Abstract
Both IL-17 and Th17 cells have been ascribed tumor promoting as well as tumor suppressing functions. We reviewed the literature on correlations between IL-17 versus Th17 cells and survival in human cancer, following the PRISMA guidelines. Serum, formalin-fixed, paraffin-embedded (FFPE) tissue and peripheral blood samples were most frequently studied. High IL-17 quantities were correlated with poor prognosis, whereas high Th17 cell frequencies were correlated with improved prognosis. Since Th17 cells are a subpopulation of IL-17+ cells and had a different correlation with prognosis than total IL-17, we substantiate that a distinction should be made between Th17 and other IL-17+ cells.
Introduction
Interleukin-17 (IL-17) was characterized in 1993 and originally named cytotoxic T lymphocyte-associated-8 (CTLA-8).Citation1 IL-17 was more recently renamed IL-17A and has five family members: IL-17B–F.Citation2 Only IL-17F shows some homology and overlapping functions with IL-17A. The main functions of IL-17 are the attraction of neutrophils and stimulation of inflammation.Citation3 The T-helper 17 (Th17) cell, one of the predominant producers of IL-17 that was characterized in 2005,Citation4 is essential to protect the host against pathogens that are not handled well by Th1 and Th2 cells.Citation5 This pro-inflammatory cell type plays a dominant role in a variety of autoimmune diseases.Citation3 Antibodies targeting IL-17 and its receptor are now used in clinical trials to treat autoimmune diseases like psoriasis, rheumatoid arthritis, and Crohn's disease.Citation6 Since IL-17 can also be produced by innate immune cell types including both lymphoid-derived (e.g., γδT cells, invariant natural killer T cells, and innate lymphoid cells)Citation7,8 and myeloid-derived cells (e.g., neutrophils, macrophages, and mast cells),Citation9 it may bridge the activities of the innate and adaptive immune system.Citation10
Much less studied is the role of IL-17 in cancer. Both tumor-suppressing and tumor-promoting functions have been ascribed to the IL-17 protein and Th17 cells.Citation11 This ambiguity about the function of IL-17 and Th17 cells in cancer has limited the potential for targeting the molecule or using cell-based immunotherapy. Part of the ambiguity may have arisen because different aspects of the IL-17 response are studied. Total protein amount or cells expressing IL-17 protein have been measured in serum and tumor-associated fluids by ELISA and in FFPE tissue by immunohistochemistry, respectively. The effect of Th17 cells has been analyzed mainly in peripheral blood, but also in tumor-associated fluids, FFPE and fresh frozen tissue by flow cytometry, immunohistochemistry or RT-PCR. A review on Th17 cells in cancer by Wilke et al. in 2011 already noted that correlations between the IL-17 protein and survival may be different from correlations with the Th17 cell population.Citation12
To systematically study the correlations between the IL-17 protein and Th17 cells and survival in human cancer, we investigated all publications in NCBI PubMed, Ovid Embase, and Web of Science addressing this subject. The aim of our study was to identify the correlations between both IL-17 protein and Th17 cells and prognosis in cancer. The studies were classified by the sample type used to study IL-17 or Th17 cells: serum, FFPE tissue, peripheral blood, tumor-associated fluids, and fresh frozen tissue. Subsequently, the effect on survival was analyzed for each of the sample types studied. The implications for further research of IL-17 and Th17 cells are discussed.
Results
Study design and selection criteria
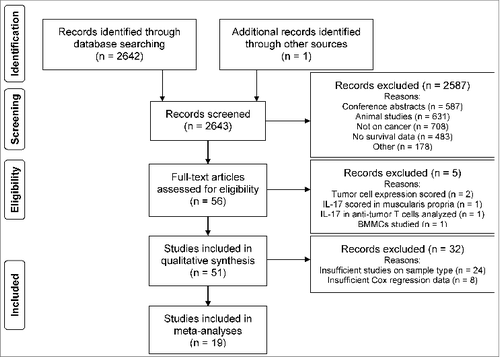
Of the 2,643 publications identified through database searching on IL-17 or Th17 and cancer, 56 studies met the inclusion criteria (). The main reasons for a publication to be excluded were: being a conference abstract (23%), an animal study (24%), no study on cancer (27%), or not reporting on survival data (19%). Two articles were excluded due to lack of other references on the same method and survival analysis. One article reported on an IL-17 polymorphism analysis,Citation13 while the other studied RNA levels of Th17 cell expressed retinoic acid receptor-related orphan receptor gamma (RORγt).Citation14 Neither of these studies found a correlation with survival. An overview of the included studies sorted by sample type and clinical outcome is shown in .
