Abstract
Natural killer (NK) cells are innate immune cells that become progressively exhausted in advanced stage cancer, crippling their ability to execute antitumor functions. We previously characterized the nature of NK cell exhaustion in metastatic melanoma patients, reporting a correlation with high expression of TIM-3. Blockade of this immune checkpoint molecule reversed the exhausted phenotype and improved NK cell function.
Among the most promising approaches to activate antitumor immunity is the blockade of the checkpoint molecules cytotoxic T lymphocyte-associated protein 4 (CTLA-4) and programmed cell death 1 (PD-1), immunotherapies which contribute to improved overall survival in the treatment of various cancers, including melanoma.Citation1,2 Immune checkpoints refer to inhibitory molecules that modulate the duration and amplitude of immune responses in peripheral tissues in order to maintain self-tolerance and minimize tissue damage. A number of studies have shown that tumors can co-opt certain immune-checkpoint pathways as a major mechanism of tumor immune escape. Despite the dramatic results obtained with CTLA-4 and PD-1 blockade, a substantial percentage of treated patients either fail to respond or eventually progress, highlighting the need to identify and target novel immune checkpoint inhibitory molecules. In this regard, the immune checkpoint T cell immunoglobulin- and mucin-domain-containing molecule-3 (TIM-3) has emerged as a key molecule in immune regulation and represents a promising novel therapeutic candidate.
TIM-3 (also known as hepatitis A virus cellular receptor 2) was initially identified on activated CD4+ T helper type 1 (Th1) and CD8+ cytotoxic T type 1 (Tc1) cells and its sustained expression has been shown to contribute to T-cell exhaustion, a state of immune cell dysfunction that prevents optimal control of infection and tumors. It is characterized by poor effector function, sustained expression of inhibitory receptors and a transcriptional state distinct from that of functional effector cells. Dysregulation of TIM-3 expression on immune cells has been linked to several diseases. While low or absent expression has been linked to excessive inflammatory responses implicated in autoimmune diseases, such as multiple sclerosis, its overexpression contributes to inhibition of the immune response in chronic viral infections and cancers. For example, TIM-3 is co-expressed with PD-1 on human tumor-specific CD8+ T cells, and studies in murine models in vivo have shown that Tim-3 blockade alone, or especially in combination with anti-PD-1, is able to reverse T-cell exhaustion and control tumor growth in several tumor models, including melanoma.Citation3,4 Natural killer (NK) cells play a key role in antitumor responses through interferon γ (IFNγ) production and direct cytotoxicity against tumor cells. However, once they have reached the tumor site, they may become exhausted and display profound functional defectsCitation5-Citation7 that render them unable to display optimal antitumor responses. While T-cell exhaustion has been extensively characterized in many contexts, this phenomenon remains underexplored in the case of NK cells. Thus, we characterized NK cell phenotypes in the context of metastatic melanoma and examined how TIM-3 expression affects the activity of these innate immune cells.Citation8
Compared to their counterparts isolated from healthy donors, we found that peripheral NK cells from patients with metastatic melanoma (Stage III and IV) display an exhausted phenotype, as indicated by higher expression of inhibitory receptors (e.g., KIR3DL1 and KIR2DL3), a lower expression of activating receptors (e.g., NKG2D, NpP46 and DNAM-1), an impaired response to IL-2 and IL-15 due to downregulation of their receptors (IL-2R and IL-15R), and functional defects in their ability to proliferate, secrete cytokines and kill target cells. Gill et al. recently described that adoptively transferred murine NK cells are able to traffic to the tumor site, yet fail to control tumor growth as they become exhausted. The authors showed that this exhausted NK cell phenotype is characterized by down regulation of the transcription factor Eomesodermin (Eomes) and can be reversed by overexpression of Eomes.Citation5 Interestingly, we also observed that NK cells from patients with metastatic melanoma expressed reduced levels of Eomes. Collectively, these findings demonstrate that NK cells are exhausted and dysfunctional in the blood of patients with metastatic melanoma.
We investigated the NK cell receptor repertoire in order to identify molecular candidates contributing to NK cell dysfunction or exhaustion that could possibly be targeted to reverse immune exhaustion. TIM-3, but not CTLA-4 or PD-1, was identified among the receptors whose expression was upregulated on NK cells isolated from patients with metastatic melanoma.Citation8 An analysis of a cohort of >80 melanoma patients demonstrated a progressive increase in TIM-3+ NK cells corresponding to advancing stages of melanoma. Notably, the expression of TIM-3 was significantly higher in melanoma patients with poor prognostic factors, such as lesion thickness >1mm, mitotic rate ≥1/mm2, ulceration and the presence of metastases. Most strikingly, blockade of TIM-3 reversed the exhausted NK cell phenotype and substantially restored NK cell cytotoxicity, cytokine production and proliferation. Altogether, these results showed that TIM-3 participates in actively inhibiting NK cell function in advanced melanoma.
