Abstract
Therapeutic targeting of the CD40 pathway may be efficacious for cancer treatment. Accumulating evidence suggests synergistic and unique antitumor responses may be achieved using CD40-based therapies in combination with other immunomodulators or targeted agents.
CD40-CD40 ligand interactions constitute a critical co-stimulatory pathway for promoting antigen presenting cell (APC) activation and CD8+ T cell-mediated protective immunity to cancer. Agonistic antibodies to CD40 (αCD40) represent promising therapeutic options to generate antitumor immunity by both direct and indirect mechanisms. We, and others, have shown that αCD40 mediates antitumor responses indirectly by stimulating APC activation, as well as directly by triggering CD40-positive tumor cells to produce cytokines,Citation1 express the death receptor Fas and induce Fas-dependent tumor cell apoptosis.Citation2
Upregulated expression of CD40 has been noted in nephritis and other renal diseases. However, until recently no correlation between renal cell carcinoma (RCC)-associated CD40 expression and patient outcome had been described. Since ligation of tumor-associated CD40 has direct anticancer effects, we speculated that CD40 expression might have prognostic value in RCC. In our retrospective study, we found that tumor-associated CD40 expression was highest among long-term survivors (>8 year follow-up time).Citation3 In contrast, short-term survivors (<4 year follow-up time) exhibited significantly reduced CD40 expression. Furthermore, the levels of CD40 expression in RCC patient samples directly correlated with incidence of tumor cell apoptosis and CD8+ T cell frequency.Citation3 Although our findings do not permit definitive attribution of antitumor responses to CD40 in RCC, they do support its value as a biomarker of patient survival and provide rationale to interrogate targeting CD40 as a therapeutic avenue in RCC. Further studies of patient tumor samples could prove valuable to determine whether responsiveness to particular immunotherapies, such as αCD40, IL-2, and interferon (IFN), relate to tumor-associated CD40 expression.
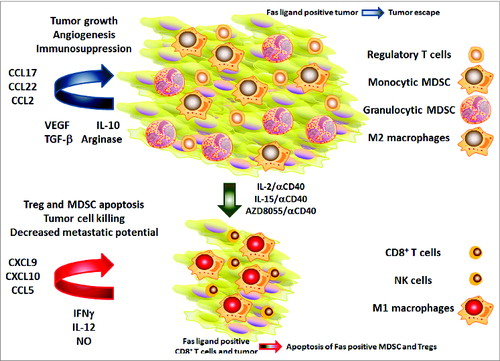
Figure 1. CD40-based combination immunotherapy restructures the tumor microenvironment. Tumor-promoting immunoregulatory cells often prevail within the tumor microenvironment (upper half). These cells contribute to tumor growth, angiogenesis and immunosuppression through the expression of immunoregulatory cytokines as well as chemokines responsible for regulatory T cell (Treg) and myeloid-derived suppressor cell (MDSC) recruitment. Tumor-associated Fas ligand expression under normal conditions may contribute to tumor escape by eliciting death of Fas-ligand positive effector T cells. After treatment with anti-CD40 agonistic antibody in combination with IL-2, IL-15 or the mTOR-kinase inhibitor AZD8055 (bottom half) therapy, the tumor environment is converted into one in which T helper type 1 (Th1) cytokines and effector immune cells predominate. These cells mediate apoptosis of Tregs and MDSCs, and drive malignant cell killing, therebyreducing tumor metastatic potential. IFNγ, interferon γ; NK, natural killer; NO, nitric oxide, TGFβ, transforming growth factor β; VEGF, vascular endothelial growth factor.
The ability of agonistic CD40 antibodies (referred to as αCD40) or CD40 ligand to stimulate immune responses and target tumors suggests such reagents have promise as cancer immunotherapeutics. Initial clinical trials of αCD40 in the treatment of pancreatic ductal adenocarcinoma, and other solid cancers, have shown promising results, both in single-agent studies and in combination with chemotherapy.Citation4 In these pilot studies, the use of agonistic CD40 antibody was found to be safe and associated with partial antitumor responses.
