Abstract
Whereas the presence of autoantibodies in cancer patients has been acknowledged, their diagnostic or therapeutic significance has yet to be established. This is due, at least in part, to the lack of robust screening techniques to detect and characterize such antibodies for further assessment. In this study, we screened colorectal cancer (CRC) patient sera for antibodies specifically targeting the key cell cycle inhibitory factor p21 encoded by the cyclin-dependent kinase inhibitor 1A (CDKN1A). Anti-p21 antibody titers were higher in CRC patient samples versus controls, correlating with a more advanced disease stage and lymph node involvement. Further, we isolated for the first time a specific human antibody fragment against p21, which could potentially be useful as a tool to study tumorigenicity in CRC patients.
Abbreviations:
- aAbs, autoantibodies
- CDKN1A, cyclin-dependent kinase inhibitor 1
- CRC, colorectal cancer
- ELISA, enzyme-linked immunosorbent assay
- Fab, fragment antigen-binding
- HER2/ERBB2, v-erb-b2 avian erythroblastic leukemia viral oncogene homologue 2
- Ni-NTA, nickel-charged nitrilotriacetic acid
- OD50, half-maximum binding titer
- p21/CIP1, CDKN1A protein
- TAAs, tumor-associated antigens
- TP53, tumor protein p53.
Emerging evidence suggests that cancer patients produce autoantibodies (aAbs) against certain tumor-associated antigens (TAAs), antibodies that can be detected in the blood.Citation1,2 In fact, cancer-associated aAbs have been proposed for various clinical aspects, such as biomarkers for early cancer detection, as tools to monitor therapy, and to predict disease progression.Citation3 TAAs and circulating aAbs have been also reported in colorectal cancer (CRC) patients.Citation4 However, our knowledge on their role in immunosurveillance or tumorigenicity, is still limited.Citation2 While some previous reports have suggested that naturally occurring human antibodies could contribute to cancer progression, others have proposed an antitumor effect of aAbs. For example, human Fab fragments, specific for breast tumor driven antigens, have been shown to induce growth of breast cancer cells,Citation5 whereas in a separate study, anti-HER2 aAbs effectively blocked breast carcinoma colony formation by attenuating downstream signal transduction.Citation6 More recently, immune responses against TAAs were found to be critical in cancer immunoediting, and potentially immunotherapy.Citation7 It is thus crucial to study aAbs raised against TAAs to better evaluate their molecular, diagnostic and therapeutic potentials.
As a proof of concept study to evaluate potential aAbs in CRC patients, we have identified and isolated, for the first time, human monoclonal antibody Fab fragments against p21 (CDKN1A), a potent cyclin-dependent kinase inhibitor and a key mediator of p53-dependent cell cycle arrest after DNA damage. p21 is implicated in colorectal carcinogenesis, and is known to have aberrant expression in some colorectal tumors.Citation8
Peripheral blood samples of 25 previously untreated CRC patients and 13 healthy individuals were included in our study. Sera of the CRC patients and the controls were screened by ELISA for the presence of anti-p21 aAbs, and the relative antibody titers were determined by calculating the serum dilutions needed for half-maximum binding, or optical density (OD50) of antibodies (Abs) to p21 (GraphPad Prism 5, USA). Cancer patients had significantly higher OD50 values, and thus antibody titers, against p21 in comparison to healthy individuals (mean OD50 of 347 vs. 7.4, respectively; P < 0.0003 ). Compared to patients with lower serologic endpoint titers, CRC patients with higher endpoint titers of p21 antibodies tended to have more aggressive (i.e., advanced stage) CRC, such that 9/12 (75%) of patients with Stages III & IV had higher titers vs. 5/16 (31.2%) of those with early stage CRCs, P = 0.054 (). Also, 7/9 (78%) of patients with lymph node positivity had higher p21 antibody titers vs. 6/16 (37.5%) of patients with lymph node negativity, P = 0.09 ().
