Abstract
Central thymic tolerance mechanisms create a formidable barrier against the generation of self-reactive T cells. While preventing autoimmunity, this barrier also limits an effective antitumor immunological response. We recently reported that anti-RANKL blocking antibody breaches this central tolerance barrier, thus increasing the repertoire of melanoma reactive T cells. Thus, central tolerance blockade may be an effective therapeutic strategy to enhance anticancer immunity.
Cancer immunotherapeutic strategies have, so far, largely targeted peripheral (i.e., extrathymic) immune tolerance mechanisms to augment T-cell immune activation. A prime example is ipilimumab, an IgG1 blocking antibody that targets the immune checkpoint protein cytotoxic T lymphocyte associated protein 4 (CTLA-4) on peripheral T cells. Although ipilimumab is approved for use in advanced melanoma patients, only a small fraction of patients derive therapeutic benefit.Citation1 Thus, there is an urgent clinical need to understand the mechanisms limiting the effects of ipilimumab. One possible factor that could account for the restricted effects of ipilimumab may be the small pool of available melanoma-reactive T cells in the setting of intact central (i.e., thymic) tolerance.
Central tolerance mechanisms are an important safeguard in preventing T cell-mediated autoimmunity and include the negative selection of self-reactive effector T cells within the thymus (reviewed inCitation2). Medullary thymic epithelial cells (mTECs) play a critical role in thymic negative selection by upregulating the ectopic expression of a wide array of tissue-specific self-antigens (TSAs), such that developing T cells that recognize these TSAs with high affinity undergo clonal deletion. Within mature mTECs, the transcription factor Autoimmune Regulator (Aire) promotes ectopic expression of a subset of TSAs. The crucial role of Aire in preventing autoimmunity is clearly illustrated by the development of spontaneous multi-organ autoimmunity in Aire-deficient mice and humans. Aire-expressing mTECs, are thus essential to maintain immune tolerance toward self-antigens.
In the course of preventing autoimmunity, mTECs also simultaneously limit the T-cell response against tumor antigens. Many TSA's expressed by mTECs are also tumor antigens targeted by T cells during an antitumor immune response. In the human thymus, MAGE-A1, MAGE-A2, MAGE–A3, NY-ESO, MART-1, tyrosinase, and other tumor-associated antigens are expressed by mTECs.Citation3 We and others have shown that ectopic expression of melanoma antigens, including TRP-1 and tyrosinase, by mTECs is under the control of Aire,Citation4,5 and Aire-regulated expression of melanoma antigens promotes clonal deletion of melanoma-reactive T cells in the thymus ().Citation4 In Aire deficient mice, decreased melanoma antigen expression rescues melanoma-reactive T cells from clonal deletion and enhances melanoma immunity.Citation4 Thus, Aire-mediated expression of TSAs in mTECs, while critical in preventing autoimmunity, also simultaneously limits the generation of T cells important in the immune rejection of cancer. These findings suggest that therapeutic strategies that break central tolerance may be key in enhancing antitumor immunity.
Figure 1. Anti-RANKL antibody rescues melanoma-reactive T cells from thymic negative selection. (A) Aire in mTECs promotes melanoma antigen expression and negative selection of melanoma-reactive T effector (Teff) cells. As a result, the immune response against melanoma is limited. (B) Anti-RANKL antibody depletes Aire-expressing mTECs; as a result, melanoma-reactive Teff cells escape thymic negative selection. The increased pool of melanoma-reactive Teff cells contributes to a more effective anti-melanoma immune response.
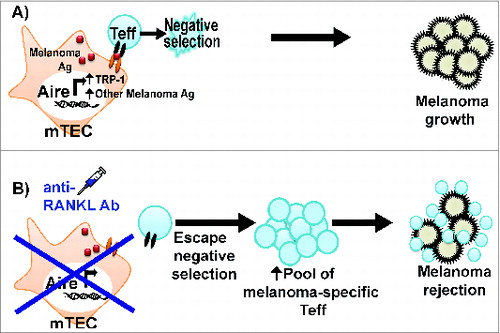
mTEC cellularity is dependent upon the signaling pathway of the tumor necrosis factor (TNF) family members RANK and RANKL.Citation6 Mice lacking RANKL have decreased numbers of Aire-expressing mTECs in the thymus, and forced expression of RANKL restores mTEC cellularity.Citation6 We recently reported that administration of anti-RANKL antibody transiently depletes >90% of Aire-expressing mTECs in adult mice.Citation7 In contrast, anti-RANKL antibody administration only modestly decreased cortical thymic epithelial cells (cTECs) and immature mTECs, which suggests selective depletion of mature Aire-expressing mTECs. The lack of Aire-expressing mTECs in anti-RANKL antibody-treated mice is associated with reduced Aire-dependent expression of melanoma antigens and other TSAs in the thymus.Citation7 As a result, clonal deletion of T cells specific for these TSAs is defective in anti-RANKL antibody treated mice (). In particular, anti-RANKL antibody administration results in decreased ectopic expression of TRP-1 in mTECs and defective negative selection of TRP-1 specific CD4+ T cells,Citation7 which have previously been shown to be sufficient for eradicating B16 melanoma tumors.Citation8 As a result, anti-RANKL antibody administration increases the pool of melanoma-reactive T cells capable of mounting an effective anti-melanoma immune response and improves survival in mice challenged with B16 melanoma. Anti-RANKL antibody therefore represents a therapeutic means of breaking through the central tolerance barrier that limits an effective anti-cancer immune response.
