111/j.1600-6143.2005.01197.x
- Ren G, Su J, Zhao X, Zhang L, Zhang J, Roberts AI, Zhang H, Das G, Shi Y. Apoptotic cells induce immunosuppression through dendritic cells: critical roles of IFN-gamma and nitric oxide. J Immunol 2008 181:3277-84; PMID:18713999; http://dx.doi.org/10.4049/jimmunol.181.5.3277
- De Gruijl TD, Luykx-de Bakker SA, Tillman BW, van den Eertwegh AJM, Buter J, Lougheed SM, van der Bij GJ, Safer AM, Haisma HJ, Curiel DT, et al. Prolonged maturation and enhanced transduction of dendritic cells migrated from human skin explants after in situ delivery of CD40-targeted adenoviral vectors. J Immunol 2002 169:5322-31; PMID:12391253; http://dx.doi.org/10.4049/jimmunol.169.9.5322
- Oosterhoff D, Heusinkveld M, Lougheed SM, Kosten I, Lindstedt M, Bruijns SCM, van Es T, van Kooyk Y, van der Burg SH, de Gruijl TD. Intradermal delivery of TLR agonists in a human explant skin model: preferential activation of migratory dendritic cells by polyribosinic-polyribocytidylic acid and peptidoglycans. J Immunol 2013 190:3338-45; PMID:23467931; http://dx.doi.org/10.4049/jimmunol.1200598
- Hooijberg E, Ruizendaal JJ, Snijders PJ, Kueter EW, Walboomers JM, Spits H. Immortalization of human CD8+ T cell clones by ectopic expression of telomerase reverse transcriptase. J Immunol 2000 165:4239-45; PMID:11035057; http://dx.doi.org/10.4049/jimmunol.165.8.4239
- Landsberg J, Kohlmeyer J, Renn M, Bald T, Rogava M, Cron M, Fatho M, Lennerz V, Wölfel T, Hölzel M, et al. Melanomas resist T-cell therapy through inflammation-induced reversible dedifferentiation. Nature 2012 490:412-6; PMID:23051752; http://dx.doi.org/10.1038/nature11538
- Segura E, Valladeau-guilemond J, Donnadieu M-H, Sastre-garau X, Soumelis V, Amigorena S. Characterization of resident and migratory dendritic cells in human lymph nodes. J Exp Med 2012 209:653-60; PMID:22430490; http://dx.doi.org/10.1084/jem.20111457
- Bayerl C, Ueltzhöffer A, Jung EG. Langerhans cells enclosing sunburn cells in acute UV erythema in vivo. Arch Dermatol Res 1999 291:303-5; PMID:10367715; http://dx.doi.org/10.1007/s004030050413
- Banchereau J, Thompson-Snipes L, Zurawski S, Blanck J-P, Cao Y, Clayton S, Gorvel J-P, Zurawski G, Klechevsky E. The differential production of cytokines by human Langerhans cells and dermal CD14(+) DCs controls CTL priming. Blood 2012 119:5742-9; PMID:22535664; http://dx.doi.org/10.1182/blood-2011-08-371245
- Banchereau J, Zurawski S, Thompson-Snipes L, Blanck J-P, Clayton S, Munk A, Cao Y, Wang Z, Khandelwal S, Hu J, et al. Immunoglobulin-like transcript receptors on human dermal CD14+ dendritic cells act as a CD8-antagonist to control cytotoxic T cell priming. Proc Natl Acad Sci U S A 2012 109:18885-90; PMID:23112154; http://dx.doi.org/10.1073/pnas.1205785109
- Chu C-C, Ali N, Karagiannis P, Meglio Di P, Skowera A, Napolitano L, Barinaga G, Grys K, Sharif-Paghaleh E, Karagiannis SN, et al. Resident CD141 (BDCA3)+ dendritic cells in human skin produce IL-10 and induce regulatory T cells that suppress skin inflammation. J Exp Med 2012 209:935-45; PMID:22547651; http://dx.doi.org/10.1084/jem.20112583
- Wickman GR, Julian L, Mardilovich K, Schumacher S, Munro J, Rath N, Al Zander S, Mleczak A, Sumpton D, Morrice N, et al. Blebs produced by actin-myosin contraction during apoptosis release damage-associated molecular pattern proteins before secondary necrosis occurs. Cell Death Differ 2013 20(10):1293-1305; PMID:23787996; http://dx.doi.org/10.1038/cdd.2013.69