Figure 1. Serum concentrations of anti-p21 (CDKN1A) autoantibodies (aAbs) are higher in CRC patients correlating with advanced disease state. (A) Ninety-six well plates were coated with 1 μg/mL of recombinant p21 antigenic peptide (ab56278, Abcam). After blocking and washing steps, plates were incubated with serial dilutions of serum samples from 25 colorectal cancer (CRC) patients vs. serum from 13 normal controls. Binding was detected using horseradish peroxidase labeled goat anti-human Fc antibody and developed per standard protocol. Plates were read on a spectrophotometer at OD450. The optimal serum dilution to obtain 50% binding (OD50) was determined by averaging the results from 3 independent experiments. Statistical analysis was performed by ANOVA. (B-C) Distribution of CRC patients with high and low serum anti-p21 aAb titers, with a threshold half maximal effective concentration (EC50) = 4000, according to the disease stage (B); early, Stages I & II; advanced, Stages III & IV, and according to lymph node (LN) involvement (C). Statistical analysis was performed by 2-sided Fisher's exact test.
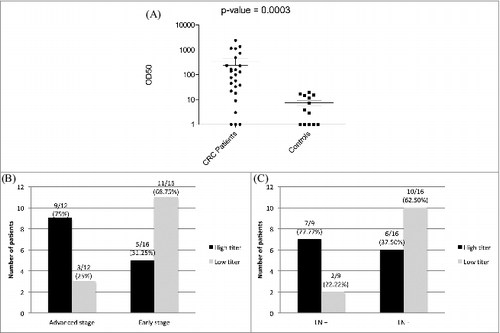
Figure 2. Colorectal cancer patient-derived Fab 26 detects p21 via western blot and Immunofluorescence assays. Colorectal cancer (CRC) patients with high antibody titers were used to derive anti-p21 Fab antibody fragments. Total RNA was isolated from freshly prepared peripheral mononuclear cells, reverse transcribed and subject to PCR to amplify variable heavy chain and light chain antibody regions. (2A) Western blot of cell lysates from C32 CRC cells overexpressing p21 and HCT116 p21−/−, a p21 null CRC cell line, probed by Fab 26 (Left panel) and mouse p21 (Right panel) revealing reliable and specific detection of p21 by Fab 26. (2B) Immunofluorescence staining of HCT116 cells using Fab 26 (upper panel) or monoclonal mouse p21 Ab (middle panel), compared to isotype control in the absence of Fab 26 and presence of secondary and fluorescence Ab (lower panel). Images were taken using confocal microscopy and the 40X objective. The DAPI staining of the stained nuclei are shown on the left for each respective immunofluorescence experiment. The depicted results are representative of 2 independent experiments.
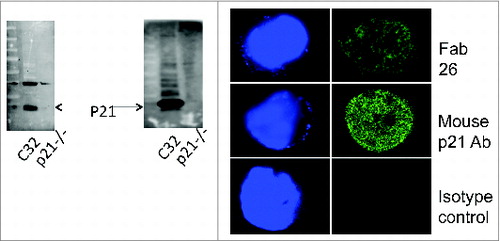
Next, we generated human Fab fragment phage surface display libraries from CRC patients with high anti-p21 antibody titers. Total RNA was isolated from peripheral blood mononuclear cells of CRC patients and subsequently analyzed by a 2100 Bioanalyzer RNA chip (Agilent Technologies). Variable antibody regions were amplified from cDNA with specific primers and converted into Fab fragments. Following panning with recombinant p21, a total of 30 p21-specific Fab fragments were identified. One of the tested crude Fab fragments (Fab26) revealed high reactivity to recombinant p21 (Fig. S1) and was used throughout this study. To check for integrity of Fab26, we sequenced and analyzed the variable and the constant regions of the heavy and light chain (Fig. S2). Fab26 was further overexpressed in E. coli and subsequently affinity purified using a nickel-charged nitrilotriacetic acid (Ni-NTA) column (Fig. S3). Purified Fab26 was subsequently used to detect p21 via Western blot analysis. Fab26 recognized p21 at the expected molecular weight in C32 cell lysate, a cell line overexpressing p21, whereas there was no immunoreactivity against p16−/−HCT116 cells (). An immunofluorescence assay and confocal microscopy with Fab26 revealed a positive nuclear localized signal in HCT-116 colon cancer cells, a staining pattern comparable to that of a mouse anti-p21 monoclonal Ab positive control (). In summary, the isolated and purified human CRC patient-derived Fab26 fragment detected p21 under native conditions confirming the validity of our approach.
