Abstract
We have recently reported that lymphocyte activation gene-3 (LAG-3,CD223) mediates the alternative, IFNα-deficient activation of plasmacytoid dendritic cells (pDCs) at tumor sites. Our findings define a novel tumor-driven strategy that promotes immunosuppression by pDCs, and we have provided more detailed information regarding the immunomodulatory role of of LAG-3. The translational relevance of our results for the treatment of tumors and autoimmune diseases is discussed herein.
Abbreviations:
- ADCC, antibody-dependent cell cytotoxicity
- APCs, antigen-presenting cells
- DDCs, dermal dendritic cells
- LAG-3, lymphocyte activation gene-3
- LNs, lymph nodes
- mAbs, monoclonal antibodies
- MDSCs, myeloid-derived suppressor cells
- PD-1, programmed cell death 1
- pDCs, plasmacytoid dendritic cells
- TLRs, toll-like receptors
- Tregs, regulatory T cells.
The formation of a permissive microenvironment is a hallmark of cancer and a required step by which tumor cells disable specific immunity. A complex network of mechanisms is responsible for establishing a tolerogenic state, in which different immunosuppressive cells are actively induced/recruited by tumors. This phenomenon has been largely documented for melanoma, which represents the paradigm of tumor immune evasion despite its intrinsic immunogenicity.
Antigen-presenting cells (APCs) initiate antitumor immune responses. Among the APCs, plasmacytoid dendritic cells (pDCs) are emerging as important regulators of tumor immunity due to their intrinsic capacity for bridging the innate and adaptive immune responses (see ). pDCs boost antitumor T cells by antigen cross-presentation and favor an adaptive response via the production of a large amount of Type I interferons (IFNs).Citation1 However, a common trait of tumor-associated pDCs is their impaired IFNα release, which is associated with their immunosuppressive and tolerogenic functions.Citation2 Indeed, pDCs at tumor sites, including those found in melanoma, are associated with a poor clinical outcome.Citation3 While the complete activation of pDCs is achieved by pathogens through toll-like receptors (TLRs), the signals/receptors that may be responsible for pDC activation in the pathogen–free inflammatory tumor environment are unknown. We have recently reported that human pDCs comprise a LAG-3+ subset that accumulates in melanoma-invaded lymph nodes (LNs) and displays a partially activated phenotype.Citation4 Lymphocyte activated gene 3 (LAG-3) is a CD4–related co-stimulatory receptor that binds to major histocompatibility complex class II (MHC-II) molecules. Our in vitro experiments have demonstrated that HLA-DR+ melanoma cells interact with LAG-3 and induce the TLR-independent activation of pDCs, which is characterized by limited IFNα and enhanced interleukin 6 (IL-6) production. pDCs activated via the alternative LAG-3 signaling pathway induce regulatory T cells (Tregs) and stimulate monocytes to release chemokine (C-C) motif ligand 2 (CCL2), which plays a crucial role in the recruitment of myeloid-derived suppressor cells (MDSCs) to tumor sites. Our data clearly showed that the LAG-3-mediated maturation pathway of pDCs also occurs in vivo. An ex vivo analysis of the cytokine profile of tumor-associated LAG-3+ pDCs revealed IL-6 enrichment and IFNα deficiency. LAG-3+ pDCs in melanoma-invaded LNs stain positively for IL-6 and preferentially localize near HLA-DR+ melanoma cells. LN areas with LAG-3+ pDCs display high densities of M2-polarized macrophages. Thus, through this alternative activation, LAG-3+ pDCs promote immunosuppression at tumor sites (see ).
