Abstract
The concept of immunogenic cancer cell death (ICD), as originally observed during the treatment with several chemotherapeutics or ionizing irradiation, has revolutionized the view on the development of new anticancer therapies. ICD is defined by endoplasmic reticulum (ER) stress response, reactive oxygen species (ROS) generation, emission of danger-associated molecular patterns and induction of antitumor immunity. Here we describe known and emerging cancer cell death-inducing physical modalities, such as ionizing irradiation, ultraviolet C light, Photodynamic Therapy (PDT) with Hypericin, high hydrostatic pressure (HHP) and hyperthermia (HT), which have been shown to elicit effective antitumor immunity. We discuss the evidence of ICD induced by these modalities in cancer patients together with their applicability in immunotherapeutic protocols and anticancer vaccine development.
Abbreviations:
- ATP, Adenosine triphosphate
- CRT, calreticulin
- DAMPs, danger-associated molecular patterns
- DC, dendritic cells
- EGFR, endothelial growth factor receptor
- eIF2α, eukaryotic translation initiation factor 2α
- ER, endoplasmic reticulum
- HHP, high hydrostatic pressure, HMGB1, high-mobility group box 1
- HT, hyperthermia
- ICD, immunogenic cell death
- HSP, heat shock protein
- Hyp-PDT, Hypericin-based Photodynamic therapy
- IFNγ, interferon-γ
- NDV, Newcastle Disease Virus
- ROS, reactive oxygen species
- RT, radiotherapy
- TLR, Toll-like receptor
- UVC, ultraviolet C light
Introduction to Immunogenic Cell Death
The contribution of the immune system to the therapeutic outcome of cancer treatment regimens involving surgery, radiotherapy (RT) or chemotherapy has been mostly neglected as the development of new therapies had primarily focused on tumor-cell killing for a long time. Of note, only in the case of RT, there was some early circumstantial evidence of contribution of immune system toward positive therapeutic response in the form of “abscopal effects.”Citation1 Reported for the first time in 1953,Citation2 abscopal effect was described as a phenomenon wherein RT could reduce tumor growth at distant sites outside the field of radiation possibly through anticancer immunity.Citation1 However, the immunological mechanism behind these abscopal effects and its reliability, or therapeutic reproducibility, remained controversial for a long time thereby impeding its establishment as a therapeutically-exploitable paradigm. Overtime though, it became evident that an antitumor immune response plays a major role in the therapeutic success of cancer treatment in general, and mediates long-term survival of experimental animals.Citation3-Citation7
Many chemotherapeutic agents exert their cytotoxic effects by the induction of tumor cell apoptosis which has been historically regarded as a non-inflammatory, immunologically silent or even tolerogenic mode of cell death.Citation8 This was challenged by a series of observations made more than a decade ago which showed that DCs can engulf apoptotic tumor cells and cross-present internalized antigens on MHC class I molecules to CD8+ T cells.Citation9 Apoptotic tumor cells were also shown to elicit an effective antitumor immune response in mice.Citation10 More recently, owing to a series of different studies, two morphologically equivalent but immunologically distinct subcategories of apoptosis, i.e., immunogenic and non-immunogenic apoptosis, were described giving rise to the new concept of immunogenic cell death (ICD).Citation4,5 Dying the immunogenic way is, however, not unique to apoptosis. Cells dying by other cell death pathways such as necrosis/necroptosis or pyroptosis also induce cell death associated with immunogenicity.Citation11,12,13 In fact, immunogenicity and inflammation associated with necrosis/necroptosis or pyroptosis was discovered before apoptotic ICD was characterized. In spite of this, it is not yet completely clear to what extent the molecular nature of the danger signals, which are mainly passively exposed by necrotic dying cancer cells, overlaps with that of immunogenic apoptosis. In this respect, it is even less clear whether during caspase-1 driven pyroptosis, a cell death pathway reported to occur predominantly in bacterially infected macrophages and dendritic cells, results in the release of similar immunogenic signals. Thus in this review we will focus on the mediators of immunogenic apoptosis.
The first immunogenic inducer doxorubicin, which belongs to the anthracycline family, was identified in 2005 by the group of Guido Kroemer and Laurence Zitvogel.Citation4 The subsequent important discoveries in the field of ICD are shown in a timeline in . Later, it was found that murine tumor cells treated not only with doxorubicin, but also with mitoxantrone, oxaliplatin or ionizing irradiation underwent ICD and elicited strong anticancer immune responses in mice in the absence of any adjuvants. The authors also showed that the capacity of dying tumor cells to generate immune response is dependent on the cell death – inducing stimulus as tumor cells treated with some other cytotoxic agents like mitomycin C, cisplatin, thapsigargin or etoposide failed to induce ICD.Citation4,5,14
Figure 1. Timeline of the most important discoveries in the field of immunogenic cell death. Abbreviations: ATP, Adenosine triphosphate; CRT, calreticulin; DC, dendritic cell; HMGB1, high mobility group box 1; HSP90, heat shock protein 90; Hyp-PDT, photodynamic therapy with hypericin; ICD, immunogenic cell death; LRP, lipoprotein receptor-related proteins; MyD88, myeloid differentiation primary response gene 88; TLR, toll like receptor; UVC, ultraviolet light C.
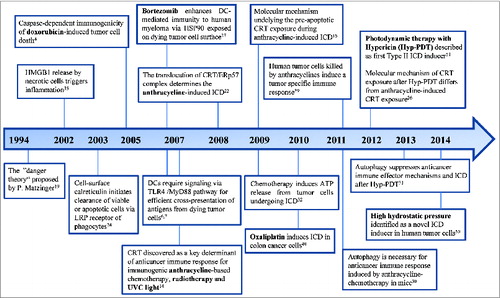
Several conditions must be fulfilled in order to define tumor cell death as immunogenic. ICD has been found to depend on the concomitant generation of ROS and activation of ER stress (either resulting from or accentuated by this ROS production).Citation15-Citation17 Stressed tumor cells undergoing ICD start to expose on their cell surface, and release or secrete into their vicinity, a variety of damage-associated molecular patterns (DAMPs)Citation18,19 which under physiological conditions have mostly non-immunological functions inside the cell. Only when exposed or emitted through complex and elaborate danger signaling trafficking moduleCitation20 these molecules act as danger signals thereby determining the immunogenicity of a dying tumor cell in a context-dependent fashion.Citation21 The list of DAMPs crucial for ICD includes (1) the pre-apoptotic surface exposure of calreticulin (CRT),Citation5,22-24 (2) the pre-apoptotic or blebbing stage-associated secretion of adenosine triphosphate (ATP),Citation25,26 (3) surface-exposure of heat shock protein 70 and 90 (HSP70 and HSP90)Citation27 and (4) the release of high mobility group box 1 (HMGB1) or other toll-like receptor (TLR) agonists like HSP70.Citation6,28 Moreover, it seems that not only the amount or diversity of immunogenic signals but also the defined spatiotemporal pattern of their emission determines the immunogenicity of dying tumor cells.Citation29 Interestingly, autophagy was shown to be indispensable for anticancer immune response induced by anthracycline-chemotherapy in mice.Citation30 On the other hand, the induction of autophagy in dying cancer cells suppressed anticancer immune effector mechanisms and ICD after photodynamic therapy with hypericin (Hyp-PDT).Citation31
The cell surface exposure of CRT or the release ATP from dying tumor cells during ICD seems to be an active process and involves the participation of several intracellular proteins.Citation15,22,26,32 Interestingly, the molecular signaling pathways which lead to CRT exposure or ATP release seem to involve an overlapping and also unique set of signaling proteins depending on the ICD inducer.Citation33,34 The release of other DAMPs such as HMGB1 seems to be rather a passive event due to the disintegration of plasma membrane of the dying tumor cells.Citation29,35 However, little is known about the intracellular mechanism of cell surface exposure or release of other danger molecules such as HSP70/90 proteins in cancer cells undergoing ICD. The immunogenic DAMPs bind to respective immune receptors e.g. pattern recognition receptors (PRRs) (TLRs for HMGB1/HSP70), phagocytosis or scavenger receptors (CD91 for surface exposed CRT/HSP90) and purinergic receptors such as P2X7R or P2Y2R (for ATP). This leads to the recruitment of innate immune cells to the tumor bed.Citation33,36 The interaction of DAMPs with their cognate immune receptors facilitates the engulfment of tumor antigens and their cross-presentation to T cells. These processes lead to a potent IL-1β- and IL-17-dependent, IFNγ-mediated immune response involving γδ T cells/cytotoxic αβ T lymphocytes and tumor eradication.Citation36 Interestingly, it has been reported that γδ T cells, in contrast to αβ T cells, may themselves possess TLRs.Citation37 However, it has not yet been analyzed whether the ICD-associated TLR-binding of DAMPs might be directly activating γδ T cells thereby partly bypassing the DC-T cell interaction route. This represents an attractive possibility that needs further analysis.
