Abstract
Targeting antigens directly to dendritic cells (DCs) in situ has emerged as a promising strategy to potentiate immunity. A recent clinical trial of antibody-mediated targeting of tumor antigen NY-ESO1 to the DC receptor DEC-205 demonstrated an induction of strong cellular and humoral responses. Recent studies with DC-targeting via nanoparticles suggest that combinatorial targeting of multiple human DC subsets may further improve the efficacy of DC targeting.
Keywords:
Abbreviations:
Protective immunity against cancer, as well as several pathogens, depends on both cellular and humoral immune responses. While vaccines against certain pathogens (such as small-pox and polio) are among the greatest successes of modern medicine, there are cases of several pathogens, including human immune-deficiency virus(HIV) and chronic diseases such as cancer, wherein strong vaccine-mediated cellular immunity would be desirable. Dendritic Cells (DCs) are the sentinels of the immune system and as specialized antigen-presenting cells, play a central role in initiating and regulating adaptive immunity. Therefore it is essential to consider the biology of DCs while designing vaccines aimed at enhancing cellular immunity.
Figure 1. Emerging strategies for targeting human DCs in situ. Dendritic cell (DC)-based therapeutic vaccines could include antibody-antigen conjugates (left) and nanoparticle-mediated delivery of DC activation (right). Activated DCs subsequently stimulate T cells and potentiate T-cell mediated anticancer immunity.
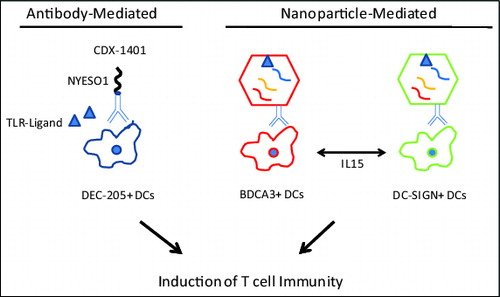
Much of the early effort at targeting protein antigens to DCs was based on adoptive transfer of antigen-loaded DCs. In most of these studies, DCs were generated ex vivo from blood monocytes, prior to adoptive transfer.Citation1 While these studies did demonstrate the capacity of such vaccines to elicit immunity in vivo, the clinical effects were modest. Another candidate approach is to target antigens to DCs directly in situ . Pioneering clinical studies by Steinman and Nussenzweig demonstrated that coupling antigens to antibodies against DEC-205, an antigen-uptake receptor on DCs, leads to enhanced activation of T-cell immunity in several models.Citation2 Importantly, targeting antigens to DEC-205 in steady state results in induction of tolerance and co-administration of adjuvant to activate DCs is essential to elicit immunity.Citation3
In order to translate these findings to the clinic, Celldex Therapeutics developed a fully human anti-DEC205 monoclonal antibody (3G9) genetically conjugated to the full length NYESO1 tumor antigen. In preclinical studies, conjugation to 3G9 enhanced cross-presentation of NYESO1 to human T cells, compared to NYESO1 protein alone.Citation4 In a human DEC205 transgenic mouse model, targeting HIV-Gag antigen to DEC205 led to robust anti-Gag cellular and humoral responses.Citation5 These data provided the basis for an early phase clinical trial to test the safety, immunogenicity and clinical efficacy of 3G9-NYESO1 conjugate (CDX1401, Celldex Therapeutics, Hampton, NJ) in patients with advanced cancer.Citation6 In this study, 45 patients with advanced cancer received escalating doses (0.1, 1 or 3 mg) of CDX1401 in combination with toll-like receptor (TLR) agonists resiquimod (TLR7/8) and Hiltenol (poly-ICLC, TLR3). Vaccine was generally well tolerated with no dose limiting toxicities. NYESO1-specific antibodies were detected following vaccination in 79% of patients. Similarly, NYESO1-specific T cells were observed in 56% of patients with evaluable pre and post-treatment samples. Cellular and humoral responses were observed at all dose levels tested and with both of the TLR agonists. There did not appear to be a particular advantage for the combination of TLR agonists, as compared to a single agonist. Although there were no objective clinical responses, 2 patients experienced minor tumor regression and 13 of the treated patients experienced stable disease with a median duration of 6.7 months. The lack of objective tumor regressions in vaccinated patients suggests the need to address suppression of vaccine-induced immune responses. Along these lines, 6 of 8 patients who received therapy with checkpoint blockade inhibitors within 3 months following CDX1401 experienced objective tumor regression. Therefore, these data suggest that the combination of CDX1401 with immune checkpoint blockade may hold promise for immunotherapy of cancer. It is notable that vaccines against NYESO1 may, in principle, also lead to the development of immune responses against other antigens more directly implicated in cancer biology. For example, a recent study demonstrated that clinical tumor regression following checkpoint blockade in patients with non-small cell lung cancer correlated with preexisting immunity against SOX2 that has emerged as an important driver oncogene in lung cancer.Citation7 The ability to target antigens derived from driver oncogenes / mutations directly to DCs in situ remains an unmet need.