- Murshid A, Gong J, Calderwood SK. The role of heat shock proteins in antigen cross presentation. Front Immunol 2012 3:63; PMID:22566944; http://dx.doi.org/10.3389/fimmu.2012.00063
- Li Y, Wang L-X, Pang P, Cui Z, Aung S, Haley D, Fox BA, Urba WJ, Hu, H-M. Tumor-derived autophagosome vaccine: mechanism of cross-presentation and therapeutic efficacy. Clin Cancer Res 2011 17:7047-57; PMID:22068657; http://dx.doi.org/10.1158/1078-0432.CCR-11-0951
- Yewdell JW. DRiPs solidify: progress in understanding endogenous MHC class I antigen processing. Trends Immunol 2011 32:548-58; PMID:21962745; http://dx.doi.org/10.1016/j.it.2011.08.001
- Van de Ven R, Lindenberg JJ, Oosterhoff D, de Gruijl T. D. Dendritic cell plasticity in tumor-conditioned skin: CD14(+) cells at the cross-roads of immune activation and suppression. Front Immunol 2013 4:403; PMID:24324467; http://dx.doi.org/10.3389/fimmu.2013.00403
- Molenkamp BG, Vuylsteke RJ, van Leeuwen PM, Meijer S, Vos W, Wijnands PG, Scheper RJ, de Gruijl TD. Matched skin and sentinel lymph node samples of melanoma patients reveal exclusive migration of mature dendritic cells. Am J Pathol 2005 167:1301-7; PMID:16251414; http://dx.doi.org/10.1016/S0002-9440(10)61217-5
- Molenkamp BG, van Leeuwen PA, Meijer S, Sluijter BJ, Wijnands PG, Baars A, van den Eertwegh AJ, Scheper RJ, de Gruijl TD. Intradermal CpG-B activates both plasmacytoid and myeloid dendritic cells in the sentinel lymph node of melanoma patients. Clin Cancer Res 2007 13:2961-9; PMID:17504997; http://dx.doi.org/10.1158/1078-0432.CCR-07-0050
- Khan S, Weterings JJ, Britten CM, de Jong AR, Graafland D, Melief CJM, van der Burg SH, van der Marel G, Overkleeft HS, Filippov DV, et al. Chirality of TLR-2 ligand Pam3CysSK4 in fully synthetic peptide conjugates critically influences the induction of specific CD8+ T-cells. Mol Immunol 2009 46:1084-91; PMID:19027958; http://dx.doi.org/10.1016/j.molimm.2008.10.006
- Akiyama K, Ebihara S, Yada A, Matsumura K, Aiba S, Nukiwa T, Takai T. Targeting apoptotic tumor cells to Fc gamma R provides efficient and versatile vaccination against tumors by dendritic cells. J Immunol 2003 170:1641-8; PMID:12574326; http://dx.doi.org/10.4049/jimmunol.170.4.1641
- Auffermann-Gretzinger S, Eger L, Bornhäuser M, Schäkel K, Oelschlaegel U, Schaich M, Illmer T, Thiede C, Ehninger G. Fast appearance of donor dendritic cells in human skin: dynamics of skin and blood dendritic cells after allogeneic hematopoietic cell transplantation. Transplantation 2006 81:866-73; PMID:16570010; http://dx.doi.org/10.1097/01.tp.0000203318.16224.57
- Perreault C, Pelletier M, Landry D, Gyger M. Study of Langerhans cells after allogeneic bone marrow transplantation. Blood 1984 63:807-11; PMID:6367851
- Vuylsteke RJ, Molenkamp BG, Gietema HA, van Leeuwen PA, Wijnands PG, Vos W, van Diest PJ, Scheper RJ, Meijer S, de Gruijl TD. Local administration of granulocyte/macrophage colony-stimulating factor increases the number and activation state of dendritic cells in the sentinel lymph node of early-stage melanoma. Cancer Res 2004 64:8456-60; PMID:15548718; http://dx.doi.org/10.1158/0008-5472.CAN-03-3251
- Van den Ancker W, van Luijn MM, Ruben JM, Westers TM, Bontkes HJ, Ossenkoppele GJ, de Gruijl TD, van de Loosdrecht AA. Targeting Toll-like receptor 7/8 enhances uptake of apoptotic leukemic cells by monocyte-derived dendritic cells but interferes with subsequent cytokine-induced maturation. Cancer Immunol Immunother 2011 60:37-47; PMID:20859626; http://dx.doi.org/10.1007/s00262-010-0917-y