From this point of view, cancer cell death can be further defined as immunogenic provided that the tumor-rejecting immunity is elicited in mice after immunization with syngeneic dying tumor cells in the absence of any adjuvant. Thus, ICD inducer must exert, at least in part, the therapeutic efficacy in vivo leading to a reduction or eradication of the tumor mass.Citation36
The growing list of the ICD inducers, exhibiting all the major checkpoints determining the immunogenicity of cell death as described above, have been recently divided into two groups. These groups are based on their ability to trigger both cancer cell death as well as danger signaling as a consequence of direct induction of ER-stress (Type II inducers), or whether the inducer evokes ER stress-based danger signaling and apoptosis/cell death through convergent, but mechanistically separate targets (Type I inducers).Citation33,38 Type I inducers of ICD such as anthracyclines,Citation4,39 oxaliplatin,Citation40 shikonin,Citation41 7A7 (murine EGFR-specific antibody),Citation42 cyclophosphamide,Citation43 bortezomib,Citation27 cardiac glycosides,Citation44 septacidin,Citation45 bleomycin,Citation46 ultraviolet C light (UVC),Citation14 wogonin,Citation47 vorinostat,Citation48 γ-irradiationCitation14 and newly described HHPCitation49,50 target mainly cytosolic proteins, plasma membrane channels or proteins, or DNA replication and repair machinery, rather than primarily targeting the ER.Citation33 On the other hand, Type II inducers which specifically target the ER include PDT with Hypericin (Hyp-PDT),Citation51 and various different oncolytic viruses. Oncolytic viruses such as adenovirus, coxsackievirus B3,Citation33,38 measles virus, vaccinia viruses, herpes simplex virus or Newcastle disease virusCitation13 have been shown to induce various modes of ICD,Citation11 however, the underlying molecular mechanisms remains to be determined. Of note, the Newcastle disease virus is the only oncolytic virus shown so far to induce both ICDCitation13 as well as “abscopal effect”-like antitumor immunity as the localized intratumoral therapy with Newcastle disease virus leads to lymphocyte infiltration and antitumor effect in distant tumors without direct contact between the latter tumors and this virus.Citation52 In , we summarize scarce data available on the induction of anticancer immunity in patients by Type I and Type II inducers as evidenced by ICD determinants or by eliciting tumor-antigen specific T cell responses. More clinical trials showing the impact of immunogenicity on the prognosis of cancer patients are awaited.
Table 1. The evidence of immunogenic cell death induction by Type I and Type II in cancer
Chemotherapeutics and targeted drug classes have received maximal clinical attention compared to most physical anticancer modalities baring RT and to a certain extent, PDT. However, the emergence of ICD and re-emergence of therapeutic relevance of immunotherapy has paved the way for the development of autologous or allogeneic cancer cell-based immunotherapy exploiting physical modality-induced immunogenic tumor cell death. Of note, physical anticancer modalities-based ICD might be preferable over the chemotherapeutically induced ICD for preparing cell-based immunotherapeutics since the former does not leave behind active drug residues. The main aim of this review is to discuss in detail the molecular and cell signaling properties of physical modalities inducing ICD such as RT, UVC-light, HHP, Hyp-PDT or HT. These cell death-inducing modalities are of a particular interest for designing or generating in situ cancer vaccines, whole cell- or DC-based vaccines for cancer immunotherapy.Citation53 We discuss the evidence of ICD induced by the physical modalities in cancer patients together with a few clinical trials exploiting the whole cell or DC-based cancer vaccines using tumor cells killed by an ICD.
Physical Modalities Inducing Tumor Immunogenicity
RT is estimated to be used as a treatment modality with curative or palliative intent in at least 50% of cancer patients.Citation54 The anti-neoplastic activity of irradiation (X- or γ-rays) was believed to lie in its capacity to damage DNA and induce apoptosis of tumor cells. The abscopal effect of RT has been known for 60 yCitation2 and observed in patients with various types of tumors. This suggests that RT induces ICD in situ Citation55,56 and stimulates T cell-mediated anticancer effect. RT has been shown to induce the surface exposure of CRTCitation14,57 and HSP70,Citation58 and HMGB1 release.Citation57,59 Irradiated tumor cells stimulate DC maturationCitation60 and induce IFNγ-producing T cells in vitro and in vivo.Citation38,61,62 Moreover, mice vaccinated with DCs loaded with irradiated cancer cells are immune to the challenge with live syngeneic cells.Citation63 Even though RT was reported to decrease the number of T regulatory cells in some settings,Citation62 regulatory T cells have been described to be more resistant to cytotoxic effect of ionizing radiation.Citation64 The latter is supported by the observation that depletion of regulatory T cells potentiates the anti-neoplastic effect of RT in murine models.Citation54,64,65 Interestingly, beside X- or γ-irradiation, vaccination with α-irradiated (bismuth-213) murine adenocarcinoma MC-38 also induces long-lasting protective antitumor response in mice which depends on tumor-specific T cells.Citation66 MC-38 cells treated with213−Bi are capable of releasing DAMPs and stimulating dendritic cells in vitro .
The evidence for ICD induction in human cancer patients undergoing RT is scarce. Frey et al.,Citation67 showed that chemotherapy treatment in combination with X-ray caused ICD in human colorectal tumor cell lines. Recently, Suzuki et al.Citation57 have shown that chemoradiotherapy induces ICD in patients with esophageal squamous cell carcinoma which triggers tumor antigen-specific T cell responses. Here, HMGB1 was significantly upregulated within the tumor microenvironment and positively correlated with patient survival. As RT improves the clinical outcome of other treatment modalities such as surgery and chemotherapy, it is likely to enhance antitumor effect of various immunotherapeutic agents such as monoclonal antibodies, whole-cell or DC-based vaccines or TLR agonists.Citation54,68,69 For example, a case of the abscopal effect in patient with melanoma treated with ipilimumab and RT has been reported.Citation71 Current clinical studies in anticancer radioimmunotherapy have been recently extensively summarized in Vacchelli et al.Citation54 Interestingly, in a recent review Frey at al.Citation68 have described the combination of RT and HHP-generated whole cell tumor vaccine with the application of IL-12 in an immunotherapeutic protocol. Similarly, Sipuleucel-T®, the currently only FDA-approved antigen presenting cell-based cancer vaccine for the treatment of asymptomatic metastatic castration resistant prostate cancerCitation71 is being evaluated in clinical trial together with RTCitation54 ().
Table 2. The list of clinical trials which involve the preparation of tumor cells killed by an ICD-inducing physical modality for the use as whole cell- or DC-based vaccines in cancer immunotherapy
Ultraviolet C light
Ultraviolet light (UV) refers to electromagnetic radiation with a wavelength shorter than visible violet light but longer than X- and γ-rays. According to the wavelength range, UV light can be divided into UVA (400–320 nm), UVB (320–280 nm) and UVC (280–200 nm).Citation72 In cells UV light affects mainly DNA which leads to the apoptosis or necrosis depending on the cell type.Citation14,59,72 However, only UVC-light treatment at 10–120 nm, technically with properties of ionizing radiation, was shown to induce ICD in tumor cells which was accompanied by the pre-apoptotic exposure of CRTCitation14 on the cell surface and HSP70 and HMGB1 release into the cell culture medium at later time points.Citation59 Various molecular determinants and pathways of UVC-light-mediated ICD await further elucidation. The ability of UVC but not UVA or UVB light to induce ICD has been an enigma which remains unexplored. UVA, UVB, and UVC are all capable of stimulating ROS productionCitation73 as well as overlapping stress response pathwaysCitation74 including ER stress.Citation75 Which particular subtle difference distinguishes between ICD and non-ICD is an avenue worth investigating.