Several mechanisms have been proposed to underlie the humoral immune response in cancer patients. Autoantibody production against tumors could be secondary to overexpression of cellular proteins, irrespective of whether the protein is located intracellularly (e.g., the tumor suppressor p53), or at the cell surface (e.g., oncogenic HER2).Citation3 Whereas antibody production against cell surface proteins is naturally anticipated, the mechanisms by which intracellular proteins elicit a humoral immune response are not clearly understood. In fact, many TAAs are intracellular products, and thus possible release by necrosis, cell lysis, or micro-vesicle shedding from the tumor site could be initiating triggers that stimulate the immune system.Citation3,9
The molecular functions of p21 are various and conflicting. Although initially considered a tumor suppressor protein, p21 has recently been shown to have oncogenic properties, depending on various factors, such as the time frame of expression within carcinogenesis, post-translational modifications of the protein, and subcellular localization. Contrary to nuclear p21, cytoplasmic p21 can have anti-apoptotic/oncogenic effects and is frequently correlated with aggressive phenotypes in human cancer.Citation10 The paraffin blocks of CRC patients in our study were not available for immunohistochemical analysis for p21 protein, and thus additional studies are needed to explore any potential correlations between the localization of p21 and the serum antibody titers in CRC patients.
Isolation and production of human antibodies against tumor antigens can provide opportunities to evaluate their functional properties more accurately. Some aAbs could exhibit anticancer molecular phenotypes, such as the occurrence of p53 aAbs shown to stabilize and even restore the function of mutant p53.Citation11 Furthermore, aAbs may have therapeutic implications. For example, it was previously shown in a mouse sarcoma model that anti-p53 antibodies inhibited engraftment of the murine sarcoma cell line MethA by acting as an anti-idiotypic vaccine.Citation12 Additionally, it must be borne in mind that adaptive immunity can potentially maintain cancer in an occult state by restoring tumor immunogenicity and restraining cancer growth.Citation13 Recent evidence indicates that TAAs are essential for escaping the immunoediting process in cancer.Citation14 Taken together, these data portend a role for antibodies generated against TAAs in immunotherapy.
Fab production and screening by phage display has been used for isolation of antibodies, or antibody fragments, to a variety of antigens and has been shown to accurately reflect the antibody response in vivo.Citation15 We found that Fab26 was reactive with both recombinant p21 and wild-type p21 from cancer cells, confirming that this approach can be successfully used in combination with other CRC specific antigens to characterize colorectal patient carcinomas.
Our study presents the first report of the isolation of circulating anti-p21 antibodies from CRC patients. We hereby demonstrate a feasible strategy for detecting and isolating aAbs from the sera of CRC patients, and ultimately generating a p21 reactive Fab fragment. This approach will likely prove useful to further study the functional significance of aAbs and potentially exploit their merits as immunotherapeutic agents in the treatment of CRC.
Material and Methods
Colorectal cancer patients and normal donors
The Scientific Review Committee of Northwestern University approved IRB protocols (Protocol Number: STU00057385) for CRC patient recruitment, and informed consent was obtained from all participants. The sera of 25 patients (median age 64 years, interquartile range 57–85) with histologic diagnosis of colon cancer and no prior therapy comprised this study. Volunteer healthy individuals (n = 13) without any known malignancy or ongoing health problem (median age 30 years, interquartile range 25–35) consented to participate and were included as a control group.