Table 1.1. Correlation between IL-17 quantification in serum. All studies describing a correlation between a measurement of IL-17 in serum by ELISA and overall or disease-free survival are shown. The analyses were sorted by clinical outcome, cancer type and study size. Sample size indicates the number of patients on which the correlation between the IL-17 measurement and survival was reported. The column ‘Correlation’ indicates whether a high measurement was correlated with poor or improved survival. A multivariate analysis including both the IL-17 measurement as well as another variable also containing this IL-17 measurement (e.g. a ratio) was not included in our analysis since the potential effect might be lost by correcting for it. An asterisk behind a reference number indicates that the study was not included in the quantitative analyses in Table 2 and Fig. 2. Whether or not the correlation found was independently correlated with survival when corrected for clinico-pathological parameters in a multivariate Cox regression analysis is also indicated. A multivariate analysis including both the IL-17 measurement as well as another variable also containing this IL-17 measurement (e.g., a ratio) was not included in our analysis since the potential effect might be lost by correcting for it. Measurement deviances are indicated under ‘Notes’. CLL = chronic lymphocytic leukemia; AC = adenocarcinoma; CRC = colorectal carcinoma; HCC = hepatocellular carcinoma; NA = not applicable or not mentioned in the article; NSCLC = non-small cell lung carcinoma.
Figure 1. PRISMA Flow diagram. Database searching identified 2642 publications on IL-17 or Th17 and cancer. One publication on this topic of our group published in this issue of OncoImmunology was added manually. Using the search term “tumor” caused studies on tumor necrosis factor to be selected regardless of whether the study was on cancer. Although tumor necrosis factor was excluded as a major topic, many publications that were not on cancer had to be excluded manually. All inclusion criteria were met by 56 articles. Five studies were excluded from analysis for using different sample types or methods than all other articles. Another 32 studies were excluded from meta-analysis because not enough studies were available on sample types other than tissue analyzed for IL-17 expression or insufficient data were provided. Figure adapted from Moher et al.Citation80
Studies reporting on survival analysis or risk of recurrence were included regardless of the outcome of the study. A potential publication bias was caused by excluding articles that reported on correlations with other clinico-pathological parameters but not survival. This bias was minimized by screening all articles that reported on correlations with clinico-pathological parameters for having performed a survival analysis. The survival criterion enabled us to focus on studies that are relevant for the potential targeting of IL-17 or Th17 cells in a clinical setting.
Generally, a random or consecutive group of patients was analyzed for relatively objective measures (see Tables S1–4) Although most studies did not provide details on the sample selection method, the majority of the studied did specify that they used a continuous variable or categorized IL-17 or Th17 cell numbers in groups based on the presence, mean or median to analyze the effect by Kaplan–Meier and Cox regression analyses. Potential risks of bias identified in categorizing IL-17 or Th17 expression were optimal cut-off values chosen arbitrarilyCitation15,16 or using a minimum p value,Citation17-23 receiver operating characteristic (ROC) curveCitation24-27 or regression tree analysis.Citation28 Furthermore, one study compared the six long (>3 y) vs. short (<1.5 y) surviving patients.Citation29 Another study reported that post-chemotherapy samples were used when no pretreatment samples were available for immunohistochemistry.Citation30 A final potential risk factor was observed in a study of leukemia patients treated with allogeneic stem cell transplantation after myeloablative conditioning, which included donors that varied from related to unrelated and different prophylaxis regimens to prevent graft-vs.-host disease.Citation31 Additional study details and concerns are listed per sample type in Tables S1–4. Clinico-pathological characteristics of the different studies per measurement method are provided in Tables S5–8.
High IL-17 serum levels are correlated with poor survival
Serum, paraffin tissue, peripheral blood mononuclear cells (PBMCs) and occasionally tumor-associated fluids or fresh frozen tissue were used to measure IL-17 protein or RNA and Th17 cells. Since the cell source and related activity measured may differ in different sample types, we sorted and analyzed the studies by sample type. The amount of IL-17 protein in serum was measured by ELISA (). Since total protein quantity was measured, the IL-17 could have been derived from Th17 cells but also from innate immune cell types. Five studies out of ten reported that a high amount of serum IL-17 protein was correlated with poor survival.Citation17,24-26,31 One study showed a correlation between high IL-17 and improved survival in leukemia.Citation32 Four studies did not observe a significant correlation between high serum IL-17 levels and survival,Citation33-36 although one group did find a trend toward poor prognosis (p = 0.05).Citation36 Overall, a high amount of IL-17 protein in serum has predominantly been correlated with poor survival ().