Figure 1 (See previous page). Role of LAG-3 in pDCs and its translational relevance in melanoma and psoriasis. (A) Under normal conditions, upon activation, plasmacytoid dendritic cells (pDCs) upregulate T cell co-stimulatory molecules (such as CD86 and CD40) and increase MHC-II and TRAIL expression; activated pDCs secrete pro-inflammatory and anti-viral cytokines, such as Type I interferons (IFNα/β), and promote B- and DC-cell differentiation/activation, T helper cell type 1 (Th1) response, and natural killer (NK) cell and CD8+ T cell cytotoxic activities. (B) In melanoma-invaded lymph nodes (LNs), MHC-II molecules on melanoma cells bind to lymphocyte activated gene 3 (LAG-3) expressed on the surface of pDCs, resulting in the tolerogenic activation of pDCs. Low levels of IFNα and high levels of interleukin 6 (IL-6) induce monocytes to produce chemokine (C-C) motif ligand 2 (CCL2) with subsequent myeloid derived suppressor cell (MDSC) accumulation and regulatory T cell (Treg) expansion. (C) In melanoma lesions, the antagonistic anti-LAG-3 mAb blocks the negative signals mediated by LAG-3 in exhausted T cells and limits the suppressor functions of Tregs. The same mAb blocks the interaction between pDCs expressing LAG-3 and melanoma cells expressing its ligand (MHC-II), allowing for pDC activation and IFNα production while limiting the frequencies of MDSCs and Tregs with subsequent T-cell expansion. Clinical-grade LAG-3Ig (IMP321) promotes DC maturation and CD8+ T-cell induction/expansion. (D) In psoriatic lesions, the agonist anti-LAG-3 mAb can trigger LAG-3-mediated signaling in pDCs, thus diminishing IFNα production and consequently hampering the activation of dermal dendritic cells (DDCs) and the development of pathogenic Th1 responses. The cytotoxic anti-LAG-3 mAb depletes autoreactive LAG-3+ T cells.
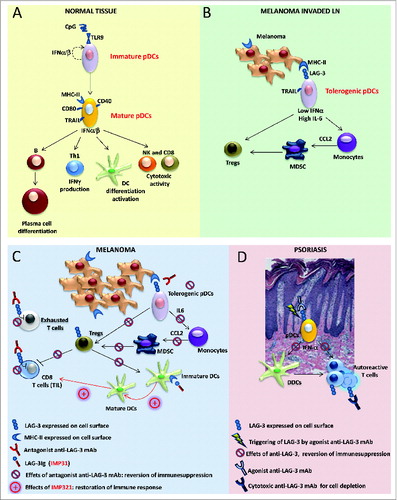
Our results suggest that therapeutic strategies aimed at restoring active antitumor immunity should consider the need for establishing fully functioning pDCs in the tumor context. The re-acquisition of high levels of IFNα via the use of TLR agonists as a monotherapy has been shown to be inefficient in promoting enduring antitumor activity.Citation5 Thus, the development of novel approaches for subverting the immunosuppressive activities of pDCs in tumors is mandatory. Our data indicate that LAG-3 is a molecule that can be targeted to counteract the tumor-driven inhibitory functions of pDCs. An antagonist monoclonal antibody (mAb) that prevents LAG-3-mediated signal transduction in pDCs, given in combination with TLR agonists, may effectively modify the immunological functions of pDCs to promote protective antitumor immunity (see ). This is a testable hypothesis as antagonistic, humanized anti-LAG-3 mAbs are currently available for clinical use. Indeed, LAG-3 plays complex immunological roles and is a negative co-stimulatory molecule that is expressed by exhausted or chronically stimulated T cells, such as those found at tumor sites.Citation6 LAG-3 also defines Tregs with enhanced suppressive functions that accumulate in melanoma-invaded LNs where it functions as selective marker for human Treg type 1 cells.Citation7,8 With the aim of reverting exhaustion and possibly limiting Treg functions, clinical trials using anti-LAG-3 mAbs with or without anti-PD1 mAbs are currently underway, and patients with solid and hematologic cancers are being recruited (NCT02061761; NCT01968109). For these clinical trials, immunological monitoring that includes assessments of the phenotypes and functions of both the circulating and tumor-infiltrating pDCs, if present, can indeed be useful to further delineate the roles of pDCs in tumor immunity.
LAG-3 expressed by pDCs may become a target for the treatment of autoimmune diseases, and agonist anti-LAG-3 mAbs, although not yet available, may have a clinical impact on the treatment of psoriasis. In psoriasis, the production of IFNα by pDCs is the initial event in the innate cascade driving pathologic inflammation.Citation9 The LAG-3-mediated activation of pDCs may represent an efficient strategy for limiting IFNα and promoting immunosuppression in the local environment. An additional therapeutic approach may rely on the active depletion of LAG-3+ pathogen-activated T cells by anti-LAG-3-specific mAbs endowed with antibody-dependent cell cytotoxicity (ADCC)-related functions (see ). Humanized cytotoxic anti-LAG-3 mAbs have recently been developed, and a Phase I study of healthy subjects and patients with psoriasis will soon be underway (NCT02195349).