UVC-light has been known for more than 30 y to induce an inflammatory response in skin.Citation76 In 1991 Begovic et al.Citation77 showed that vaccination of immunocompetent mice with UVC-irradiated tumor cells conferred immunity to subsequent re-challenge with live tumor cells in contrast to immunodeficient mice which developed tumors. This tumor-growth inhibiting effect was mediated by CD8+ T cells and NK cells. UVC-treated tumor cells were shown to stimulate phagocytosis and DC maturation which in turn lead to the stimulation of IFNγ producing CD8+ T cells.Citation59 Moreover, DCs stimulated with UVC-treated cancer cells upregulated genes connected to antigen processing and proinflammatory cytokines.Citation38,59 The effect of UVC irradiation on tumor cells has recently been tested in a model of superficial brain cancer and metastasis.Citation78 UVC irradiation, beamed through the craniotomy open window, induced apoptosis in tumor cells which led to a significantly extended survival of experimental animals. In humans, there are no clinical studies involving UVC treatment of tumors, possibly due to a high pro-tumorigenic mutation rate induced by UVC light.Citation38
High hydrostatic pressure
HHP between 1 and 100 megapascal (MPa) is considered to be physiological, and it induces reversible morphological changes and a mild stress response. HHP between 100–150 MPa induces apoptosis of murine cells, HHP between 150–250 MPa affects the viability of human cells, whereas HHP treatment between 300–400 MPa (dependant of the cell type) leads to cell necrosis.Citation79-Citation81 HHP treatment causes cell rounding, cytoplasmic gelification, the inhibition of enzymatic functions and synthesis of cellular proteins. However, DNA does not seem to be affected by HHP below 1000 MPa.Citation81 In biotechnology, HHP is applied to sterilize food, human transplants and pharmaceuticals.Citation81,82 The use of HHP as a cancer treatment modality was described in 1972 for the treatment of bladder carcinoma.Citation83 The hydrostatic bladder dilatation method was subsequently tested in cancer patients in a small clinical trial.Citation84 Later the group of Shinitzky showed that vaccination by HHP-killed tumors cells treated with a chemical crosslinker adenosine dialdehyde alone and in combination with a reducing agent N-acetyl-L-cysteine induced antitumor immunity in miceCitation85,86 and exhibited immunogenicity in vitro.Citation87,88 The cell death induced by HHP was investigated in greater detail by the group of Udo Gaipl who suggested that HHP might be a promising technique for a generation of whole cell-based anticancer vaccines.Citation79-Citation81 Apoptotic cells treated with HHP were shown to release HSP70 and HMGB1 and possess immunogenicity in vivo which was determined by tumor-specific antibodies.Citation49,80 Recently, Fucikova et al.Citation89 have shown that HHP is a potent inducer of ICD of human prostate and ovarian cancer cell lines as well as in acute lymphocytic leukemia cells which leads to the exposure of CRT, HSP70 and HSP90 molecules on the cell surface and the release of HMGB1 and ATP from the dying cells. More importantly, DCs loaded with HHP-killed tumor cells displayed an enhanced phagocytic capacity, expressed high levels of co-stimulatory molecules, and stimulated high numbers of tumor-specific T lymphocytes without inducing T regulatory cells in the absence of any additional immunostimulants.Citation89 HHP-induced tumor cell death was shown to fulfill all currently described molecular criteria of ICD, including the activation of analogous intracellular signaling pathways similar to anthracyclinesCitation15 and Hyp-PDT (see below).Citation26 Accordingly, an increased production of ROS, phosphorylation of eIF2α, the activation of caspase-8 and caspase-8-mediated cleavage of BAP31 was detected.Citation89 The immunogenicity of HHP-killed tumor cells in vivo is currently being evaluated in therapeutic as well as prophylactic settings in mouse cancer models.
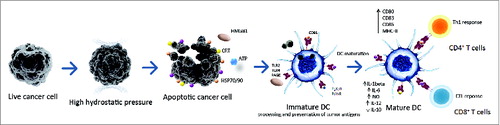
HHP treatment of tumor cells can be easily standardized and performed in GMP conditions to allow its incorporation into manufacturing protocols for cancer DC-based immunotherapy product. Multiple clinical trials for prostate and ovarian cancerCitation90 have now been initiated to evaluate the potential of DC-based cancer vaccine loaded with HHP-treated cancer cells to induce tumor cell-specific immune responses and modify the clinical course of the disease (). A schematic representation of DC-based vaccine preparation using immunogenic HHP-treated tumor cells which could be applied to other physical tumor cell death-inducing modalities is shown in .
Figure 2. A schematic representation of DC-based vaccine preparation using immunogenic HHP-killed tumor cells. Tumor cells treated with HHP (or other physical ICD-inducing modalities) expose various danger signals, so called DAMPs, in different stages of apoptosis. These DAMPs include calreticulin (CRT), heat shock proteins 70/90 (HSP70/90), HMGB1 and ATP. These molecules bind to respective cognate receptors like CD91 (for CRT/HSPs), TLR2/TLR4 (for HMGB1 or HSP70), P2RX7/P2RY2 (for ATP), respectively, on the cell surface of DCs. This leads to an enhanced engulfment of tumor cells and DC maturation characterized by upregulation of costimulatory molecules such as CD80, CD83, CD86 and HLA-DR, and by a distinct pro-inflammatory cytokine pattern. Activated DCs efficiently present tumor-specific antigens in the context of MHC class I and II molecules to T cells, inducing antitumor CD4+ and CD8+ T cell responses. Abbreviations: ATP, Adenosine triphosphate; CRT, calreticulin; CTL, cytotoxic T lymphocytes; DAMPs, danger-associated molecular patterns; DC, dendritic cell; HHP, high hydrostatic pressure; HMGB1, high mobility group box 1 HSP70, heat shock protein 70; HSP90, heat shock protein 90; ICD, immunogenic cell death; P2RX7, P2X purinoceptor 7; P2RY2, P2Y purinoceptor 2; RAGE, receptor for advanced glycation endproducts; TLR, toll like receptor.
Photodynamic therapy
Over the last decades, PDT has been explored as a promising anticancer treatment due to its relative specificity as well as the absence of harmful side effects usually associated with chemotherapy and RT. PDT has a two-step modus operandi involving administration of mainly tumor-localizing photosensitizer followed by its activation with a light of specific wavelength, which ultimately leads to the photochemical production of ROS, thereby causing oxidative stress-based cell death. The most attractive attribute of PDT is that, this oxidative stress can be directed toward a particular subcellular organelle or locale within the cancer cells, due to the tendency of a given photosensitiser to exhibit a certain degree of “tropism” toward a particular subcellular site e.g., Hypericin tends to be reticulotropic since it mainly localizes in the ER membrane.Citation91 The extent of PDT-induced damage is multifactorial depending on, among others, the type of tumor cell, photosensitizer type and its subcellular localization, and the cellular oxygen levels as well as light irradiation fluency. PDT-induced antitumor effects include cytotoxicity toward tumor cells, tumor-infiltrating cells and vasculature as well as the activation of the complement cascade and recruitment of immune cells like DCs or neutrophils to the tumor site.Citation92,93 The molecular mechanisms of PDT-mediated cell death depend strongly on the subcellular localization of the photosensitizer and the PDT dosage.Citation94 At high fluence PDT in general tends to induce necrosis while high to medium fluence induce either a mixture of apoptosis and necrosis or predominantly apoptosis in a dose-dependent fashion.Citation91
It is noteworthy though, that due to the essential involvement of a chemical component, namely a photosensitizer/pro-drug, PDT unlike RT or HHP, cannot be considered as an exclusively physical modality but rather a physicochemical anticancer modality. However, since in the absence of the physical component (i.e., specific wavelength of light used for activating a particular photosensitizer), PDT would be unable to exert its bona fide anticancer effects (i.e., high cancer cell death, ICD or antitumor immunity in general),Citation26,93 we have included a discussion of the relevant immunogenic features of this physicochemical anticancer modality along with the actual physical procedures, like RT and HHP.
Since early work in 1970s, there have been over 200 clinical trials involving PDT alone or in combination with other treatment modalities of various cancers.Citation93 However, very little is known about the impact of PDT on the human immune system. It has been shown that local tumor PDT can enhance systemic antigen-specific immune responses against tumors in patientsCitation95 and can also induce clinical abscopal effect-like immune response against distant non-treated tumors.Citation96 Importantly, Garg et al.Citation26,51 have recently shown that specifically Hyp-PDT induces ICD in murine and human systems. Hyp-PDT is the first Type II ICD inducer to be characterized, and it is by far the most effective inducer of ROS-based ER stress among all the known ICD inducers.Citation51 Since Hypericin localizes prevalently in the ER, its light-activation causes ROS-based ER stress that culminates into mitochondrial apoptosis.Citation97 Hyp-PDT has been observed to induce signatures of ER stress in a treated bladder carcinoma tumor in vivo.Citation98 Moreover, Hyp-PDT has been applied in clinical trials with some success for the treatment of patients with non-melanoma skin cancer,Citation99 cutaneous T-cell lymphomaCitation100 mesotheliomaCitation101 and basal or squamous cell carcinoma.Citation102
Hyp-PDT induces all the major molecular and immunological hallmarks of ICD. Uniquely, Hyp-PDT induces pre-apoptotic active emission of four crucial DAMPs i.e., surface exposed CRT, surface exposed HSP70, surface exposed HSP90 and secreted ATPCitation26,51,103 (Dudek et al. unpublished results). This is followed by passive, late apoptotic, release of chaperokines like HSP70/HSP90. Hyp-PDT-treated cancer cells are preferentially phagocytosed in a surface CRT-dependent fashion by various innate immune cells including murine and human DCs which undergo efficient phenotypic and functional maturation.Citation26 These fully mature DCs thereafter induce efficient proliferation and clonal expansion of human IFNγ-producing CD4+ and CD8+ T lymphocytesCitation104 – an important sign of activation of anticancer immune effector mechanisms. In line with this, Hyp-PDT elicited ICD has been found to be capable of mediating efficient tumor rejection in vivo in murine prophylactic as well as therapeutic/curative vaccination models.Citation16,91
PDT, in general, has been shown to be suitable for vaccine generation, as immunization with PDT-killed tumor cells or cell lysate induces strong antitumor immunity in miceCitation105,106 also in the absence of any adjuvants.Citation107 In addition, photoimmunotherapy with DCs loaded with PDT-treated tumor cells has been shown to stimulate the cytotoxicity of T and NK cells toward tumors in miceCitation108 suggesting its clinical potential. However, despite some clinical success in using PDT in cancer treatment, there are no clinical data on the use of PDT-based cancer vaccines in immunotherapy. There is currently a clinical trial in preparation for application of PDT-based vaccines (). Of note, Hyp-PDT-induced ICD based DC vaccines are currently being tested in preclinical trials for glioblastoma and ovarian cancer (Garg et al. unpublished data; Immunotherapy Platform Leuven or ITPL, UZLeuven, Belgium). The success of such preclinical trials and preclinical optimizations would define the possible clinical translation of PDT-based immunotherapy in the near future.