Unfortunately, αCD40 also has the potential to elicit undesirable effects that may limit its usefulness for cancer therapy. For example, CD40 ligation may induce the production of vascular endothelial growth factor (VEGF) by endothelial cells and monocytes, thereby promoting angiogenesis in vivo,Citation5. CD40 agonists may also stimulate the genesis of regulatory B cells (Bregs), known to induce T-cell tolerance and control inflammation.Citation6 The CD40-dependent effects in stimulating Breg generation may thus limit the utility of an αCD40-based single agent in cancer therapy. In our own studies, we found that monotherapy with αCD40 elicited (C-C) motif chemokine ligand/receptor 2 (CCL2/CCR2)-dependent leukocyte responses, but failed to induce removal of regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs) within the kidney tumor microenvironment.Citation7 Furthermore, αCD40 antibody therapy only marginally reduced tumor burden, with no significant effect upon lung metastases or long-term survival.
Despite the potential limitations for αCD40 monotherapy, over the past 13 years our studies suggest αCD40 may be effective when used in combination with other immune- or molecularly targeting agents. For example, when used alone, αCD40 was completely dependent upon CCR2, the CCL2/MCP-1 receptor, which is associated with MDSC recruitment. However, when IL-2 or IL-15 were administered in combination with αCD40, the result was an interferon γ (IFNγ)-dependent, T helper type 1 (Th1) cytokine and chemokine profile associated with elevated levels of nitric oxide (NO),Citation8 reduced frequency of tumor-associated Tregs and MDSCs, and more durable antitumor responses.Citation7
In another approach, we hypothesized that the combination of tumor-targeting agents with αCD40 would achieve more durable antitumor responses via both direct cancer cell killing and activation of APC for enhanced tumor antigen uptake. In collaboration with AstraZeneca, we showed that the first in-class orally bioavailable mTOR kinase inhibitor, AZD8055 administered in combination with αCD40 inhibited metastatic renal cancer tumor progression in liver metastatic foci.Citation9 Interestingly, AZD8055/αCD40, but not rapamycin/αCD40, significantly increased immune cell infiltration into the tumor microenvironment and induced the expression of Th1-associated cytokines. To our knowledge, this is one of the first demonstrations that molecularly targeted agents, when used in combination with immunotherapeutics such as αCD40, elicit Th1-associated antitumor responses not achievable using either agent alone. Overall, our findings demonstrate the feasibility of preferentially redistributing immune effector and regulatory cells specifically within the tumor microenvironment by IFNγ-dependent combinatorial immunotherapy.
Using 2 different murine tumor models (Renca and 4T1) we found that Treg and MDSC loss within the tumor microenvironment after IL-2/αCD40 treatment occurs through a Fas-dependent cell death pathway.Citation10 Tumor-infiltrating CD8+ T cells, neutrophils and immature myeloid cells expressed Fas ligand following treatment, immune cells which could potentially engage Fas expressed on tumor-associated Tregs and MDSCs. Adoptive transfer and persistence of Fas-deficient Tregs or MDSC into wild-type, Treg or MDSC-depleted hosts, resulted in the loss of antitumor efficacy in response to IL-2/αCD40. These results confirmed that successful immunotherapy is dependent upon Fas-mediated depletion of Tregs and MDSCs. Although tumor cell Fas ligand expression has been associated with a more advanced disease stage in RCCCitation10 and Fas expression apparently correlates with worse outcome in RCC, our results suggest that removal of tumor-associated, Fas-sensitive immunoregulatory cells could contribute to efficacious treatment.
Another component underlying IL-2/αCD40 synergy is the IFNγ-dependent upregulation of nitric oxide synthase (NOS2) expression within the tumor microenvironment, a response that is critical for the control of lung metastases.Citation8 NOS2 inhibition, or clodronate-mediated macrophage depletion, eliminated the ability of IL-2/αCD40 to reduce lung metastases, but had no effect on reduction of primary tumor burden. These results discriminate the NO-independent effects of IL-2/αCD40 against primary tumors from the macrophage-derived, NO-dependent effects required for inhibition of lung metastases. We also showed that the anti-metastatic effects of IL-2/αCD40 therapy involve the NO-dependent decrease in matrix metalloproteinase (MMP) expression and activity, concomitant with an increase in the expression of E-cadherin and tissue-inhibitor of metalloproteinase 1 (TIMP1) within the tumor microenvironment, a molecular response which would presumably reduce the metastatic potential of tumors.Citation8 In support, in an in vivo model of metastatic disease, we found that NOS2 expression within the tumor microenvironment is essential to regulate the expression and activity of MMP and E-cadherin, and corresponding reduced metastatic burden, in response to IL-2/αCD40 therapy.