Serum ELISA for p21 reactive antibodies
Ninety-six well plates (Costar) were coated with p21 recombinant protein (ab56278, Abcam, Cambridge, MA) at 1 μg/mL (50 ng/well) over night at 4°C. Plates were washed and blocked for 1 h at room temperature (RT) with phosphate buffered saline (PBS) containing 4% non fat dry milk supplemented with 0.05% Tween-20. After washing, serial dilutions of patient serum samples and normal controls were added to the plate and incubated for an additional h at RT. After washing, bound antibodies were detected via secondary labeling with a horse-radish peroxidase (HRP)-labeled goat anti human Fc antibody (102652, Jackson ImmunoResearch, West Grove, PA) for 1 h at RT. Plates were then washed and developed using TMB (3,3′,5,5′-tetramethylbenzidine) solution. The reaction was finally stopped with 2N H2SO4 before reading at OD450 nm with a BIOTEK plate reader.
Fab screening ELISA
Plates were coated with 1 μg/mL p21 (50 ng/well) and incubated overnight at 4°C. After washing and blocking the plates, serially diluted patient-derived synthetic Fab fragments (see below) were added and incubated for 1 h at RT. Fab binding was detected with a HRP labeled goat anti-human Fab fragment specific antibody. After 1 h at RT plates were washed, developed, stopped, and read at OD450 nm, as described above.
Fab antibody phage libraries
Total RNA was isolated from freshly prepared peripheral mononuclear cells obtained from 10 mL of whole blood from CRC patient samples. The quality of the RNA was assessed using a 2100 Bioanalyzer RNA nano chip (Agilent Technologies, Inc. Santa Clara, CA). Next, 250 ng of total RNA was reverse transcribed into cDNA using a SuperScript III Reverse Transcriptase kit (Invitrogen, Carlsbad, CA). Variable heavy chain and light chain antibody regions were amplified with specific primers, as previously described.Citation16 The constant regions (CH1, kappa, and lambda) were then fused to the variable regions by overlap extension PCR. Finally, the synthesized heavy and light chains were ligated into the phagemid vector pCOMB3X. Electro-competent XL-1 blue cells were transformed with the Fab pCOMB3X constructs and infected with helper phage to assemble the Fab fragment on the surface of phage particles. Phage was isolated by polyethylene glycol precipitation and stored for subsequent panning rounds at 4°C.
Enriching antibody phage libraries for p21-reactive Fab fragments
Wells were coated o/n with 1 μg/mL of p21. Phage particles were amplified overnight in XL-1 blue cells and used the next day for the first round of selection. Prior to phage incubation, plates were blocked with 5% non-fat dry milk in PBS supplemented with 0.1% Tween-20 for 1 h at RT. Phage suspension was added and incubated for another 2 h at 37°C. Meanwhile, XL-1 blue cells were grown to an OD600 of 1.0. Wells were rigorously washed 3 times with PBS containing 0.1% Tween-20. The p21-bound phage was eluted using 0.1 M HCl (pH 2.0) and enriched by infecting XL-1 blue cells. The next day, the phage was isolated and subsequently used for a second round of selection. A total of 4 rounds were applied differing in the number of wash steps (i.e., 2 more wash cycles were added per round of panning).
Western blot
HCT116 p21−/− cells were kindly provided by B Vogelstein (Johns Hopkins, Baltimore, MD). A total amount of 25–50 μg C32 (sc-2205, Santa Cruz Biotechnology, Santa Cruz, CA) and HCT-116 p21−/− whole cell lysate was loaded onto a 4–20% gradient polyacrylamide gel. Proteins were electro-blotted onto nitrocellulose membranes and were further incubated with Fab26 (1:50) or mouse monoclonal p21 antibody (1:200 dilution, sc-817, Santa Cruz Biotechnology) overnight at 4°C. Bound antibodies were detected by an HRP-linked anti-human Fab and anti-mouse antibody conjugate (1:2000, Santa Cruz Biotechnology). Finally, secondary antibodies were detected by Enhanced Chemiluminescence detection reagent (ECL; Amersham, Little Chalfont, UK), and visualized with a LAS-3000 imager (FujifilmUSA, Valhalla, NY), as previously described.