Hyperthermia
HT refers to the administration of heat locally as well as systemically (whole-body HT). Since the 1970s numerous pre-clinical studies on the effects of heat on tumor cells have been performed in vitro as well as in experimental animal models. In clinical oncology, HT has been shown to be a potent sensitizer for the conventional chemo- or RT-treatment and improved patient's survival in various clinical trials.Citation109 Clinical HT was shown to affect innate and adaptive immunity; stimulating antigen presentation, maturation and migration of DCs, as well as homing of T lymphocytes to lymph nodes thereby facilitating T cell priming. The efficiency of heat-induced killing of tumor cells depends mainly on the applied temperature (ranging in most studies from 41°C to 44°C), the duration of heat treatment, tumor cell type and the cell cycle phase. Malignant cells are capable of thermotolerance induced by heat-shock response which is accompanied by the expression of HSPs and other post-translational adaptation processes.Citation109 HT was shown to cause apoptosis mainly at lower temperatures (41–43°C) (and predominantly necrosis at higher temperatures (>43°C).Citation110 Increased immunogenicity of mouse and human tumor cells subjected to HT as well as the induction of tumor-antigen specific T cell responses in vitro and in vivo has been well documented.Citation111-Citation113 The major technical problem with HT application is the difficulty to heat specifically only the tumor region without inducing damage to the normal tissue. In recent years, the development of new techniques based on magnetic nanoparticlesCitation114 allowed the induction of such tumor-specific HT. The group of Kobayashi has developed a HT system based on liposomes containing magnetic nanoparticles which caused necrotic tumor cell death and the release of HSP70, thereby stimulating an antitumor immune response in vivo.Citation115,116 Recently, magnet-mediated HT at high therapeutic temperatures (50–55°C) was shown to induce abscopal antitumor immune effects on Walker-256 carcinosarcomas in rats.Citation117
Currently, it is unclear, whether HT treatment alone can induce bona fide ICD. HT-treatment (<43°C) was shown to induce ER stress.Citation118 Also HMGB1 release was detected after HT treatment of tumor cells lines at high temperature of 56°C.Citation59 The current paradigm of the immunogenicity of HT lies in the action of HSP70 and/or other released heat shock proteins which via TLR4 signaling play the main role in the initiation of tumor-specific immune responses.Citation111,112,119,120 It has been shown that the combination of HT and RT (X-rays or UVC) induces an inflammatory necrotic tumor death which can be monitored by the release of HMGB1 and HSP70Citation121,122 and stimulation of DC maturation and release of pro-inflammatory cytokines.Citation59,123 Currently there are no clinical data on the use of HT-killed tumor cells alone in clinical protocols.
Conclusion
Malignant diseases represent a major challenge in human medicine. Combined therapeutic regimens like surgery, RT and chemotherapy can efficiently reduce the tumor volume and render cancer cells visible for immune attack, thereby improving the prognosis of cancer patients. However, the occurrence of metastatic disease and the reservoir of cancer stem cells still remain the greatest challenge in combating cancer. Therefore the combination of the cancer cell killing with the concomitant help of the host immune system to mount a competent anticancer immune response is an attractive therapeutic aim. Physical cell death-inducing modalities like PDT or HT have been proven to be able to act as in situ vaccines, and to aid in inducing antitumor immunity in human patients; PDT most likely via ICD induction. Moreover, these modalities, especially HHP which is a potent inducer of ICD in tumor cells, might have a great potential in the development of new whole cell-based or DC-based vaccines. More research is however needed on the molecular mechanism of ICD induction by PDT, HHP and possibly by HT, as well as by the currently used chemotherapeutic agents and irradiation to optimize the therapeutic approaches. Efforts should also be made to incorporate the design of new modern immunotherapeutic strategies based on ICD inducers into current multimodal therapeutic protocols.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by research grants IGA NT12402-5 and IGA NT1159 provided by the Ministry of Health of the Czech Republic. A.D.G. is supported by a FWO post-doctoral fellowship. Research in the lab of P.A. is supported by FWO (G0584.12N and K202313N), GOA/11/2009 grant of the KU Leuven and IAP7/32, funded by the Interuniversity Attraction Poles Programme, initiated by the Belgian State.
References
- Demaria S, Ng B, Devitt ML, Babb JS, Kawashima N, Liebes L, Formenti SC. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol, Biol, Phys 2004; 58:862-70; PMID:14967443; http://dx.doi.org/10.1016/j.ijrobp.2003.09.012
- Mole RH. Whole body irradiation; radiobiology or medicine? Br J Radiol 1953; 26:234-41; PMID:13042090; http://dx.doi.org/10.1259/0007-1285-26-305-234
- Korbelik M, Krosl G, Krosl J, Dougherty GJ. The role of host lymphoid populations in the response of mouse EMT6 tumor to photodynamic therapy. Cancer Res 1996; 56:5647-52; PMID:8971170
- Casares N, Pequignot MO, Tesniere A, Ghiringhelli F, Roux S, Chaput N, Schmitt E, Hamai A, Hervas-Stubbs S, Obeid M et al. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J Exp Med 2005; 202:1691-701; PMID:16365148; http://dx.doi.org/10.1084/jem.20050915
- Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, Castedo M, Mignot G, Panaretakis T, Casares N et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med 2007; 13:54-61; PMID:17187072; http://dx.doi.org/10.1038/nm1523
- Apetoh L, Ghiringhelli F, Tesniere A, Criollo A, Ortiz C, Lidereau R, Mariette C, Chaput N, Mira JP, Delaloge S et al. The interaction between HMGB1 and TLR4 dictates the outcome of anticancer chemotherapy and radiotherapy. Immunol Rev 2007; 220:47-59; PMID:17979839; http://dx.doi.org/10.1111/j.1600-065X.2007.00573.x
- Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E, Saulnier P et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med 2007; 13:1050-9; PMID:17704786; http://dx.doi.org/10.1038/nm1622
- Savill J, Fadok V. Corpse clearance defines the meaning of cell death. Nature 2000; 407:784-8; PMID:11048729; http://dx.doi.org/10.1038/35037722
- Albert ML, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature 1998; 392:86-9; PMID:9510252; http://dx.doi.org/10.1038/32183
- Nowak AK, Lake RA, Marzo AL, Scott B, Heath WR, Collins EJ, Frelinger JA, Robinson BW et al. Induction of tumor cell apoptosis in vivo increases tumor antigen cross-presentation, cross-priming rather than cross-tolerizing host tumor-specific CD8 T cells. J Immunol 2003; 170:4905-13; PMID:12734333; http; http://dx.doi.org/10.4049/jimmunol.170.10.4905
- Guo ZS, Liu Z, Bartlett DL. Oncolytic immunotherapy: dying the right way is a key to eliciting potent antitumor immunity. Front Oncol 2014; 4:74; PMID:24782985; http://dx.doi.org/10.3389/fonc.2014.00074
- Inoue H, Tani K. Multimodal immunogenic cancer cell death as a consequence of anticancer cytotoxic treatments. Cell Death Differ 2014; 21:39-49; PMID:23832118; http://dx.doi.org/10.1038/cdd.2013.84
