Abstract
Programmed cell death ligand 1 (PDL1) expression was recently shown to correlate with tumor-infiltrating lymphocytes (TILs) in a subset of osteosarcoma patients. Among clinical factors evaluated across human osteosarcoma samples, a pulmonary origin of metastases correlated with high PDL1 expression and prominent TILs. Considering that multiple agents targeting PD-1/PDL1 are under development, targeting this immune checkpoint may be a novel immunotherapeutic route for osteosarcoma in future clinical trials.
Keywords:
Commentary
Osteosarcoma is an aggressive malignant tumor of the bone that is the eighth most common form of childhood cancer. The 5-year survival rate with current multimodal therapies is approximately 70%. However, progress has slowed over the last 30 years, and treatments with intensifying regimens or adding novel agents have shown limited improvement. Moreover, for patients with metastatic osteosarcoma at diagnosis and for those with relapsed disease, the 4- to 5-year overall survival rate is less than 20%.Citation1 Therefore, the development of novel therapeutic strategies is critical for this cancer patient population.
In the oncology community, there has been an increasing interest in the mechanisms of cancer cell immune tolerance and escape, with a particular focus on the T-cell coinhibitory receptor programmed cell death 1 (PDCD1, better known as PD-1) and its corresponding B7 family of ligands, including programmed cell death ligand 1 (PDL1), (). T-cell responses follow a complex signaling sequence through the T-cell receptor to either stimulate, or inhibit, the immune response. PD-1 is a cell-surface receptor expressed on subsets of T and B lymphocytes, as well as other immune cells. In the inflammatory microenvironment, stimuli such as interferon γ (IFNγ) may upregulate PDL1 expression on tumor cells to suppress the immune response. A number of malignancies, including melanoma, lung cancer, renal cell carcinoma, ovarian cancer, and colorectal cancers, have been demonstrated to exploit these inhibitory signals by upregulating PDL1 constitutively or in response to cancer-associated inflammation.Citation2 The expression of PDL1 expression in osteosarcoma, however, had, so far, remained obscure.
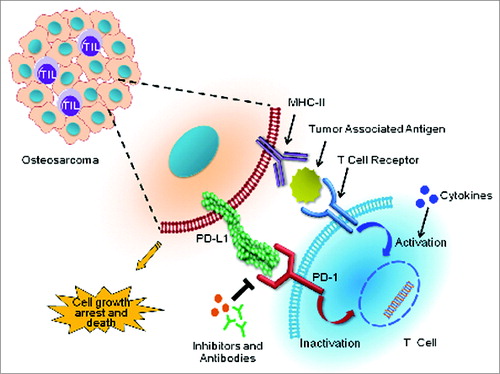
Figure 1. Immunotherapeutic targeting of PD-1 and PDL1 reciprocal interactions between osteosarcoma cells and T cells. Binding of the T-cell inhibitory receptor programmed cell death 1 (PD-1) to its cognate ligand PDL1 on the surface of cancer cells inactivates T-cell cytotoxic functions that otherwise would induce osteosarcoma cell death. Abrogating this pathway with anti-PD-1 or PDL1 blocking antibodies (or inhibitors) prevents this inhibition, and allows the reactivation of T-cell anticancer cytotoxic functions. Adding immune activators, such as cytokines, may elicit a synergistic response to anti-PD-1/PDL1 targeted therapies in osteosarcoma.
Recent immunohistochemical analyses suggest that PDL1 expressionmay be an important prognostic biomarker, perhaps particularly when combined with T-cell activation signals. Despite these indications, diagnostics for PDL1 are limited by the lack of reliable protein-based assays, and the literature remains controversial. Prior research has shown a correlation between PDL1 mRNA and protein expression. Accordingly, we sought to develop an RNA-based PDL1 assay to circumvent the technical issues constraining the IHC-based strategies. To our knowledge, our study was the first to investigate PDL1 expression in osteosarcoma.
Using our newly developed RNA-based assay to examine PDL1 expression, we showed PDL1 expression in 84.2% of osteosarcoma patient samples, with high levels in 24% of patient tumors. Expression levels were evaluated against clinical factors such as age/gender, eventual metastasis or recurrence, chemotherapy, percentage of necrosis, and survival, as well as origin of metastasis, if present. There was a slight trend for poorer overall survival for osteosarcoma patients with high PDL1 expression, but no other correlation between PDL1 expression and the manifestation of clinicopathologic features was found. However, these data are likely confounded by the small, heterogeneous sample size. Furthermore, we examined the presence of tumor-infiltrating lymphocytes (TILs), and found a positive correlation between TILs and PDL1 expression in our tumor samples. We found higher levels of expression of PDL1 and corresponding TILs in metastases that originated in osteosarcoma of the lung versus nonpulmonary sites. Of note, the PDL1 expression detected may arise either from osteosarcoma cells or the associated TILs. Regardless, the presence of PDL1 itself remains relevant to determine potential application of immune checkpoint-based therapies. Future studies will examine the subtypes of lymphocytes present in biopsies, as well as search for evidence of T-cell activation. Taken together, these findings suggest that there is a subset of patients for whom PDL1-directed therapies could be relevant, and sets an important framework to design anti-PD-1 or anti-PDL1 immune checkpoint therapies in osteosarcoma patients.