In addition to antibody-antigen conjugates, another emerging approach for targeting DCs in situ involves nanoparticles (NPs).Citation8 The clinical safety of these polymers is already well established in the clinic and NPs are particularly suited to developing complex polyepitope vaccines that can be personalized to each patient. In addition to the flexibility with loading of immunogenic cargo, NPs can also be readily decorated with specific antibodies, allowing targeting to defined cell types. These considerations have encouraged several investigators to explore targeting NPs to DCs. However which specific DC subset should be targeted remains unclear.Citation8 Human DCs consist of several subsets including DC-SIGN+ myeloid DCs, plasmacytoid DCs, and BDCA3+ DCs, which are thought to be human equivalents of murine CD8α+ DC subset. In a recent study, Sehgal et al. demonstrated that simultaneously targeting both DC-SIGN+ and BDCA3+ DC subsets via NPs synergistically stimulated the activation of T cell-mediated immunity, as compared to targeting each DC subset alone.Citation9 The mechanism underlying this synergy involved IL15-mediated DC-DC cross talk. These studies also demonstrated that DC-targeted NPs could be loaded with complex antigens, and simultaneously enhance immunity to multiple antigens/epitopes, including to tumor antigens such as SOX2. Thus, together these data set the stage for the next generation of DC-targeting studies simultaneously targeting multiple DC subsets. It is notable that potent human vaccines such as the Yellow Fever vaccine do, indeed, simultaneously engage multiple DC subsets.Citation10 In other words, as opposed to current approaches targeting a single DC subset, it should be of interest to explore the optimal combination of DC subsets to target in vivo in order to optimize the generation of T cell immunity. In the last 2 decades, much has been discovered regarding the biology and functional properties of various DC subsets.Citation2 Translating this knowledge to the clinic should enhance the efficacy of vaccines against pathogens and cancer.
References
- Palucka K, Banchereau J. Dendritic-cell-based therapeutic cancer vaccines. Immun. 2013; 39(1):38-48; PMID:23890062; http://dx.doi.org/10.1016/j.immuni.2013.07.004
- Steinman RM. Decisions about dendritic cells: past, present, and future. Annu Rev Immunol. 2012; 30:1-22; PMID:22136168; http://dx.doi.org/10.1146/annurev-immunol-100311-102839
- Hawiger D, Inaba K, Dorsett Y, Guo M, Mahnke K, Rivera M, Ravetch JV, Steinman RM, Nussenzweig MC. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J Exp Med. 2001; 194(6):769-79; PMID:11560993; http://dx.doi.org/10.1084/jem.194.6.769
- Tsuji T, Matsuzaki J, Kelly MP, Ramakrishna V, Vitale L, He LZ, Keler T, Odunsi K, Old LJ, Ritter G, et al. Antibody-targeted NY-ESO-1 to mannose receptor or DEC-205 in vitro elicits dual human CD8+ and CD4+ T cell responses with broad antigen specificity. J Immunol. 2011; 186(2):1218-27; PMID:21149605; http://dx.doi.org/10.4049/jimmunol.1000808
- Cheong C, Choi JH, Vitale L, He LZ, Trumpfheller C, Bozzacco L, Do Y, Nchinda G, Park SH, Dandamudi DB, et al. Improved cellular and humoral immune responses in vivo following targeting of HIV Gag to dendritic cells within human anti-human DEC205 monoclonal antibody. Blood. 2010; 116(19):3828-38; PMID:20668230; http://dx.doi.org/10.1182/blood-2010-06-288068
- Dhodapkar MV, Sznol M, Zhao B, Wang D, Carvajal RD, Keohan ML, Chuang E, Sanborn RE, Lutzky J, Powderly J, et al. Induction of antigen-specific immunity with a vaccine targeting NY-ESO-1 to the dendritic cell receptor DEC-205. Sci Transl Med. 2014; 6(232):232ra51; PMID:24739759; http://dx.doi.org/10.1126/scitranslmed.3008068
- Dhodapkar KM, Gettinger SN, Das R, Zebroski H, Dhodapkar MV. SOX2-specific adaptive immunity and response to immunotherapy in non-small cell lung cancer. Oncoimmunology. 2013; 2(7):e25205; PMID:24073380; http://dx.doi.org/10.4161/onci.25205
- Paulis LE, Mandal S, Kreutz M, Figdor CG. Dendritic cell-based nanovaccines for cancer immunotherapy. Curr Opin Immunol. 2013; 25(3):389-95; PMID:23571027; http://dx.doi.org/10.1016/j.coi.2013.03.001
- Sehgal K, Ragheb RTF, Dhodapkar M, Dhodapkar K. Nanoparticle-mediated combinatorial targeting of multiple human dendritic cell (DC) subsets leads to enhanced T cell activation via IL15-dependent DC cross-talk. J Immunol. 2014; in press; PMID:25080481
- Pulendran B, Tang H, Denning TL. Division of labor, plasticity, and crosstalk between dendritic cell subsets. Curr Opin Immunol. 2008; 20(1):61-7; PMID:18082389; http://dx.doi.org/10.1016/j.coi.2007.10.009