- Koks CA, Garg AD, Ehrhardt M, Riva M, Vandenberk L, Boon L, Vleeschouwer SD, Agostinis P, Graf N, Van Gool SW. Newcastle disease virotherapy induces long-term survival and tumor-specific immune memory in orthotopic glioma through the induction of immunogenic cell death. Int J Cancer 2015; 136:E313-E325; PMID:25208916; http://dx.doi.org/10.1002/ijc.29202
- Obeid M, Panaretakis T, Joza N, Tufi R, Tesniere A, van Endert P, Zitvogel L, Kroemer G. Calreticulin exposure is required for the immunogenicity of gamma-irradiation and UVC light-induced apoptosis. Cell Death Differ 2007; 14:1848-50; PMID:17657249; http://dx.doi.org/10.1038/sj.cdd.4402201
- Panaretakis T, Kepp O, Brockmeier U, Tesniere A, Bjorklund AC, Chapman DC, Durchschlag M, Joza N, Pierron G, van Endert P et al. Mechanisms of pre-apoptotic calreticulin exposure in immunogenic cell death. EMBO J 2009; 28:578-90; PMID:19165151; http://dx.doi.org/10.1038/emboj.2009.1
- Garg AD, Krysko DV, Vandenabeele P, Agostinis P. The emergence of phox-ER stress induced immunogenic apoptosis. Oncoimmunology 2012; 1:786-8; PMID:22934283; http://dx.doi.org/10.4161/onci.19750
- Kepp O, Menger L, Vacchelli E, Locher C, Adjemian S, Yamazaki T, Martins I, Sukkurwala AQ, Michaud M, Senovilla L et al. Crosstalk between ER stress and immunogenic cell death. Cytokine Growth Factor Rev 2013; 24:311-8; PMID:23787159; http://dx.doi.org/10.1016/j.cytogfr.2013.05.001
- Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukocyte Biol 2007; 81:1-5; PMID:17032697; http://dx.doi.org/10.1189/jlb.0306164
- Matzinger P. Tolerance, danger, and the extended family. Ann Rev Immunol 1994; 12:991-1045; PMID:8011301; http://dx.doi.org/10.1146/annurev.iy.12.040194.005015
- Garg AD, Martin S, Golab J, Agostinis P. Danger signalling during cancer cell death: origins, plasticity and regulation. Cell Death Differ 2014; 21:26-38; PMID:23686135; http://dx.doi.org/10.1038/cdd.2013.48
- Garg AD, Dudek AM, Agostinis P. Cancer immunogenicity, danger signals, and DAMPs: what, when, and how? BioFactors 2013; 39:355-67; PMID:23900966; http://dx.doi.org/10.1002/biof.1125
- Panaretakis T, Joza N, Modjtahedi N, Tesniere A, Vitale I, Durchschlag M, Fimia GM, Kepp O, Piacentini M, Froehlich KU et al. The co-translocation of ERp57 and calreticulin determines the immunogenicity of cell death. Cell Death Differ 2008; 15:1499-509; PMID:18464797; http://dx.doi.org/10.1038/cdd.2008.67
- Kepp O, Gdoura A, Martins I, Panaretakis T, Schlemmer F, Tesniere A, Fimia GM, Ciccosanti F, Burgevin A, Piacentini M et al. Lysyl tRNA synthetase is required for the translocation of calreticulin to the cell surface in immunogenic death. Cell Cycle 2010; 9:3072-7; PMID:20699648; http://dx.doi.org/10.4161/cc.9.15.12459
- Gardai SJ, Xiao YQ, Dickinson M, Nick JA, Voelker DR, Greene KE, Henson PM. By binding SIRPalpha or calreticulinCD91, lung collectins act as dual function surveillance molecules to suppress or enhance inflammation. Cell 2003; 115 13-23; PMID:14531999; http://dx.doi.org/10.1016/S0092-8674(03)00758-X
- Ghiringhelli F, Apetoh L, Tesniere A, Aymeric L, Ma Y, Ortiz C, Vermaelen K, Panaretakis T, Mignot G, Ullrich E et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med 2009; 15:1170-8; PMID:19767732; http://dx.doi.org/10.1038/nm.2028
- Garg AD, Krysko DV, Verfaillie T, Kaczmarek A, Ferreira GB, Marysael T, Rubio N, Firczuk M, Mathieu C, Roebroek AJ et al. A novel pathway combining calreticulin exposure and ATP secretion in immunogenic cancer cell death. EMBO J 2012; 31:1062-79; PMID:22252128; http://dx.doi.org/10.1038/emboj.2011.497
- Spisek R, Charalambous A, Mazumder A, Vesole DH, Jagannath S, Dhodapkar MV. Bortezomib enhances dendritic cell (DC)-mediated induction of immunity to human myeloma via exposure of cell surface heat shock protein 90 on dying tumor cells: therapeutic implications. Blood 2007; 109:4839-45; PMID:17299090; http://dx.doi.org/10.1182/blood-2006-10-054221
- Tesniere A, Panaretakis T, Kepp O, Apetoh L, Ghiringhelli F, Zitvogel L, Kroemer G. Molecular characteristics of immunogenic cancer cell death. Cell Death Differ 2008; 15:3-12; PMID:18007663; http://dx.doi.org/10.1038/sj.cdd.4402269
- Kepp O, Galluzzi L, Martins I, Schlemmer F, Adjemian S, Michaud M, Sukkurwala AQ, Menger L, Zitvogel L, Kroemer G. Molecular determinants of immunogenic cell death elicited by anticancer chemotherapy. Cancer Metast Rev 2011; 30:61-9; PMID:21249425; http://dx.doi.org/10.1007/s10555-011-9273-4
- Michaud M, Martins I, Sukkurwala AQ, Adjemian S, Ma Y, Pellegatti P, Shen S, Kepp O, Scoazec M, Mignot G et al. Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science 2011; 334:1573-7; PMID:22174255; http://dx.doi.org/10.1126/science.1208347
- Garg AD, Dudek AM, Agostinis P. Autophagy-dependent suppression of cancer immunogenicity and effector mechanisms of innate and adaptive immunity. Oncoimmunology 2013; 2:e26260; PMID:24353910; http://dx.doi.org/10.4161/onci.26260
- Martins I, Tesniere A, Kepp O, Michaud M, Schlemmer F, Senovilla L, Séror C, Métivier D, Perfettini JL, Zitvogel L et al. Chemotherapy induces ATP release from tumor cells. Cell Cycle 2009; 8:3723-8; PMID:19855167; http://dx.doi.org/10.4161/cc.8.22.10026
- Krysko DV, Garg AD, Kaczmarek A, Krysko O, Agostinis P, Vandenabeele P. Immunogenic cell death and DAMPs in cancer therapy. Nat Rev Cancer 2012; 12:860-75; PMID:23151605; http://dx.doi.org/10.1038/nrc3380
- Martins I, Wang Y, Michaud M, Ma Y, Sukkurwala AQ, Shen S, Kepp O, Métivier D, Galluzzi L, Perfettini JL et al. Molecular mechanisms of ATP secretion during immunogenic cell death. Cell Death Differ 2014; 21:79-91; PMID:23852373; http://dx.doi.org/10.1038/cdd.2013.75
- Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 2002; 418:191-5; PMID:12110890; http://dx.doi.org/10.1038/nature00858
- Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Ann Rev Immunol 2013; 31:51-72; PMID:23157435; http://dx.doi.org/10.1146/annurev-immunol-032712-100008
- Wesch D, Peters C, Oberg HH, Pietschmann K, Kabelitz D. Modulation of gammadelta T cell responses by TLR ligands. Cell Mol Life Sci: CMLS 2011; 68:2357-70; PMID:21560072; http://dx.doi.org/10.1007/s00018-011-0699-1
- Dudek AM, Garg AD, Krysko DV, De Ruysscher D, Agostinis P. Inducers of immunogenic cancer cell death. Cytokine Growth Factor Rev 2013; 24:319-33; PMID:23391812; http://dx.doi.org/10.1016/j.cytogfr.2013.01.005
- Fucikova J, Kralikova P, Fialova A, Brtnicky T, Rob L, Bartunkova J, Spísek R. Human tumor cells killed by anthracyclines induce a tumor-specific immune response. Cancer Res 2011; 71:4821-33; PMID:21602432; http://dx.doi.org/10.1158/0008-5472.CAN-11-0950
- Tesniere A, Schlemmer F, Boige V, Kepp O, Martins I, Ghiringhelli F, Aymeric L, Michaud M, Apetoh L, Barault L et al. Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene 2010; 29:482-91; PMID:19881547; http://dx.doi.org/10.1038/onc.2009.356
- Chen HM, Wang PH, Chen SS, Wen CC, Chen YH, Yang WC, Yang NS. Shikonin induces immunogenic cell death in tumor cells and enhances dendritic cell-based cancer vaccine. Cancer Immunol, Immunother: CII 2012; 61:1989-2002; PMID:22527248; http://dx.doi.org/10.1007/s00262-012-1258-9
- Garrido G, Rabasa A, Sanchez B, Lopez MV, Blanco R, Lopez A, Hernández DR, Pérez R, Fernández LE. Induction of immunogenic apoptosis by blockade of epidermal growth factor receptor activation with a specific antibody. J Immunol 2011; 187:4954-66; PMID:21984704; http://dx.doi.org/10.4049/jimmunol.1003477
- Schiavoni G, Sistigu A, Valentini M, Mattei F, Sestili P, Spadaro F, Sanchez M, Lorenzi S, D'Urso MT, Belardelli F et al. Cyclophosphamide synergizes with type I interferons through systemic dendritic cell reactivation and induction of immunogenic tumor apoptosis. Cancer Res 2011; 71:768-78; PMID:21156650; http://dx.doi.org/10.1158/0008-5472.CAN-10-2788