Currently, there are multiple agents targeting the PD-1/PDL1 system at various stages of clinical development. Several early-stage studies of inhibitory antibodies aimed at PD-1 (pembrolizumab, nivolumab) or PDL1 (BMS-936559, MPDL3280A) have shown remarkable clinical activity and, importantly, durability across a range of malignancy subtypes, including renal cell carcinoma, colorectal cancer, melanoma, and small cell and non-small cell lung cancer.Citation3-8 These PD-1/PDL1 targeted therapies also hold potential in adjuvant settings. Preliminary data have suggested that there may be a relationship between the activity of these agents, PDL1 expression, and/or immune cells in the tumor microenvironment. An example of this corollary includes coordinated expression of PDL1, CD8+ T-cells, and a T-cell activation gene signature among cancer patients with treatment response versus nonresponders who exhibited minimal CD8+ T-cell infiltration and an absence of T-cell activation.Citation9
These observations have led to additional studies exploring combination therapies involving immunoregulatory pathways. These include immune activating cytokines (e.g., interferons, IL-21, IL-2), vascular endothelial growth factor (VEGF) inhibitors (e.g., bevacizumab, sunitinib, pazopanib), and other immune checkpoint antibody blockers (e.g., ipilimumab, anti-LAG-3, and anti-TIM-3) in lung cancers and renal cell carcinoma.Citation6,8,10 Anti-PD-1 or anti-PDL1 blocking antibody in combination with BRAF inhibition induced a positive synergistic response in melanoma patients, and significantly prolonged survival, slowed tumor growth, and increased both the number and activity of TILs.Citation5
Osteosarcoma remains one of the deadliest malignancies among children and adults, as the heterogeneity of osteosarcoma has made it difficult to develop targeted therapies. Therefore, immunotherapeutic approaches may be an attractive alternative treatment option for osteosarcoma patients. Immunotherapy can be used either alone or in combination with traditional chemotherapies. From the results of our study demonstrating the correlation of PDL1 expression and TILs in osteosarcoma patients, immune checkpoint blockade holds great potential to improve patient outcomes through the development of rational combinations of molecularly targeted therapies and immunotherapies.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We would like to thank Yan Gao for her contribution to the design of the figure.
Funding
This work was supported in part by grants from the Gattegno and Wechsler funds. JK Shen is supported by the Jennifer Hunter Yates Foundation and the Kenneth Stanton Osteosarcoma Research Fund. Z Duan is supported, in part, through a grant from Sarcoma Foundation of America, a developmental research award from Sarcoma SPORE (NCI), a grant from NCI/NIH, UO1, CA151452–01, and a grant from an Academic Enrichment Fund of MGH Orthopedic Surgery.
References
- Shen JK, Cote GM, Choy E, Yang P, Harmon D, Schwab J, Nielsen GP, Chebib I, Ferrone S, Wang X, et al. Programmed cell death ligand 1 expression in osteosarcoma. Cancer Immunol Res 2014; 2(7):690-8; PMID:24866169; http://dx.doi.org/10.1158/2326-6066.CIR-13-0224
- Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 2002; 8:793-800; PMID:12091876; http://dx.doi.org/10.1038/nm0902-1039c
- Herbst RS, Gordon MS, Fine GD, Sosman JA, Soria J-C, Hamid O, Powderly JD, Burris HA, Mokatrin A, Kowanetz M, et al. A study of MPDL3280A, an engineered PD-L1 antibody in patients with locally advanced or metastatic tumors. ASCO Meeting Abstr 2013; 31:3000.
- Sullivan RJ, Lorusso PM, Flaherty KT. The intersection of immune-directed and molecularly targeted therapy in advanced melanoma: where we have been, are, and will be. Clin Cancer Res: Official J Am Assoc Cancer Res 2013; 19:5283-91; PMID:24089441; http://dx.doi.org/10.1158/1078-0432.CCR-13-2151
- Cooper ZA, Juneja VR, Sage PT, Frederick DT, Piris A, Mitra D, Lo JA, Hodi FS, Freeman GJ, Bosenberg MW, et al. Response to BRAF inhibition in melanoma is enhanced when combined with immune checkpoint blockade. Cancer Immunol Res 2014; 2(7):643-54; PMID:24903021; http://dx.doi.org/10.1158/2326-6066
- Aerts JG, Hegmans JP. Tumor-specific cytotoxic T cells are crucial for efficacy of immunomodulatory antibodies in patients with lung cancer. Cancer Res 2013; 73:2381-8; PMID:23580578; http://dx.doi.org/10.1158/0008-5472.CAN-12-3932
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012; 366:2443-54; PMID:22658127; http://dx.doi.org/10.1056/NEJMoa1200694
- Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012; 366:2455-65; PMID:22658128; http://dx.doi.org/10.1056/NEJMoa1200694
- Powderly JD, Koeppen H, Hodi FS, Sosman JA, Gettinger SN, Desai R, Tabernero J, Soria J-C, Hamid O, Fine GD, et al. Biomarkers and associations with the clinical activity of PD-L1 blockade in a MPDL3280A study. ASCO Meeting Abstr 2013; 31:3001.
- Philips GK, Atkins MB. New agents and new targets for renal cell carcinoma. ASCO Educ Book 2014:e222-7; PMID:24857106; http://dx.doi.org/10.14694/EdBook_AM.2014.34.e222