p21 immunofluorescence staining and confocal microscopy
HCT-116 cell lines were seeded in chambered slides at 1,500 cells per chamber. Cells were fixed in 4% paraformaldehyde. After washing with ice cold PBS, the fixed cells were permeabilized by incubating with 0.5% Triton X-100 in PBS for 10 min. The chambers were emptied, washed and blocked with 20% bovine serum albumin (w/v) in PBS for 1 h. Cells were then stained by overnight incubation with purified Fab26 (1:25 dilution) or monoclonal mouse p21 (1:200 dilution, sc-817, Santa Cruz Biotechnology). The next day, the cells incubated with Fab26 were washed with PBS, and were incubated with goat-anti human Fab (1:2000, Santa Cruz Biotechnology, Santa Cruz, CA) for 1 h. As a control experiment, cells were only incubated with goat-anti human Fab in the absence of Fab26. All the chambers, were washed and incubated for 1 h at RT with anti-human or anti-mouse Alexa Fluor 488 (Invitrogen) to detect Fab26 and p21, respectively. Cells were mounted in Prolong Gold Antifade with 4,6-diamidino-2-phenylindole (DAPI; Invitrogen) and cured at RT for 1 h before visualizing. Images were taken with a 40X objective lens (40X DIC MN2 Plan APO, NA:0.9, WD: 140 micrometer).
Fab26 purification
Purification was done using AKTAxpress FPLC automated system (GE Healthcare). Sample was applied to HisTrap FF crude Ni column (cat # 17-5286-01, GE Healthcare) at flow rate of 5 mL/min. Purified fractions were collected into 96 Well Standard Assay Block (cat# 3961, Corning Inc.) at 2 mL per well. Binding buffer contained 20 mM sodium phosphate, 500 mM NaCl, and 20 mM imidazole. Elution buffer contained 20 mM sodium phosphate, 500 mM NaCl, and 500 mM imidazole.
Statistical analyses
All statistical analyses were performed using GraphPad Prism version 6.00 for Windows (GraphPad Software, La Jolla, Calif; www.graphpad.com). 2-sided Fisher's exact test and analysis of variance (ANOVA) were used to compare categorical and numerical variables respectively, as appropriate. A p-value of less than 0.05 was considered statistically significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplemental Materials
Supplemental materials for this article are available at the publisher's website.
952202_Supplementary_Materials.zip
Download Zip (541.4 KB)952202_Supplementary_Materials.zip
Download Zip (522.6 KB)Acknowledgments
We thank Bert Vogelstein for the HCT116 p21 -/- cell line. Recombinant Protein Production Core (rPPC), CLP at Northwesten University helped in purifying Fab 26 using Ni-NTA column. We thank Khashayarsha Khazaie for providing the samples, and Nancy Krett for editing the manuscript. FB and JSG were involved in study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, and statistical analysis. NA, MKW and JB were involved in acquisition of data, technical support and approval of final draft. BJ was involved in study concept and design, interpretation of data, critical revision of the manuscript for important intellectual content, material support and study supervision, and also obtained funding.
Funding
This work was supported by the National Institutes of Health, grant R01CA141057. Faraz Bishehsari was supported by National Institutes of Health, Institutional NRSA, Clinical Oncology Research Training Program T32 CA079447. Imaging work was performed at the Northwestern University Center for Advanced Microscopy supported by NCI CCSG P30 CA060553 awarded to the Robert H Lurie Comprehensive Cancer Center and the Nikon Imaging Center at Northwestern University.
References
- Jaras K, Anderson K. Autoantibodies in cancer: prognostic biomarkers and immune activation. Expert Rev Proteomics 2011; 8(5):577-89; PMID:21999829; http://dx.doi.org/10.1586/epr.11.48
- Chen H, Werner S, Tao S, Zörnig I, Brenner H. Blood autoantibodies against tumor-associated antigens as biomarkers in early detection of colorectal cancer. Cancer Lett 2014; 346(2):178-87; PMID:24462820; http://dx.doi.org/10.1016/j.canlet.2014.01.007
- Heo CK, Bahk YY, Cho EW. Tumor-associated autoantibodies as diagnostic and prognostic biomarkers. BMB Rep 2012; 45(12):677-85; PMID:23261052; http://dx.doi.org/10.5483/BMBRep.2012.45.12.236
- Pedersen JW, Gentry-Maharaj A, Nøstdal A, Fourkala EO, Dawnay A, Burnell M, Zaikin A, Burchell J, Papadimitriou JT, Clausen H, et al. Cancer associated auto-antibodies to MUC1 and MUC4 - A blinded case-control study of colorectal cancer in UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS). Int J Cancer 2014; 134(9):2180-8; PMID:24122770.
