Abstract
Tumor recurrence remains a major problem for patients with cancer, even after initial beneficial responses to standard-of-care chemotherapeutic agents. With the recent advances in immunotherapy strategies, there is growing interest in synergistically combining immunotherapy with conventional chemotherapy to achieve durable antitumor effects. In some cases, chemotherapy-induced myeloid suppressor cells represent a critical obstacle to achieving this goal.
Cytotoxic chemotherapy has been historically viewed as being incompatible with immunotherapy due to its generally lymphodepleting effects. This perception was later challenged by mounting evidence from animal studies showing that the host immune system actually contributes to the efficacy of a number of anticancer drugs. In recent years, the cellular and molecular mechanisms by which certain anticancer drugs elicit antitumor immune responses have begun to be unraveled. The alkylating agent cyclophosphamide (CTX) represents a prototypical immune-potentiating anticancer drug. The immunostimulatory effects of CTX are attributable to its ability to: (i) provoke immunogenic cell death (ICD); ii) deplete/inactivate T regulatory cells (Tregs); and (iii) induce lymphopenia and accompanying cytokine storm.Citation1 These properties justify selecting CTX as a popular choice for chemo-immunotherapy combinatorial regimens in which it has been used to debulk tumors and condition the host for subsequent immunotherapies.
While much attention has been focused on the immunostimulatory effects of CTX, the immunosuppressive aspect of this chemodrug has been noticed for years. Early studies showed that in addition to its direct lymphodepletive effect, CTX can induce cells with immunosuppressive activities.Citation2 Later studies identified these suppressor cells as myeloid-derived suppressor cells (MDSCs).Citation3,4 We recently reported that CTX can induce the expansion of myeloid cells consisting of monocytic and granulocytic subsets, and only the monocytic subset harbors T-cell suppressive activity.Citation5 We herein term these CTX-induced immunosuppressive monocytic myeloid cells “CTX-MDSCs”. We subsequently showed that selective depletion of CTX-MDSCs following chemo-immunotherapy in a mouse model of B-cell lymphoma significantly improved long-term survival, providing evidence that CTX-MDSCs contribute to tumor immune evasion and relapse.Citation5
Our study provides new insights regarding the ontogeny of CTX-MDSCs. We found that the doses of CTX that result in significant myeloid cell expansion in mice fell into the medium-high range (100–300 mg/kg), whereas metronomic CTX doses (10–40 mg/kg) failed to induce myeloid cells. Paradoxically, the same dose range of CTX that elicits myeloid suppressor cells also engenders immunostimulatory effects. We speculate that the same mechanisms that underlie CTX's immunostimulatory effects may also lead to the manifestation of CTX's immunosuppressive effect. Our hypothesized scenario is that following CTX-induced myelo-leukodepletion, immature myeloid cells undergo homeostatic proliferation in the midst of a surge of inflammatory cytokines, chemokines and growth factors. These events converge to drive myeloid cell expansion along with the unfortunate acquisition of suppressive activity as well. We showed that this phenomenon occurred in mice after chemotherapy even in the absence of tumor, suggesting that induction of MDSCs is a fundamental counter-regulatory mechanism employed by the host in response to chemical insults to prevent excessive, and potentially detrimental, inflammatory responses. At the molecular level, granulocyte macrophage colony stimulating factor (GM-CSF), colony stimulating factor 3 (G-CSF/Csf3), IL-1β, IL-6, and (C-C) motif chemokine ligand 2 (CCL2) are among inflammatory mediators induced after CTX treatment,Citation6,7 candidate effector molecules that may promote the expansion of CTX-MDSCs. We showed that this inflammatory milieu was further heightened by the presence of T helper type 1 (Th1) CD4+ effector cells, which might explain why CD4+ effector cells can amplify CTX-MDSCs.Citation5,7 The exact inflammatory mediators and signaling pathways involved in this process remain to be defined.