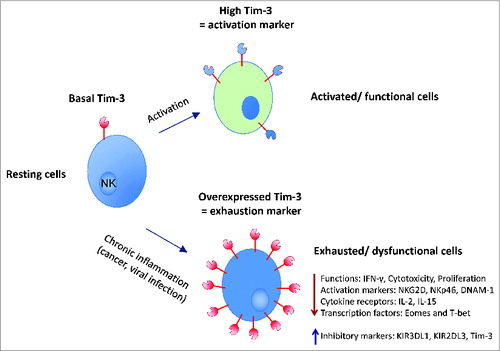
While investigating TIM-3 regulation on NK cells isolated from normal donors, we and other groups found that the receptor is present on resting cells but its expression increases significantly after stimulation with a number of cytokines, including IL-2, IL-12, Il-15, IL-18, IFNα.Citation8-Citation10 Moreover, NK cells with the highest TIM-3 levels corresponded to the most active cells, in other words, the most cytotoxic subset with the highest IFNγ production. To determine the function of TIM-3 on NK cells, we developed different systems to engage and cross-link the receptor in order to activate its signaling pathway including: beads coated with anti-TIM-3 antibody; ligation with a putative TIM-3 ligand, Galectin-9; co-culture with melanoma cell lines lacking or expressing Galectin-9; and a reverse-antibody dependent cell cytotoxicity assay. Using these tools, we determined that upon activation, Tim-3 functions as an inhibitory receptor on NK cells by reducing their cytotoxicity and cytokine production. Like PD-1 on T cells, our findings indicate that TIM-3 limits the duration and magnitude of NK cell responses. In conclusion, our study indicates that TIM-3 is not only a marker for NK cell activation/maturation, but that it also curtails NK cell function to prevent chronic activation and inflammation. In certain contexts such as cancer, sustained TIM-3 expression and function contributes to their exhaustion state (), much like it does on T cells.
Figure 1. The expression and role of TIM-3 in natural killer (NK) cells. NK cell activation, through exposure to different cytokines (e.g., IL-2, IL-15. Il-18 and IL-12), leads to an increase in TIM-3 expression (activation marker – upper part of the figure). In the context of chronic inflammation, such as occurs in cancer or chronic viral infection, TIM-3 is overexpressed on NK cells and plays an important role in inducing exhaustion of these cells (exhaustion marker – lower part of the figure). TIM-3, T cell immunoglobulin- and mucin-domain-containing molecule-3; Eomes, eomesodermin.
Our study provides the first demonstration that NK cells from patients with advanced melanoma display an exhausted phenotype, and, that in this context, TIM-3 blockade is able to reverse NK cell exhaustion and improve function. These findings support the development of TIM-3-targeted therapies to restore antitumor responses that are dependent not only upon T cells, but also the innate immune system. One can speculate that compared with CTLA-4 and PD-1 blockade, fewer adverse events might be expected with TIM-3 blockade given that Tim-3 deficient mice do not show any evidence of auto-immunity. Although the reversal of NK cell exhaustion is significant, it is not complete and it is likely that other molecules or pathways are involved. Therefore, combinatorial strategies might be the most promising approach to restore effective antitumor immune responses and limit cancer cell immune escape. Unraveling the mechanisms underlying the role of TIM-3 on other immune cells and the TIM-3 signaling pathway will open the way to developing new therapeutic strategies for immune-related disorders.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
These studies were supported by the Cancer Research Institute.
References
- Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363:711-23; PMID:20525992; http://dx.doi.org/10.1056/NEJMoa1003466
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med 2012; 366:2443-54; PMID:22658127; http://dx.doi.org/10.1056/NEJMoa1200690
- Fourcade J, Sun Z, Benallaoua M, Guillaume P, Luescher IF, Sander C, Kirkwood JM, Kuchroo V, Zarour HM. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J Exp Med 2010; 207:2175-86; PMID:20819923; http://dx.doi.org/10.1084/jem.20100637
- Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med 2010; 207:2187-94; PMID:20819927; http://dx.doi.org/10.1084/jem.20100643
- Gill S, Vasey AE, De Souza A, Baker J, Smith AT, Kohrt HE, Florek M, Gibbs KD Jr, Tate K, Ritchie DS, et al. Rapid development of exhaustion and down-regulation of eomesodermin limit the antitumor activity of adoptively transferred murine natural killer cells. Blood 2012; 119:5758-68; PMID:22544698; http://dx.doi.org/10.1182/blood-2012-03-415364
- Mamessier E, Sylvain A, Thibult ML, Houvenaeghel G, Jacquemier J, Castellano R, Gonlves A, Andr P, Romagn F, Thibault G, et al. Human breast cancer cells enhance self tolerance by promoting evasion from NK cell antitumor immunity. J Clin Invest 2011; 121:3609-22; PMID:21841316; http://dx.doi.org/10.1172/JCI45816
- Platonova S, Cherfils-Vicini J, Damotte D, Crozet L, Vieillard V, Validire P, Andr P, Dieu-Nosjean MC, Alifano M, Rnard JF, et al. Profound coordinated alterations of intratumoral NK cell phenotype and function in lung carcinoma. Cancer Res 2011; 71:5412-22; PMID:21708957; http://dx.doi.org/10.1158/0008-5472.CAN-10-4179
- da Silva IP, Gallois A, Jimenez-Baranda S, Khan S, Anderson AC, Kuchroo VK, Osman I, Bhardwaj N, et al. Reversal of NK-cell exhaustion in advanced melanoma by tim-3 blockade. Cancer Immunol Res 2014; 2:410-22; PMID:24795354; http://dx.doi.org/10.1158/2326-6066.CIR-13-0171
- Gleason MK, Lenvik TR, McCullar V, Felices M, O'Brien MS, Cooley SA, Verneris MR, Cichocki F, Holman CJ, Panoskaltsis-Mortari A, et al. Tim-3 is an inducible human natural killer cell receptor that enhances interferon gamma production in response to galectin-9. Blood 2012; 119:3064-72; PMID:22323453; http://dx.doi.org/10.1182/blood-2011-06-360321
- Ndhlovu LC, Lopez-Verg S, Barbour JD, Jones RB, Jha AR, Long BR, Schoeffler EC, Fujita T, Nixon DF, Lanier LL. Tim-3 marks human natural killer cell maturation and suppresses cell-mediated cytotoxicity. Blood 2012; 119:3734-43; PMID:22383801; http://dx.doi.org/10.1182/blood-2011-11-392951