Table 1.2. Correlation between IL-17+ cells in tissue and survival. A representation of studies on tumor infiltrated IL-17+ cells quantified by immunohistochemistry on FFPE tissue slides or tissue microarrays. If ‘intratumoral’ is indicated, peritumoral cells were scored as well. CT = chemotherapy; NS = not significant; RT = radiotherapy; SCC = squamous cell carcinoma
Table 1.3. Correlation between Th17 cells and survival. Analyses of Th17 quantification on FFPE tissue, peripheral blood PBMCs and fresh frozen samples. Bref = brefeldin A; FC = flow cytometry; GVHD = graft-versus-host disease; Iono = ionomycin; Mon = monensin; PMA = phorbol 12-myristate 13-acetate
Table 1.4. Correlation between IL-17 and Th17 cells in tumor-associated fluids and survival sorted by measurement type. MPE = malignant pleural effusion
Table 2. Correlations per measurement type. The number of analyses per sample and measurement type of IL-17 protein or Th17 cells showing a correlation with improved or poor prognosis or no effect is indicated. The final column denotes the ratio of the number of analyses showing a correlation with improved prognosis over the number of analyses showing a correlation with poor prognosis, as an indication of the factor difference. A white box indicates a correlation with improved survival, a dark gray box a correlation with poor survival and a light gray box no clear correlation
A high number of IL-17+ cells in tissue is correlated with poor survival
The total number of IL-17+ cells was quantified on cancer tissue FFPE whole slides or tissue microarrays using immunohistochemistry. This type of analysis allows for quantification of the total number of IL-17+ cells within the tumor microenvironment. IL-17 is expressed by different types of tumor infiltrating immune cells in cancer, predominantly neutrophils and mast cells.Citation37-39 The total number of IL-17+ cells was correlated with poor prognosis in 18 out of 27 studies ().Citation15,16,18-20,30,37,40-50 Five studies reported on a correlation between a high number of IL-17+ cells and improved survival.Citation21,28,29,51,52 It is important to note that in two of these five studies, the IL-17+ cells were scored in areas with the densest lymphocytic infiltrate, one of which was on pancreatic ductal adenocarcinoma patients who had received immunotherapy (the correlation between IL-17 and survival was based on 12 patients).Citation21,29 Four studies did not observe a significant correlation between total IL-17+ cells in the tumor and survival.Citation22,23,53,54 Again the scoring in 2 of these 4 studies had been performed in hot-spot or dense lymphocytic infiltrate areas, while only 3 of the 18 studies reporting on a negative correlation had focused on hot-spots. Three more studies did not focus on IL-17+ tumor-infiltrating immune cells and are included with their reported correlations in for completeness, but not in the quantitative analyses.Citation39,55,56
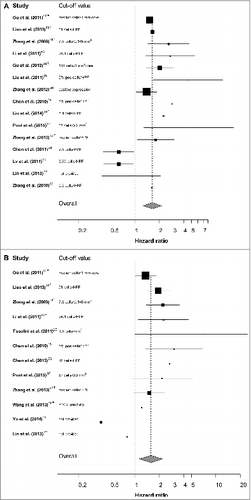
Collectively, 18 studies reported on a significant correlation between high IL-17 and poor prognosis, over 3.5 times more than the studies showing a correlation with improved prognosis (n = 5, ). To visualize the overall correlation, forest plots are shown for the hazard ratio of a high number of IL-17+ cells on overall (OS) () and disease-free survival (DFS) (). Of the 22 studies reporting on overall survival, 7 were excluded from the meta-analysis due to insufficient Cox regression data. Of the 16 studies reporting on DFS, 4 were excluded due to insufficient Cox regression data.
Figure 2. Forest plots for IL-17+ cells in tissue. Schematic quantitative analyses of the studies on the number of IL-17+ cells in FFPE tissue are shown by forest plots. Cox regression hazard ratios and 95% confidence intervals for the correlation between a high number of IL-17+ cells and overall survival (A) and disease-free survival (B) were obtained from the articles or via personal communication with the authors. An asterisk (*) indicates that a multivariate Cox regression analysis was used because a univariate analysis was not provided. A dagger (†) indicates that part of the data were obtained via e-mail. The cut-off value used to divide the IL-17+ cell frequency in a high and low group is indicated for comparison. The center of the random effects model represents the pooled hazard ratio, while the 95% confidence interval is represented by the diamond horizontal borders.