The immunomodulatory functions of LAG-3 include additional levels of complexity. LAG-3 also exists as a soluble monomer that is shed from the cell surfaces of activated T cells or secreted as an alternatively spliced protein isoform. Taking advantage of a soluble recombinant LAG-3 fusion protein (LAG-3Ig), we and others have shown that the LAG-3-mediated triggering of MHC-II molecules induces the maturation process in immature DCs as well as the production of chemokines that target the DCs to LNs (see ).Citation10 Clinical-grade LAG-3Ig, known as IMP321, is currently in use in different clinical trials and has shown encouraging results, both at low doses as a vaccine adjuvant to cancer antigens and at high doses as a systemic APC activator to further boost chemotherapy-induced immunogenic tumor cell death (NCT00354263; NCT00365937; NCT00349934).
In conclusion, our results reveal novel immunological functions of LAG-3 and suggest that the manipulation of LAG-3-mediated signaling to target different immune cell subsets, including pDCs, shows promise as an effective tool for either restoring immune functions in patients with melanoma (see ) or correcting aberrant immune activation in patients affected by autoimmune skin diseases, such as psoriasis (see ).
References
- Tel J, Sittig SP, Blom RA, Cruz LJ, Schreibelt G, Figdor CG, de Vries IJ. Targeting uptake receptors on human plasmacytoid dendritic cells triggers antigen cross-presentation and robust type I IFN secretion. J Immunol 2013; 191:5005-12; PMID:24127556; http://dx.doi.org/10.4049/jimmunol.1300787
- Reizis B, Bunin A, Ghosh HS, Lewis KL, Sisirak V. Plasmacytoid dendritic cells: recent progress and open questions. Annu Rev Immunol 2011; 29:163-83; PMID:21219184; http://dx.doi.org/10.1146/annurev-immunol-031210-101345
- Jensen TO1, Schmidt H, Møller HJ, Donskov F, Høyer M, Sjoegren P, Christensen IJ, Steiniche T. Intratumoral neutrophils and plasmacytoid dendritic cells indicate poor prognosis and are associated with pSTAT3 expression in AJCC stage I/II melanoma. Cancer. 2012;118:2476-85; PMID:21953023; http://dx.doi.org/10.1002/cncr.26511
- Camisaschi C, De Filippo A, Beretta V, Vergani B, Villa A, Vergani E, Santinami M, Cabras AD, Arienti F, Triebel F, et al. Alternative activation of human plasmacytoid DCs in vitro and in melanoma lesions: involvement of LAG-3. J Invest Dermatol 2014; 134:1893-902; PMID:24441096; http://dx.doi.org/10.1038/jid.2014.29
- Guha M. Anticancer TLR agonists on the ropes. Nat Rev Drug Discov 2012; 11:503-5; PMID:22743965; http://dx.doi.org/10.1038/nrd3775
- Matsuzaki J, Gnjatic S, Mhawech-Fauceglia P, Beck A, Miller A, Tsuji T, Eppolito C, Qian F, Lele S, Shrikant P, et al. Tumor-infiltrating NY-ESO-1-specific CD8+ T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proc Natl Acad Sci U S A 2010; 107:7875-80; PMID:20385810; http://dx.doi.org/10.1073/pnas.1003345107
- Camisaschi C, Casati C, Rini F, Perego M, De Filippo A, Triebel F, Parmiani G, Belli F, Rivoltini L, Castelli C. LAG-3 expression defines a subset of CD4(+)CD25(high)Foxp3(+) regulatory T cells that are expanded at tumor sites. J Immunol 2010; 184:6545-51; PMID:20421648; http://dx.doi.org/10.4049/jimmunol.0903879
- Gagliani N, Magnani CF, Huber S, Gianolini ME, Pala M, Licona-Limon P, Guo B, Herbert DR, Bulfone A, Trentini F, et al. Coexpression of CD49b and LAG-3 identifies human and mouse T regulatory type 1 cells. Nat Med 2013; 19:739-46; PMID:23624599; http://dx.doi.org/10.1038/nm.3179
- Boyman O, Conrad C, Tonel G, Gilliet M, Nestle FO. The pathogenic role of tissue-resident immune cells in psoriasis. Trends Immunol 2007; 28:51-7; PMID:17197238; http://dx.doi.org/10.1016/j.it.2006.12.005
- Casati C, Camisaschi C, Rini F, Arienti F, Rivoltini L, Triebel F, Parmiani G, Castelli C. Soluble human LAG-3 molcule amplifies the in vitro generation of type 1 tumor-specific immunity. Cancer Res 2006; 66:4450-60; PMID:16618772; http://dx.doi.org/10.1158/0008-5472.CAN-05-2728