Because RANK and (or) RANKL are expressed by multiple cancer cell types, the decreased melanoma growth in anti-RANKL antibody treated mice could potentially be due to direct effects of anti-RANKL antibody on melanoma cells rather than through its effects on the immune system. To exclude this possibility, we treated donor mice with anti-RANKL antibody prior to transfer of donor splenocytes into immunodeficient recipients. In so doing, the effects of antibody treatment are confined to the transferred immune cells without affecting melanoma cells implanted in the recipients. Anti-RANKL antibody treatment of donor mice increased recipient survival in response to melanoma challenge, consistent with an important role for anti-RANKL antibody in modulating the melanoma targeting immune response.
In sum, our findings point to central tolerance blockade with anti-RANKL antibody as an efficacious immunotherapeutic strategy in the treatment of melanoma. A number of questions remain to be addressed regarding the effects of anti-RANKL antibody. First, it is well recognized that the thymus involutes with age; thus whether anti-RANKL antibody will be equally effective in older animals remains to be determined. Furthermore, the antigen specificity of T cells generated by the thymus is age-dependent.Citation9 Therefore, whether melanoma-reactive T cells continue to be generated in older mice requires clarification. Second, whether anti-RANKL antibody treatment will result in autoimmune side effects is not known. Transplantation of RANK-deficient thymic stroma into athymic nude mice resulted in autoimmune liver disease, weight loss, and diarrhea.Citation10 Although overt autoimmunity was not seen in anti-RANKL antibody treated mice in our studies, mice were followed for a relatively short duration following treatment. Thus following mice treated with anti-RANKL antibody for a longer time course is warranted. Third, the potential efficacy of anti-RANKL antibody in other tumor types remains to be explored. Fourth, the mechanism by which anti-RANKL antibody results in decreased mTEC cellularity is not clear. Previous work has suggested that RANK-RANKL signaling is required for mature mTEC proliferation, which suggests that anti-RANKL antibody may function to block cell division.Citation6 An alternative possibility is that RANK-RANKL provides a necessary survival signal in adult mice, and anti-RANKL antibody administration results in mTEC apoptosis. Fifth, anti-RANKL antibody (denosumab) is currently approved by the FDA for use in osteoporosis and other bone-related diseases in humans. Whether denosumab has similar effects on mTECs in humans remains to be explored. Finally, the combinatorial effects of central tolerance blockade with currently available agents that block peripheral tolerance (e.g., ipilimumab) remains to be determined. The potential synergism between anti-RANKL antibody and ipilimumab is an exciting possibility that necessitates further investigation. These studies will provide a framework for the potential use of central tolerance blockade for cancer immunotherapy.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363:711-23; PMID:20525992; http://dx.doi.org/10.1056/NEJMoa1003466
- Anderson MS, Su MA. Aire and T cell development. Curr Opin Immunol 2011; 23:198-206; PMID:21163636; http://dx.doi.org/10.1016/j.coi.2010.11.007
- Gotter J, Brors B, Hergenhahn M, Kyewski B. Medullary epithelial cells of the human thymus express a highly diverse selection of tissue-specific genes colocalized in chromosomal clusters. J Exp Med 2004; 199:155-66; PMID:14734521
- Zhu M-L, Nagavalli A, Su MA. Aire deficiency promotes TRP-1-specific immune rejection of melanoma. Cancer Res 2013; 73:2104-16; PMID:23370329; http://dx.doi.org/10.1158/0008-5472.CAN-12-3781
- Träger U, Sierro S, Djordjevic G, Bouzo B, Khandwala S, Meloni A, Mortensen M, Simon AK. The immune response to melanoma is limited by thymic selection of self-antigens. PLoS One 2012; 7:e35005; PMID:22506061; http://dx.doi.org/10.1371/journal.pone.0035005
- Hikosaka Y, Nitta T, Ohigashi I, Yano K, Ishimaru N, Hayashi Y, Matsumoto M, Matsuo K, Penninger JM, Takayanagi H, et al. The cytokine RANKL produced by positively selected thymocytes fosters medullary thymic epithelial cells that express autoimmune regulator. Immunity 2008; 29:438-50; PMID:18799150; http://dx.doi.org/10.1016/j.immuni.2008.06.018
- Khan IS, Mouchess ML, Zhu M-L, Conley B, Fasano KJ, Hou Y, Fong L, Su MA, Anderson MS. Enhancement of an anti-tumor immune response by transient blockade of central T cell tolerance. J Exp Med 2014; 211:761-8; PMID:24752296; http://dx.doi.org/10.1084/jem.20131889
- Xie Y, Akpinarli A, Maris C, Hipkiss EL, Lane M, Kwon EKM, Muranski P, Restifo NP, Antony PA. Naive tumor-specific CD4+ T cells differentiated in vivo eradicate established melanoma. J Exp Med 2010; 207:651-67; PMID:20156973; http://dx.doi.org/10.1084/jem.20091921
- He Q, Morillon YM, Spidale NA, Kroger CJ, Liu B, Sartor RB, Wang B, Tisch R. Thymic development of autoreactive T cells in NOD mice is regulated in an age-dependent manner. J Immunol 2013; 191:5858-66; PMID:24198282; http://dx.doi.org/10.4049/jimmunol.1302273
- Rossi SW, Kim MY, Leibbrandt A, Parnell SM, Jenkinson WE, Glanville SH, McConnell FM, Scott HS, Penninger JM, Jenkinson EJ, et al. RANK signals from CD4+3 inducer cells regulate development of Aire-expressing epithelial cells in the thymic medulla. J Exp Med 2007; 204:1267-72; PMID:17502664