- Menger L, Vacchelli E, Adjemian S, Martins I, Ma Y, Shen S, Yamazaki T, Sukkurwala AQ, Michaud M, Mignot G et al. Cardiac glycosides exert anticancer effects by inducing immunogenic cell death. Sci Translational Med 2012; 4:143ra99; PMID:22814852; http://dx.doi.org/10.1126/scitranslmed.3003807
- Abdul Qader Sukkurwala SA, Laura S, Mickaël M, Sabrina S, Erika V, Elisa Elena B, Lorenzo G, Laurence Z, Oliver K, Guido K. Screening of novel immunogenic cell death inducers within the NCI Mechanistic Diversity Set. Oncoimmunology 2014; 3:e28473; PMID:25050214
- Bugaut H, Bruchard M, Berger H, Derangere V, Odoul L, Euvrard R, Ladoire S, Chalmin F, Végran F, Rébé C et al. Bleomycin exerts ambivalent antitumor immune effect by triggering both immunogenic cell death and proliferation of regulatory T cells. PloS One 2013; 8:e65181; PMID:23762310; http://dx.doi.org/10.1371/journal.pone.0065181
- Yang Y, Li XJ, Chen Z, Zhu XX, Wang J, Zhang LB, Qiang L, Ma YJ, Li ZY, Guo QL et al. Wogonin induced calreticulinannexin A1 exposure dictates the immunogenicity of cancer cells in a PERKAKT dependent manner. PloS One 2012; 7:e50811; PMID:23251389; http://dx.doi.org/10.1371/journal.pone.0050811
- West AC, Mattarollo SR, Shortt J, Cluse LA, Christiansen AJ, Smyth MJ, Johnstone RW. An intact immune system is required for the anticancer activities of histone deacetylase inhibitors. Cancer Res 2013; 73:7265-76; PMID:24158093; http://dx.doi.org/10.1158/0008-5472.CAN-13-0890
- Weiss EM, Meister S, Janko C, Ebel N, Schlucker E, Meyer-Pittroff R, Fietkau R, Herrmann M, Gaipl US, Frey B. High hydrostatic pressure treatment generates inactivated mammalian tumor cells with immunogeneic features. J Immunotoxicol 2010; 7:194-204; PMID:20205624; http://dx.doi.org/10.3109/15476911003657414
- Fucikova J, Moserova I, Truxova I, Hermanova I, Vancurova I, Partlova S, Fialova A, Sojka L, Cartron PF, Houska M et al. High hydrostatic pressure induces immunogenic cell death in human tumor cells. Int J Cancer J Int du Cancer 2014; 135:1165-77; PMID:24500981; http://dx.doi.org/10.1002/ijc.28766
- Garg AD, Krysko DV, Vandenabeele P, Agostinis P. Hypericin-based photodynamic therapy induces surface exposure of damage-associated molecular patterns like HSP70 and calreticulin. Cancer Immunol, Immunother: CII 2012; 61:215-21; PMID:22193987; http://dx.doi.org/10.1007/s00262-011-1184-2
- Zamarin D, Holmgaard RB, Subudhi SK, Park JS, Mansour M, Palese P, Merghoub T, Wolchok JD, Allison JP. Localized ncolytic virotherapy overcomes systemic tumor resistance to immune checkpoint blockade immunotherapy. Sci Translational Med 2014; 6:226ra32; PMID:24598590; http://dx.doi.org/10.1126/scitranslmed.3008095
- Vacchelli E, Vitale I, Eggermont A, Fridman WH, Fucikova J, Cremer I, Galon J, Tartour E, Zitvogel L, Kroemer G, et al. Trial watch: dendritic cell-based interventions for cancer therapy. Oncoimmunology 2013; 2:e25771; PMID:24286020; http://dx.doi.org/10.4161/onci.25771
- Vacchelli E, Vitale I, Tartour E, Eggermont A, Sautes-Fridman C, Galon J, Zitvogel L, Kroemer G, Galluzzi L. Trial watch: anticancer radioimmunotherapy. Oncoimmunology 2013; 2:e25595; PMID:24319634; http://dx.doi.org/10.4161/onci.25595
- Golden EB, Pellicciotta I, Demaria S, Barcellos-Hoff MH, Formenti SC. The convergence of radiation and immunogenic cell death signaling pathways. Front Oncol 2012; 2:88; PMID:22891162; http://dx.doi.org/10.3389/fonc.2012.00088
- Rubner Y, Wunderlich R, Ruhle PF, Kulzer L, Werthmoller N, Frey B, Weiss EM, Keilholz L, Fietkau R, Gaipl US. How does ionizing irradiation contribute to the induction of anti-tumor immunity? Front Oncol 2012; 2:75; PMID:22848871; http://dx.doi.org/10.3389/fonc.2012.00075
- Suzuki Y, Mimura K, Yoshimoto Y, Watanabe M, Ohkubo Y, Izawa S, Murata K, Fujii H, Nakano T, Kono K. Immunogenic tumor cell death induced by chemoradiotherapy in patients with esophageal squamous cell carcinoma. Cancer Res 2012; 72:3967-76; PMID:22700877; http://dx.doi.org/10.1158/0008-5472.CAN-12-0851
- Stangl S, Themelis G, Friedrich L, Ntziachristos V, Sarantopoulos A, Molls M, Skerra A, Multhoff G. Detection of irradiation-induced, membrane heat shock protein 70 (Hsp70) in mouse tumors using Hsp70 Fab fragment. Radiother Oncol: J Eur Soc Therap Radiol Oncol 2011; 99:313-6; PMID:21704400; http://dx.doi.org/10.1016/j.radonc.2011.05.051
- Brusa D, Migliore E, Garetto S, Simone M, Matera L. Immunogenicity of 56 degrees C and UVC-treated prostate cancer is associated with release of HSP70 and HMGB1 from necrotic cells. Prostate 2009; 69:1343-52; PMID:19496055; http://dx.doi.org/10.1002/pros.20981
- Prasad SJ, Farrand KJ, Matthews SA, Chang JH, McHugh RS, Ronchese F. Dendritic cells loaded with stressed tumor cells elicit long-lasting protective tumor immunity in mice depleted of CD4+CD25+ regulatory T cells. J Immunol 2005; 174:90-8; PMID:15611231; http://dx.doi.org/10.4049/jimmunol.174.1.90
- Strome SE, Voss S, Wilcox R, Wakefield TL, Tamada K, Flies D, Chapoval A, Lu J, Kasperbauer JL, Padley D et al. Strategies for antigen loading of dendritic cells to enhance the antitumor immune response. Cancer Res 2002; 62:1884-9; PMID:11912169
- Schaue D, Ratikan JA, Iwamoto KS, McBride WH. Maximizing tumor immunity with fractionated radiation. Int J Radiat Oncol, Biol, Phys 2012; 83:1306-10; PMID:22208977; http://dx.doi.org/10.1016/j.ijrobp.2011.09.049
- Carr-Brendel V, Markovic D, Smith M, Taylor-Papadimitriou J, Cohen EP. Immunity to breast cancer in mice immunized with X-irradiated breast cancer cells modified to secrete IL-12. J Immunother 1999; 22:415-22; PMID:10546157; http://dx.doi.org/10.1097/00002371-199909000-00005
- Kachikwu EL, Iwamoto KS, Liao YP, DeMarco JJ, Agazaryan N, Economou JS, McBride WH, Schaue D. Radiation enhances regulatory T cell representation. Int J Radiat Oncol, Biol, Phys 2011; 81:1128-35; PMID:21093169; http://dx.doi.org/10.1016/j.ijrobp.2010.09.034
- Baba J, Watanabe S, Saida Y, Tanaka T, Miyabayashi T, Koshio J, Ichikawa K, Nozaki K, Koya T, Deguchi K et al. Depletion of radio-resistant regulatory T cells enhances antitumor immunity during recovery from lymphopenia. Blood 2012; 120:2417-27; PMID:22806892; http://dx.doi.org/10.1182/blood-2012-02-411124
- Gorin JB, Menager J, Gouard S, Maurel C, Guilloux Y, Faivre-Chauvet A, Morgenstern A, Bruchertseifer F, Chérel M, Davodeau F et al. Antitumor immunity induced after alpha irradiation. Neoplasia 2014; 16:319-28; PMID:24862758; http://dx.doi.org/10.1016/j.neo.2014.04.002
- Frey B, Stache C, Rubner Y, Werthmoller N, Schulz K, Sieber R, Semrau S, Rödel F, Fietkau R, Gaipl US. Combined treatment of human colorectal tumor cell lines with chemotherapeutic agents and ionizing irradiation can in vitro induce tumor cell death forms with immunogenic potential. J Immunotoxicol 2012; 9:301-13; PMID:22800185; http://dx.doi.org/10.3109/1547691X.2012.693547
- Frey B, Rubner Y, Kulzer L, Werthmoller N, Weiss EM, Fietkau R, Gaipl US. Antitumor immune responses induced by ionizing irradiation and further immune stimulation. Cancer Immunol, Immunother: CII 2014; 63:29-36; PMID:24052136; http://dx.doi.org/10.1007/s00262-013-1474-y
- Formenti SC, Demaria S. Combining radiotherapy and cancer immunotherapy: a paradigm shift. J Nat Cancer Inst 2013; 105:256-65; PMID:23291374; http://dx.doi.org/10.1093/jnci/djs629
- Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S, Mu Z, Rasalan T, Adamow M, Ritter E et al. Immunologic correlates of the abscopal effect in a patient with melanoma. New Engl J Med 2012; 366:925-31; PMID:22397654; http://dx.doi.org/10.1056/NEJMoa1112824
- Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. New Engl J Med 2010; 363:411-22; PMID:20818862; http://dx.doi.org/10.1056/NEJMoa1001294
- Maverakis E, Miyamura Y, Bowen MP, Correa G, Ono Y, Goodarzi H. Light, including ultraviolet. J Autoimmun 2010; 34:J247-57; PMID:20018479; http://dx.doi.org/10.1016/j.jaut.2009.11.011
- Widel M, Krzywon A, Gajda K, Skonieczna M, Rzeszowska-Wolny J. Induction of bystander effects by UVA, UVB, and UVC radiation in human fibroblasts and the implication of reactive oxygen species. Free Radical Biol Med 2014; 68:278-87; PMID:24373962; http://dx.doi.org/10.1016/j.freeradbiomed.2013.12.021
- Zhong JL, Yang L, Lu F, Xiao H, Xu R, Wang L, Zhu F, Zhang Y. UVA, UVB and UVC induce differential response signaling pathways converged on the eIF2alpha phosphorylation. Photochem Photobiol 2011; 87:1092-104; PMID:21707633; http://dx.doi.org/10.1111/j.1751-1097.2011.00963.x
- Anand S, Chakrabarti E, Kawamura H, Taylor CR, Maytin EV. Ultraviolet light (UVB and UVA) induces the damage-responsive transcription factor CHOPgadd153 in murine and human epidermis: evidence for a mechanism specific to intact skin. J Invest Dermatol 2005; 125 323-33; PMID:16098044
- Eaglstein WH, Sakai M, Mizuno N. Ultraviolet radiation-induced inflammation and leukocytes. J Invest Dermatol 1979; 72:59-63; PMID:422868; http://dx.doi.org/10.1111/1523-1747.ep12530248
- Begovic M, Herberman RB, Gorelik E. Ultraviolet light-induced increase in tumor cell susceptibility to TNF-dependent and TNF-independent natural cell-mediated cytotoxicity. Cell Immunol 1991; 138:349-59; PMID:1934075; http://dx.doi.org/10.1016/0008-8749(91)90159-9
- Momiyama M, Suetsugu A, Kimura H, Kishimoto H, Aki R, Yamada A, Sakurada H, Chishima T, Bouvet M, Endo I et al. Imaging the efficacy of UVC irradiation on superficial brain tumors and metastasis in live mice at the subcellular level. J Cell Biochem 2013; 114:428-34; PMID:22961687; http://dx.doi.org/10.1002/jcb.24381
- Frey B, Franz S, Sheriff A, Korn A, Bluemelhuber G, Gaipl US, Voll RE, Meyer-Pittroff R, Herrmann M. Hydrostatic pressure induced death of mammalian cells engages pathways related to apoptosis or necrosis. Cell Mol Biol (Noisy-Le-Grand) 2004; 50:459-67; PMID:15529755
- Korn A, Frey B, Sheriff A, Gaipl US, Franz S, Meyer-Pittroff R, Bluemelhuberh G, Herrmann M. High hydrostatic pressure inactivated human tumour cells preserve their immunogenicity. Cell Mol Biol (Noisy-Le-Grand) 2004; 50:469-77; PMID:15529756
- Frey B, Janko C, Ebel N, Meister S, Schlucker E, Meyer-Pittroff R, Fietkau R, Herrmann M, Gaipl US. Cells under pressure - treatment of eukaryotic cells with high hydrostatic pressure, from physiologic aspects to pressure induced cell death. Curr Med Chem 2008; 15:2329-36; PMID:18855663; http://dx.doi.org/10.2174/092986708785909166
- Diehl P, Schauwecker J, Mittelmeier W, Schmitt M. High hydrostatic pressure, a novel approach in orthopedic surgical oncology to disinfect bone, tendons and cartilage. Anticancer Res 2008; 28:3877-83; PMID:19192644
- Helmstein K. Treatment of bladder carcinoma by a hydrostatic pressure technique. Report on 43 cases. Brit J Urol 1972; 44:434-50; PMID:5070147; http://dx.doi.org/10.1111/j.1464-410X.1972.tb10103.x
- Coombes GB. A clinical evaluation of hydrostatic pressure treatment for carcinoma of the bladder. Brit J Urol 1975; 47:177-83; PMID:1097023; http://dx.doi.org/10.1111/j.1464-410X.1975.tb03943.x
- Eisenthal A, Ramakrishna V, Skornick Y, Shinitzky M. Induction of cell-mediated immunity against B16-BL6 melanoma in mice vaccinated with cells modified by hydrostatic pressure and chemical crosslinking. Cancer Immunol, Immunother: CII 1993; 36:300-6; PMID:8477416; http://dx.doi.org/10.1007/BF01741168
- Goldman Y, Peled A, Shinitzky M. Effective elimination of lung metastases induced by tumor cells treated with hydrostatic pressure and N-acetyl-L-cysteine. Cancer Res 2000; 60:350-8; PMID:10667587
- Eisenthal A, Goldman Y, Skornick Y, Gelfand A, Buyaner D, Kaver I, Yellin A, Yehoshua H, Lifschitz-Mercer B, Gonnene A et al. Human tumor cells, modified by a novel pressurecrosslinking methodology, promote autologous lymphocyte proliferation and modulate cytokine secretion. Cancer Immunol, Immunother: CII 1998; 46:304-10; PMID:9756414; http://dx.doi.org/10.1007/s002620050491
- Shinitzky M, Goldman Y. Immunotherapy of cancer with pressure modified cells. Isr Med Assoc J 2000; 2:615-20; PMID:10979358
- Fucikova J, Moserova I, Truxova I, Hermanova I, Vancurova I, Partlova S, Sojka L, Cartron PF, Houska M, Rob L et al. Article title: high hydrostatic pressure induces immunogenic cell death in human tumor cells. Int J Cancer 2014; 135:1165-77; PMID:24500981; http://dx.doi.org/10.1002/ijc.28766
- Rozkova D, Tiserova H, Fucikova J, Last’ovicka J, Podrazil M, Ulcova H, Budínský V, Prausová J, Linke Z, Minárik I et al. FOCUS on FOCIS: combined chemo-immunotherapy for the treatment of hormone-refractory metastatic prostate cancer. Clinical Immunol 2009; 131:1-10; PMID:19201656; http://dx.doi.org/10.1016/j.clim.2009.01.001
- Garg AD, Agostinis P. ER stress, autophagy and immunogenic cell death in photodynamic therapy-induced anti-cancer immune responses. Photochem Photobiol Sci: Off J Eur Photochem Assoc Euro Soc Photobiol 2014; 13:474-87; PMID:24493131; http://dx.doi.org/10.1039/c3pp50333j
- Garg AD, Nowis D, Golab J, Agostinis P. Photodynamic therapy: illuminating the road from cell death towards anti-tumour immunity. Apoptosis 2010; 15:1050-71; PMID:20221698; http://dx.doi.org/10.1007/s10495-010-0479-7
- Agostinis P, Berg K, Cengel KA, Foster TH, Girotti AW, Gollnick SO, Hahn SM, Hamblin MR, Juzeniene A, Kessel D et al. Photodynamic therapy of cancer: an update. CA Cancer J Clin 2011; 61:250-81; PMID:21617154; http://dx.doi.org/10.3322/caac.20114
- Buytaert E, Dewaele M, Agostinis P. Molecular effectors of multiple cell death pathways initiated by photodynamic therapy. Biochimica et Biophysica Acta 2007; 1776:86-107; PMID:17693025
- Kabingu E, Oseroff AR, Wilding GE, Gollnick SO. Enhanced systemic immune reactivity to a Basal cell carcinoma associated antigen following photodynamic therapy. Clin Cancer Res: Off J Am Assoc Cancer Res 2009; 15:4460-6; PMID:19549769; http://dx.doi.org/10.1158/1078-0432.CCR-09-0400
- Thong PS, Ong KW, Goh NS, Kho KW, Manivasager V, Bhuvaneswari R, Olivo M, Soo KC. Photodynamic-therapy-activated immune response against distant untreated tumours in recurrent angiosarcoma. Lancet Oncol 2007; 8:950-2; PMID:17913664; http://dx.doi.org/10.1016/S1470-2045(07)70318-2
- Verfaillie T, Rubio N, Garg AD, Bultynck G, Rizzuto R, Decuypere JP, Piette J, Linehan C, Gupta S, Samali A et al. PERK is required at the ER-mitochondrial contact sites to convey apoptosis after ROS-based ER stress. Cell Death Differ 2012; 19:1880-91; PMID:22705852; http://dx.doi.org/10.1038/cdd.2012.74
- Verfaillie T, van Vliet A, Garg AD, Dewaele M, Rubio N, Gupta S, de Witte P, Samali A, Agostinis P. Pro-apoptotic signaling induced by photo-oxidative ER stress is amplified by Noxa, not Bim. Biochem Biophys Res Commun 2013; 438:500-6; PMID:23916707; http://dx.doi.org/10.1016/j.bbrc.2013.07.107
- Kacerovska D, Pizinger K, Majer F, Smid F. Photodynamic therapy of nonmelanoma skin cancer with topical hypericum perforatum extract+a pilot study. Photochem Photobiol 2008; 84:779-85; PMID:18179625; http://dx.doi.org/10.1111/j.1751-1097.2007.00260.x