- Wen YJ, Mancino A, Pashov A, Whitehead T, Stanley J, Kieber-Emmons T. Antigen binding of human IgG Fabs mediate ERK-associated proliferation of human breast cancer cells. DNA Cell Biol 2005; 24(2):73-84; PMID:15699628; http://dx.doi.org/10.1089/dna.2005.24.73
- Montgomery RB, Makary E, Schiffman K, Goodell V, Disis ML. Endogenous anti-HER2 antibodies block HER2 phosphorylation and signaling through extracellular signal-regulated kinase. Cancer Res 2005; 65(2):650-6; PMID:15695410
- DuPage M, Mazumdar C, Schmidt LM, Cheung AF, Jacks T. Expression of tumour-specific antigens underlies cancer immunoediting. Nature 2012; 482(7385):405-9; PMID:22318517; http://dx.doi.org/10.1038/nature10803
- Watanabe T, Wu TT, Catalano PJ, Ueki T, Satriano R, Haller DG, Benson AB 3rd, Hamilton SR. Molecular predictors of survival after adjuvant chemotherapy for colon cancer. N Engl J Med 2001; 344(16):1196-206; PMID:11309634; http://dx.doi.org/10.1056/NEJM200104193441603
- Somers VA, Brandwijk RJ, Joosten B, Moerkerk PT, Arends JW, Menheere P, Pieterse WO, Claessen A, Scheper RJ, Hoogenboom HR, et al. A panel of candidate tumor antigens in colorectal cancer revealed by the serological selection of a phage displayed cDNA expression library. J Immunol 2002; 169(5):2772-80; PMID:12193752; http://dx.doi.org/10.4049/jimmunol.169.5.2772
- Abbas T, Dutta A. p21 in cancer: intricate networks and multiple activities. Nat Rev Cancer 2009; 9(6):400-14; PMID:19440234; http://dx.doi.org/10.1038/nrc2657
- Hansen S, Hupp TR, Lane DP. Allosteric regulation of the thermostability and DNA binding activity of human p53 by specific interacting proteins. CRC Cell Transformation Group. J Biol Chem 1996; 271(7):3917-24; PMID:8632013
- Ruiz PJ, Wolkowicz R, Waisman A, Hirschberg DL, Carmi P, Erez N, Garren H, Herkel J, Karpuj M, Steinman L, et al. Idiotypic immunization induces immunity to mutated p53 and tumor rejection. Nat Med 1998; 4(6):710-2; PMID:9623981; http://dx.doi.org/10.1038/nm0698-710
- Koebel CM, Vermi W, Swann JB, Zerafa N, Rodig SJ, Old LJ, Smyth MJ, Schreiber RD. Adaptive immunity maintains occult cancer in an equilibrium state. Nature 2007; 450(7171):903-7; PMID:18026089; http://dx.doi.org/10.1038/nature06309
- Vesely MD, Schreiber RD. Cancer immunoediting: antigens, mechanisms, and implications to cancer immunotherapy. Ann N Y Acad Sci 2013; 1284:1-5; PMID: 23651186; http://dx.doi.org/10.1111/nyas.12105
- Coomber DW, Hawkins NJ, Clark MA, Ward RL. Generation of anti-p53 Fab fragments from individuals with colorectal cancer using phage display. J Immunol 1999; 163(4):2276-83; PMID:10438972
- Rader C. Generation of human Fab libraries for phage display. Methods Mol Biol 2012; 901:53-79; PMID:22723094; http://dx.doi.org/10.1007/978-1-61779-931-0_4