Numerous studies have shown that constituent tumor cells and tumor-derived factors drive aberrant differentiation of immature myeloid cells toward MDSCs. Tumor-induced MDSCs also contain 2 major subsets, mononuclear MDSCs (MO-MDSC) and polymorphonuclear MDSCs (PMN-MDSC). Intriguingly, the known markers for CTX-MDSCs and tumor-induced MO-MDSCs are similar (CD11b+Ly6ChiLy6G−GR1int), raising the question of whether CTX-MDSCs and tumor-induced MO-MDSCs are one and the same. However, despite their phenotypical resemblance, CTX-MDSCs and tumor-induced MO-MDSCs differ in their induction kinetics and immunosuppression mechanisms. Specifically, tumor-induced MO-MDSCs appear and accumulate as tumors progress, whereas CTX-induced myeloid suppressor cells emerge after chemotherapy. Moreover, tumor-induced MO-MDSCs mediate T-cell suppression via nitric oxide synthase (iNOS) and arginase, whereas CTX-induced myeloid suppressor cells attenuate T-cell activation in a programmed cell death 1 (PD-1)-dependent manner. Our data, in line with the study by Mikyskova et al,Citation4 support the notion that CTX-MDSCs are not identical to tumor-induced MDSCs. Future studies should address the relationship and interplay between these 2 suppressor cell populations.
Besides CTX, we found that 2 other widely used anticancer drugs (doxorubicin and melphalan) exhibit a similar effect,Citation5 suggesting that induction of myeloid suppressor cells is an inherent property of certain anticancer drugs. Future studies should identify additional MDSC-inducing chemotherapeutic agents. In humans, chemotherapy-driven MDSC expansion has been observed in cancer patients and appears to correlate with increased metastatic tumor burden.Citation8 In the broader context of cancer therapy, other treatment modalities can also induce MDSCs, including radiation therapy and adoptive T-cell therapy.Citation9,10 Taken together, these data suggest the presence of a previously unrecognized obstacle to successful cancer therapies − a robust inflammatory response, which is an expected outcome of many seemingly effective cancer therapies. Therapy-induced inflammation may elicit myeloid suppressor cells that counteract the ultimate efficacy. Our study underscores the importance and necessity of controlling therapy-induced myeloid suppressor cells. We found that treatment with gemcitabine or 5’fluorouracil (5-FU), drugs known to deplete tumor-induced MDSCs, was able to deplete CTX-MDSCs. Moreover, targeting (C-C) chemokine receptor 2 (CCR2) with monoclonal antibody or small molecule antagonist appeared to be effective in reducing the accumulation of CTX-MDSCs. Furthermore, all-trans-retinoic acid (ATRA) or IL-12 can be used to reduce the number and alter the function of CTX-MDSCs.Citation4 Finally, our data have implicated the potential clinical benefit of amelioration via PD-1 blockade therapy. We found that PD-1 blockade can abrogate CTX-MDSC-mediated suppression, providing a rationale for combining PD-1 blockade with conventional chemotherapy.
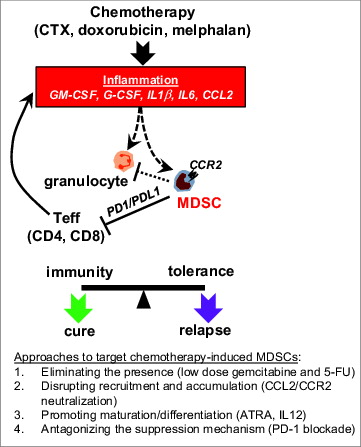
Based on our results, we propose that certain anticancer drugs are bi-functional in immunomodulation (). On one hand, chemotherapy can “reset” the tumor microenvironment, helping to create an immunogenic milieu conducive to immunotherapies. On the other hand, chemotherapy-induced inflammatory responses may induce MDSCs that counter-regulate antitumor immune responses. Therefore, the net impact of chemotherapy on tumor immunity is a dynamic balancing act between its 2 opposing immunomodulatory effects. Targeting therapy-induced myeloid suppressor cells is conducive to robust responses to immunotherapies during the post-chemotherapy window, thereby tilting the balance toward a durable, and beneficial, clinical outcome.
