Abstract
Type I interferon inducers may potentially be engineered to function as antiviral and anticancer agents, or alternatively, vaccine adjuvants, all of which may have clinical applications. We recently described a simple strategy to convert a Toll-like receptor 9 (TLR9) agonist devoid of interferon α (IFNα) stimulating activity into a robust Type I interferon inducer with potent vaccine adjuvant activity.
Keywords:
Single stranded synthetic oligodeoxynucleotides containing unmethylated cytosine-phosphate-guanine dinucleotide motifs (CpG ODN) mimic the immune stimulatory effect of bacterial DNA and constitute a family of immunotherapeutics that stimulate the cells of the innate immune system expressing the endosomal pattern recognition receptor Toll-like receptor 9 (TLR9).Citation1 CpG ODNs are classified into 4 distinct subtypes on the basis of their sequence, the nature of the ODN backbone, the presence of secondary structures and differential immune activation patterns observed among stimulated human peripheral blood mononuclear cells (PBMCs).Citation2 Of these, K-class CpG ODNs (referred to as CpG-B by other groups) have linear phosphorothioate (PS) backbones, express multiple TCGTT and/or TCGTA motifs and strongly activate B cells.Citation3 K-ODNs promote the survival, activation and maturation of plasmacytoid dendritic cells (pDC) but induce no IFNα secretion.Citation3-5 To date, the majority of clinical trials have been based on this ODN class.Citation6 The D-class ODN (referred to as CpG-A by other groups), contain a single palindromic phosphodiester purine/pyrimidine/CG/purine/pyrimidine motif linked to a poly(G) tail at their 3’ end.Citation3 The palindromic sequence and the poly(G) tail enable D type ODNs to form Hoogsteen base paired G-quadruplexes and higher order structures that can be globular (∼50 nm size), linear (∼100 nm size) or 2 forked.Citation7 Formation of such multimeric structures permits D- but not K-type ODN to bind to the transmembrane form of the chemokine and scavenger receptor chemokine (C-X-C) ligand 16 (CXCL16) expressed on the surface of pDCs.Citation8 This interaction directs the ODN into early endosomes, triggering a TLR9-MyD88-IRF7-mediated signaling pathway, leading to robust IFNα production.Citation9 The D-ODN-induced vigorous IFNα response may have potential benefits in the prevention/treatment of viral infections and/or malignancies. However, formation of spontaneous, uncontrollable higher order structures with D-class ODN complicates their pharmaceutical manufacturing process, precluding them from human clinical trials. The remaining 2 CpG ODN classes are either weak Type I interferon-inducers (C-class), or are dependent upon “high-salt buffers” to form IFNα stimulating concatameric structures (P-class), making them less potent or unpredictable for use as alternates of D-ODN.
Recently, we have demonstrated a simple strategy to convert a conventional K-type ODN into a potent Type I interferon inducer by multimerizing the ODN into ordered nanostructures through complexation with a cationic peptide.Citation10 In general, polycation induced condensation of ODN generates aggregates with a heterogeneous distribution. To avoid such an outcome, we hypothesized that a very short and hence non-flexible ODN would condense into well-defined spheres when mixed with a short peptide of high positive charge density. Our results demonstrated that among the cationic peptides tested, the 11-mer, +8 charged HIV-derived peptide Tat(47–57) condensed a 12-mer but not a more flexible 20-mer ODN into rigid individual building blocks that subsequently re-organized to forge stable, monodispersed nanorings ().Citation10 The nanorings generated a vigorous pDC-dependent IFNα response and an indirect monocyte-dependent CXCL10/IP-10 response in human blood that reciprocated D-ODN activity.Citation10 Since differences in subcellular distribution of ODN directly affect the type of response (i.e, the IFNα eliciting capacity), we further analyzed the endosomal localization patterns. Results showed that condensation with Tat peptide redirected K-ODN to early endosomes (). This subcellular distribution pattern is consistent with efficient IFNα production.
Figure 1. Assembly and mechanism of action of CpG ODN/Tat nanorings. (A). The cationic peptide condenses K-type CpG ODN into rigid individual building blocks that re-organize to form nanoring structures. Nanorings are internalized by pDC and translocate to early endosomes where they initiate a TLR9-MyD88-IRF7-mediated signaling pathway, leading to IFNα production. In free form, K-ODN localize to late endosomes, and are not qualified to trigger an interferon response. (B). Nanoring-stimulated pDCs secrete Type I interferons, supporting antigen-specific humoral and cellular immunity in vivo. Ag, antigen; CpG ODN, cytosine-guanine oligodeoxynucleotides; IFNα, interferon α; IFNγ, interferon γ; pDC, plasmacytoid dendritic cells; Tat, 8 residue charged HIV-derived peptide Tat(47–57); Th1, T helper type 1; TLR9, Toll-like receptor 9.