In conclusion, therapeutic targeting of the CD40 pathway may be efficacious for the therapy of kidney cancer, and possibly other types of malignancy, by virtue of its broad immunologic and tumor-specific effects. More studies are needed to discern the synergistic and unique antitumor responses achieved in the context of αCD40-based combinatorial therapies.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Shorts L, Weiss JM, Lee JK, Welniak LA, Subleski J, Back T, Murphy WJ, Wiltrout RH. Stimulation through CD40 on mouse and human renal cell carcinomas triggers cytokine production, leukocyte recruitment, and antitumor responses that can be independent of host CD40 expression. J Immunol 2006; 176 (11):6543-52; PMID:16709811; http://dx.doi.org/10.4049/jimmunol.176.11.6543
- Eliopoulos AG, Davies C, Knox PG, Gallagher NJ, Afford SC, Adams DH Young LS. CD40 induces apoptosis in carcinoma cells through activation of cytotoxic ligands of the tumor necrosis factor superfamily. Mol Cell Biol 2000; 20 (15):5503-15; PMID:10891490; http://dx.doi.org/10.1128/MCB.20.15.5503-5515.2000
- Weiss JM, Gregory Alvord W, Quinones OA, Stauffer JK, Wiltrout RH. CD40 expression in renal cell carcinoma is associated with tumor apoptosis, CD8 T cell frequency and patient survival. Hum Immunol 2014; 75 (7):614-620; PMID:24801648; http://dx.doi.org/10.1016/j.humimm.2014.04.018
- Vonderheide RH, Glennie MJ. Agonistic CD40 antibodies and cancer therapy. Clin Cancer Res 2013; 19 (5):1035-43; PMID:23460534; http://dx.doi.org/10.1158/1078-0432.CCR-12-2064
- Melter M, Reinders ME, Sho M, Pal S, Geehan C, Denton MD Mukhopadhyay D, Briscoe DM. Ligation of CD40 induces the expression of vascular endothelial growth factor by endothelial cells and monocytes and promotes angiogenesis in vivo. Blood 2000; 96 (12):3801-8; PMID:11090063
- DiLillo DJ, Matsushita T, Tedder TF. B10 cells and regulatory B cells balance immune responses during inflammation, autoimmunity, and cancer. Ann N Y Acad Sci 2010; 1183:38-57; PMID:20146707; http://dx.doi.org/10.1111/j.1749-6632.2009.05137.x
- Weiss JM, Back TC, Scarzello AJ, Subleski JJ, Hall VL, Stauffer JK, Chen X, Micic D, Alderson K, Murphy WJ, et al. Successful immunotherapy with IL-2/anti-CD40 induces the chemokine-mediated mitigation of an immunosuppressive tumor microenvironment. Proc Natl Acad Sci U S A 2009; 106 (46):19455-60; PMID:19892741; http://dx.doi.org/10.1073/pnas.0909474106
- Weiss JM, Ridnour LA, Back T, Hussain SP, He P, Maciag AE, Keefer LK, Murphy WJ, Harris CC, Wink DA, et al. Macrophage-dependent nitric oxide expression regulates tumor cell detachment and metastasis after IL-2/anti-CD40 immunotherapy. J Exp Med 2010; 207 (11):2455-67; PMID:20921282; http://dx.doi.org/10.1084/jem.20100670
- Jiang Q, Weiss JM, Back T, Chan T, Ortaldo JR, Guichard S, Wiltrout RH. mTOR kinase inhibitor AZD8055 enhances the immunotherapeutic activity of an agonist CD40 antibody in cancer treatment. Cancer Res 2011; 71 (12):4074-84; PMID:21540234; http://dx.doi.org/10.1158/0008-5472.CAN-10-3968
- Weiss JM, Subleski JJ, Back T, Chen X, Watkins SK, Yagita H Sayers TJ, Murphy WJ, Wiltrout RH. Regulatory T Cells and Myeloid-Derived Suppressor Cells in the Tumor Microenvironment Undergo Fas-Dependent Cell Death during IL-2/alphaCD40 Therapy. J Immunol 2014; 192 (12):5821-9; PMID:24808361; http://dx.doi.org/10.4049/jimmunol.1400404