Figure 1. Chemotherapy-induced MDSCs represent a critical obstacle to successful chemo-immunotherapy. Some standard-of-care chemotherapeutic agents, exemplified by cyclophosphamide (CTX), doxorubicin and melphalan, can induce the expansion of myeloid-derived suppressor cells (MDSCs), possibly through the action of inflammatory mediators including GM-CSF, G-CSF, IL1β, IL6 and CCL2. These therapy-induced MDSCs are highly proliferative and express high levels of CCR2. Effector T cells (particularly Th1-type CD4+ effector cells) can amplify chemotherapy-induced MDSCs, likely by intensifying the inflammatory milieu. These chemotherapy-induced MDSCs suppress T-cell activation in a PD-1-dependent manner. Chemotherapy also leads to expansion of granulocytes, which contribute to tumor rejection. Chemotherapy-induced MDSCs may suppress the antitumor activity of granulocytes. Targeting therapy-induced MDSCs can thus prevent immune tolerance and tip the balance toward durable antitumor immunity. Approaches that can potentially target chemotherapy-induced MDSCs are listed. Dotted lines indicate hypotheses yet to be tested.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Additional information
Funding
References
- Sistigu A, Viaud S, Chaput N, Bracci L, Proietti E, Zitvogel L. Immunomodulatory effects of cyclophosphamide and implementations for vaccine design. Semin Immunopathol 2011; 33:369-83; PMID:21611872; http://dx.doi.org/10.1007/s00281-011-0245-0
- McIntosh, KR, Segre, M, Segre, D. Characterization of cyclophosphamide-induced suppressor cells. Immunopharmacology 1982; 4:279-89; PMID:6181012; http://dx.doi.org/10.1016/0162-3109(82)90049-2
- Angulo I, de las Heras FG, García-Bustos JF, Gargallo D, Muñoz-Fernández MA, Fresno M. Nitric oxide-producing CD11b(+)Ly-6G(Gr-1)(+)CD31(ER-MP12)(+) cells in the spleen of cyclophosphamide-treated mice: implications for T-cell responses in immunosuppressed mice. Blood 2000; 95:212-20; PMID:10607705
- Mikyskova R, Indrová M, Polláková V, Bieblová J, Símová J, Reiniš M. Cyclophosphamide-induced myeloid-derived suppressor cell population is immunosuppressive but not identical to myeloid-derived suppressor cells induced by growing TC-1 tumors. J Immunother 2012; 35:374-84; PMID:22576342; http://dx.doi.org/10.1097/CJI.0b013e318255585a
- Ding ZC, Lu X, Yu M, Lemos H, Huang L, Chandler P, Liu K, Walters M, Krasinski A, Mack M, et al. Immunosuppressive Myeloid Cells Induced by Chemotherapy Attenuate Antitumor CD4+ T-Cell Responses through the PD-1-PD-L1 Axis. Cancer Res 2014; 74(13):3441-53; PMID:24780756; http://dx.do.org/10.1158/0008-5472.CAN-13-3596
- Moschella F, Valentini M, Aricò E, Macchia I, Sestili P, D'Urso MT, Alessandri C, Belardelli F, Proietti E. Unraveling cancer chemoimmunotherapy mechanisms by gene and protein expression profiling of responses to cyclophosphamide. Cancer Res 2011; 71:3528-39; PMID:21444678; http://dx.doi.org/10.1158/0008-5472.CAN-10-4523
- Ding ZC, Huang L, Blazar BR, Yagita H, Mellor AL, Munn DH, Zhou G. Polyfunctional CD4(+) T cells are essential for eradicating advanced B-cell lymphoma after chemotherapy. Blood 2012; 120:2229-39; PMID:22859605; http://dx.doi.org/10.1182/blood-2011-12-398321
- Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother 2009; 58:49-59; PMID:18446337; http://dx.doi.org/10.1007/s00262-008-0523-4
- Kodumudi, KN, Weber, A, Sarnaik AA, Pilon-Thomas S. Blockade of myeloid-derived suppressor cells after induction of lymphopenia improves adoptive T cell therapy in a murine model of melanoma. J Immunol 2012; 189:5147-54; PMID:23100512; http://dx.doi.org/10.4049/jimmunol.1200274
- Hosoi A, Matsushita H, Shimizu K, Fujii S, Ueha S, Abe J, Kurachi M, Maekawa R, Matsushima K, Kakimi K. Adoptive cytotoxic T lymphocyte therapy triggers a counter-regulatory immunosuppressive mechanism via recruitment of myeloid-derived suppressor cells. Int J Cancer 2014; 134:1810-22; PMID:24150772; http://dx.doi.org/10.1002/ijc.28506