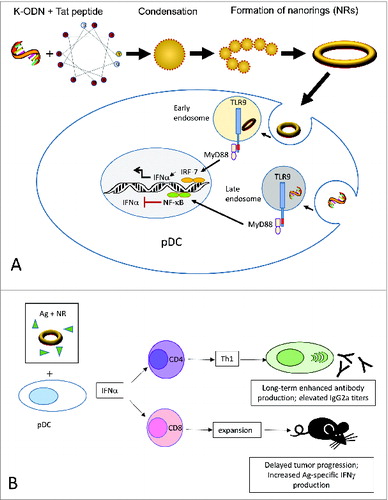
The nanorings were also found to be more effective than K-ODN in stimulating long-term antigen-specific antibody production in vivo when ad-mixed with a suboptimal dose of the inactivated foot and mouse disease vaccine.Citation10 The response was characterized by an isotype switching toward IgG2a, suggestive of T helper type 1 (Th1) support (). In a different setting, C57BL/6 mice bearing ovalbumin (OVA)-expressing EG.7 thymoma tumors were therapeutically vaccinated with the model tumor antigen OVA plus the adjuvants. The nanoring adjuvanted group displayed significant reduction in tumor size and progression.Citation10 Type I interferons can directly trigger clonal expansion and memory formation in CD8+ T cells. Consistent with this view, nanorings triggered expansion in the CD8+ T cell pool and elicited superior tumor-specific immunity () characterized by increased OVA-specific interferon γ (IFNγ) production. Next, to assess whether the nanorings required the presence of pDCs for their activity, we depleted this cell population in vivo and examined the induction of antibody response to the model antigen OVA. In pDC undepleted mice, both the nanoring and the D-ODN adjuvanted groups generated approximately 80-fold higher OVA-specific IgG2a titers when compared to antigen alone. This activity was severely impaired in mice depleted of pDCs, suggesting that pDC activation and ensuing Type I IFN production is critical for the adjuvanticity.Citation10
Clinical use of Type I interferon-inducing TLR agonists such as the TLR7/8 agonist R837 is currently limited to the topical treatment of genital warts, basal cell carcinoma, and bladder cancer. Systemic administration of imidazoquinolines incite a TLR7-independent immunotoxicity by antagonizing the adenosine receptors. Therefore, the development of other TLR-based Type I interferon inducers suitable for systemic use as adjuvants is highly desirable. We believe that our recent findings in regards to the performance of the nanorings in vivo are encouraging and may prove to be of value as antiviral or anticancer agents and vaccine adjuvants in the clinic. However, whether the nanorings would withstand testing in non-human primates where the cellular expression of TLR9 is more restricted than in mice remains to be seen.
Disclosure of Potential Conflicts of Interest
MG and IG are among the co-inventors of patents concerning the activity of CpG ODN, including their use as vaccine adjuvants. The rights to all such patents have been transferred to the US government. The authors declare no competing financial interests.
Additional information
Funding
References
- Klinman DM. Immunotherapeutic uses of CpG oligodeoxynucleotides. Nat Rev Immunol 2004;4:249-58; PMID:15057783; http://dx.doi.org/10.1038/nri1329
- Hanagata N. Structure-dependent immunostimulatory effect of CpG oligodeoxynucleotides and their delivery system. Int J Nanomedicine 2012;7:2181-95; PMID:22619554; http://dx.doi.org/10.2147/IJN.S30197
- Verthelyi D, Ishii KJ, Gursel M, Takeshita F, Klinman DM. Human peripheral blood cells differentially recognize and respond to two distinct CpG motifs. J Immunol 2001;166:2372-7; PMID:11160295; http://dx.doi.org/10.4049/jimmunol.166.4.2372
- Gursel M, Verthelyi D, Gursel I, Ishii KJ, Klinman DM. Differential and competitive activation of human immune cells by distinct classes of CpG oligodeoxynucleotide. J Leukoc Biol 2002;71:813-20; PMID:11994506
- Gursel M, Verthelyi D, Klinman DM. CpG oligodeoxynucleotides induce human monocytes to mature into functional dendritic cells. Eur J Immunol 2002;32:2617-22; PMID:12207346; http://dx.doi.org/10.1002/1521-4141(200209)32:9%3c2617::AID-IMMU2617%3e3.0.CO;2-F
- Krieg AM. CpG still rocks! Update on an accidental drug. Nucleic Acid Ther 2012;22:77-89; PMID:22352814; http://dx.doi.org/10.1089/nat.2012.0340
- Kerkmann M, Costa LT, Richter C, Rothenfusser S, Battiany J, Hornung V, Johnson J, Englert S, Ketterer T, Heckl W, et al. Spontaneous formation of nucleic acid-based nanoparticles is responsible for high interferon-alpha induction by CpG-A in plasmacytoid dendritic cells. J Biol Chem 2005;280:8086-93; PMID:15591070; http://dx.doi.org/10.1074/jbc.M410868200
- Gursel M, Gursel I, Mostowski HS, Klinman DM. CXCL16 influences the nature and specificity of CpG-induced immune activation. J Immunol 2006;177:1575-80; PMID:16849465; http://dx.doi.org/10.4049/jimmunol.177.3.1575
- Honda K, Ohba Y, Yanai H, Negishi H, Mizutani T, Takaoka A, Taya C, Taniguchi T. Spatiotemporal regulation of MyD88-IRF-7 signalling for robust type-I interferon induction. Nature 2005;434:1035-40; PMID:15815647; http://dx.doi.org/10.1038/nature03547
- Gungor B, Yagci FC, Tincer G, Bayyurt B, Alpdundar E, Yildiz S, Ozcan M, Gursel I, Gursel M. CpG ODN nanorings induce IFNα from plasmacytoid dendritic cells and demonstrate potent vaccine adjuvant activity. Sci Transl Med 2014;6:235ra61; PMID:24807558; http://dx.doi.org/10.1126/scitranslmed.3007909