- Rook AH, Wood GS, Duvic M, Vonderheid EC, Tobia A, Cabana B. A phase II placebo-controlled study of photodynamic therapy with topical hypericin and visible light irradiation in the treatment of cutaneous T-cell lymphoma and psoriasis. J Am Acad Dermatol 2010; 63:984-90; PMID:20889234; http://dx.doi.org/10.1016/j.jaad.2010.02.039
- Koren H, Schenk GM, Jindra RH, Alth G, Ebermann R, Kubin A, Koderhold G, Kreitner M. Hypericin in phototherapy. J Photochem Photobiol B, Biol 1996; 36:113-9; PMID:9002247; http://dx.doi.org/10.1016/S1011-1344(96)07357-5
- Alecu M, Ursaciuc C, Halalau F, Coman G, Merlevede W, Waelkens E, de Witte P. Photodynamic treatment of basal cell carcinoma and squamous cell carcinoma with hypericin. Anticancer Res 1998; 18:4651-4; PMID:9891535
- Garg AD, Dudek AM, Agostinis P. Calreticulin surface exposure is abrogated in cells lacking, chaperone-mediated autophagy-essential gene, LAMP2A. Cell Death Dis 2013; 4:e826; PMID:24091669; http://dx.doi.org/10.1038/cddis.2013.372
- Garg AD, Dudek AM, Ferreira GB, Verfaillie T, Vandenabeele P, Krysko DV, Mathieu C, Agostinis P. ROS-induced autophagy in cancer cells assists in evasion from determinants of immunogenic cell death. Autophagy 2013; 9:1292-307; PMID:23800749; http://dx.doi.org/10.4161/auto.25399
- Gollnick SO, Vaughan L, Henderson BW. Generation of effective antitumor vaccines using photodynamic therapy. Cancer Res 2002; 62:1604-8; PMID:11912128
- Zhang H, Ma W, Li Y. Generation of effective vaccines against liver cancer by using photodynamic therapy. Lasers Med Sci 2009; 24:549-52; PMID:18780140; http://dx.doi.org/10.1007/s10103-008-0609-4
- Korbelik M, Sun J. Photodynamic therapy-generated vaccine for cancer therapy. Cancer Immunol, Immunother: CII 2006; 55:900-9; PMID:16215717; http://dx.doi.org/10.1007/s00262-005-0088-4
- Jalili A, Makowski M, Switaj T, Nowis D, Wilczynski GM, Wilczek E, Chorazy-Massalska M, Radzikowska A, Maslinski W, Biały L et al. Effective photoimmunotherapy of murine colon carcinoma induced by the combination of photodynamic therapy and dendritic cells. Clin Cancer Res: Off J Am Assoc Cancer Res 2004; 10:4498-508; PMID:15240542; http://dx.doi.org/10.1158/1078-0432.CCR-04-0367
- Hildebrandt B, Wust P, Ahlers O, Dieing A, Sreenivasa G, Kerner T, Felix R, Riess H. The cellular and molecular basis of hyperthermia. Crit Rev Oncol Hematol 2002; 43:33-56; PMID:12098606; http://dx.doi.org/10.1016/S1040-8428(01)00179-2
- Harmon BV, Corder AM, Collins RJ, Gobe GC, Allen J, Allan DJ, Kerr JF. Cell death induced in a murine mastocytoma by 42-47 degrees C heating in vitro: evidence that the form of death changes from apoptosis to necrosis above a critical heat load. Int J Radiat Biol 1990; 58:845-58; PMID:1977828; http://dx.doi.org/10.1080/09553009014552221
- Feng H, Zeng Y, Graner MW, Likhacheva A, Katsanis E. Exogenous stress proteins enhance the immunogenicity of apoptotic tumor cells and stimulate antitumor immunity. Blood 2003; 101:245-52; PMID:12393411; http://dx.doi.org/10.1182/blood-2002-05-1580
- Shi H, Cao T, Connolly JE, Monnet L, Bennett L, Chapel S, Bagnis C, Mannoni P, Davoust J, Palucka AK et al. Hyperthermia enhances CTL cross-priming. J Immunol 2006; 176:2134-41; PMID:16455969; http://dx.doi.org/10.4049/jimmunol.176.4.2134
- Masse D, Ebstein F, Bougras G, Harb J, Meflah K, Gregoire M. Increased expression of inducible HSP70 in apoptotic cells is correlated with their efficacy for antitumor vaccine therapy. Int J Cancer J Int du Cancer 2004; 111:575-83; PMID:15239136; http://dx.doi.org/10.1002/ijc.20249
- Gordon RT, Hines JR, Gordon D. Intracellular hyperthermia. A biophysical approach to cancer treatment via intracellular temperature and biophysical alterations. Med Hypotheses 1979; 5:83-102; PMID:459972; http://dx.doi.org/10.1016/0306-9877(79)90063-X
- Ito A, Shinkai M, Honda H, Yoshikawa K, Saga S, Wakabayashi T, Yoshida J, Kobayashi T. Heat shock protein 70 expression induces antitumor immunity during intracellular hyperthermia using magnetite nanoparticles. Cancer Immunol, Immunother: CII 2003; 52:80-8; PMID:12594571
- Ito A, Honda H, Kobayashi T. Cancer immunotherapy based on intracellular hyperthermia using magnetite nanoparticles: a novel concept of “heat-controlled necrosis” with heat shock protein expression. Cancer Immunol, Immunother: CII 2006; 55:320-8; PMID:16133113; http://dx.doi.org/10.1007/s00262-005-0049-y
- Wang H, Zhang L, Shi Y, Javidiparsijani S, Wang G, Li X, Ouyang W, Zhou J, Zhao L, Wang X et al. Abscopal antitumor immune effects of magnet-mediated hyperthermia at a high therapeutic temperature on Walker-256 carcinosarcomas in rats. Oncol Lett 2014; 7:764-70; PMID:24527084
- Xu X, Gupta S, Hu W, McGrath BC, Cavener DR. Hyperthermia induces the ER stress pathway. PloS One 2011; 6:e23740; PMID:21876766; http://dx.doi.org/10.1371/journal.pone.0023740
- Chen T, Guo J, Han C, Yang M, Cao X. Heat shock protein 70, released from heat-stressed tumor cells, initiates antitumor immunity by inducing tumor cell chemokine production and activating dendritic cells via TLR4 pathway. J Immunol 2009; 182:1449-59; PMID:19155492; http://dx.doi.org/10.4049/jimmunol.182.3.1449
- Frey B, Weiss EM, Rubner Y, Wunderlich R, Ott OJ, Sauer R, Fietkau R, Gaipl US. Old and new facts about hyperthermia-induced modulations of the immune system. Int J Hyperther: Off J Eur Soc Hyperther Oncol, N Am Hyperther Group 2012; 28:528-42; PMID:22690925; http://dx.doi.org/10.3109/02656736.2012.677933
- Weiss EM, Frey B, Rodel F, Herrmann M, Schlucker E, Voll RE, Fietkau R, Gaipl US. Ex vivo- and in vivo-induced dead tumor cells as modulators of antitumor responses. Ann New York Acad Sci 2010; 1209:109-17; PMID:20958323; http://dx.doi.org/10.1111/j.1749-6632.2010.05743.x
- Mantel F, Frey B, Haslinger S, Schildkopf P, Sieber R, Ott OJ, Lödermann B, Rödel F, Sauer R, Fietkau R et al. Combination of ionising irradiation and hyperthermia activates programmed apoptotic and necrotic cell death pathways in human colorectal carcinoma cells. Strahlenther Onkol 2010; 186:587-99; PMID:21069267; http://dx.doi.org/10.1007/s00066-010-2154-x
- Schildkopf P, Frey B, Ott OJ, Rubner Y, Multhoff G, Sauer R, Fietkau R, Gaipl US. Radiation combined with hyperthermia induces HSP70-dependent maturation of dendritic cells and release of pro-inflammatory cytokines by dendritic cells and macrophages. Radiother Oncol: J Eur Soc Therap Radiol Oncol 2011; 101:109-15; PMID:21704416; http://dx.doi.org/10.1016/j.radonc.2011.05.056
- Qin Y, Lu J, Bao L, Zhu H, Li J, Li L, Jiang B, Huang X. Bortezomib improves progression-free survival in multiple myeloma patients overexpressing preferentially expressed antigen of melanoma. Chinese Med J 2014; 127:1666-71; PMID:24791872
- Audia S, Nicolas A, Cathelin D, Larmonier N, Ferrand C, Foucher P, Fanton A, Bergoin E, Maynadie M, Arnould L et al. Increase of CD4+ CD25+ regulatory T cells in the peripheral blood of patients with metastatic carcinoma: a Phase I clinical trial using cyclophosphamide and immunotherapy to eliminate CD4+ CD25+ T lymphocytes. Clin Exp Immunol 2007; 150:523-30; PMID:17956583; http://dx.doi.org/10.1111/j.1365-2249.2007.03521.x
- Viaud S, Flament C, Zoubir M, Pautier P, LeCesne A, Ribrag V, Soria JC, Marty V, Vielh P, Robert C et al. Cyclophosphamide induces differentiation of Th17 cells in cancer patients. Cancer Res 2011; 71:661-5; PMID:21148486; http://dx.doi.org/10.1158/0008-5472.CAN-10-1259