Correlation between IL-17 RNA expression in fresh frozen tissue and survival inconclusive
Two studies have analyzed IL-17 RNA expression in fresh frozen samples using RT-PCR (). Both studies, on small study populations, did not find associations with survival. One study analyzed IL-17 expression in tumor adjacent normal appearing biopsies (∼10 cm from the tumor center) from 19 colorectal cancer patients.Citation57 The other study in 17 ovarian cancer patients only measured the presence of PCR products on agarose gel.Citation58 Insufficient data were available to conclude on an association between IL-17 RNA expression in fresh frozen tissue and survival.
A high number of Th17 cells in tissue is correlated with improved survival
The total number of Th17 cells can be quantified using a combination of a T-cell marker and IL-17 in FFPE slides. Using immunohistochemistry, our group has shown that a high number of Th17 cells was correlated with improved prognosis in squamous cervical cancer,Citation37 while another study found a trend toward improved DFS (p = 0.06) in differentiated thyroid cancer ().Citation59 We did not include analyses on the ratio of the number of IL-17+ cells over the number of CD3+ or CD4+ T cells, because we do not regard this as a measure for Th17 cells since IL-17 is also produced by other cell types.
A high number of Th17 cells in peripheral blood is correlated with improved survival
Flow cytometry was used to quantify the Th17 cell frequency among PBMCs, usually defined as CD4+IL-17+ cells (see for details). A high number of Th17 cells was correlated with improved survival in four studies.Citation32,38,60,61 Two studies found a correlation with poor survival,Citation41,62 while three studies did not find a significant correlation.Citation33,63,64
Two studies focused on a different aspect of the Th17 response and are included in the overview in for completeness, but not in the quantitative analyses.Citation65,66 Notably, while two studies reported on a correlation between a high number of Th17 cells and poor prognosis, twice as many studies (n = 4) reported on a correlation with improved prognosis (see ).
A high number of Th17 cells in tumor-associated fluids is correlated with improved survival
Tumor-associated fluids have infrequently been studied for the correlation between IL-17 or Th17 numbers and survival (). Two studies have analyzed the number of Th17 cells in lung cancer malignant pleural effusion by flow cytometry.Citation67,68 Both found a significant correlation between a high number of Th17 cells and improved overall survival.
One group has studied the correlation between high IL-17 protein levels in lung cancer malignant pleural effusion measured by ELISA and described a correlation with poor survival.Citation27 Another study found a correlation between high IL-17 protein levels in ovarian carcinoma ascites and improved survival.Citation69 Finally, a study in gastric cancer patients showed a significant correlation between high IL-17 RNA expression measured by qRT-PCR and improved survival in patients treated with curative resection.Citation70
Collectively, of the five studies on tumor-associated fluids, the studies quantifying Th17 cells using flow cytometry (n = 2) and qRT-PCR (n = 1) found a correlation with improved prognosis. Of the two studies quantifying IL-17 using ELISA, one found a correlation with improved, and one with poor prognosis.
Differences between cancer types
While functional differences between IL-17 and Th17 cells may be due to the cellular source of IL-17 and the accompanying immune response, this might also depend on the cancer type. In studies on liver cancer (n = 13), a negative (n = 9), or no significant (n = 3) correlation was found between high IL-17 or Th17 cells and prognosis, except for the study of hepatocellular cancer treated with transarterial chemoembolization. All studies on colorectal cancer (n = 6) also found a correlation between high IL-17 and poor prognosis (n = 4) or no significant correlation (n = 1), except for one study that reported on IL-17 being expressed mainly by tumor rather than tumor infiltrating immune cells. The studies on non-small cell lung cancer (n = 3) reported a significant correlation between IL-17 and poor prognosis as well.
In contrast, all analyses described in six leukemia studies (n = 8) showed a significant correlation between PBMC Th17 cells or serum IL-17 and improved prognosis (n = 3) or no effect (n = 4), except for one study of serum IL-17 in patients that received stem cell transplantation after myeloablative conditioning. This might indicate that the immune response in haematological malignancies may differ from solid tumors. Of the studies on ovarian cancer (n = 4), two described a correlation between high IL-17 and improved survival. The other two groups did not find a significant correlation with disease-specific survival, but one of the studies described a correlation between high IL-17 and improved progression-free survival.
These findings indicate that there may be context specific effects on the IL-17 or Th17 cell immune response, although the number of studies per cancer type was too limited to determine whether the cancer type or sample type is more important for the effect on survival.
Discussion
The clinical impact of Th17 cells has remained unresolved in cancer.Citation71 The aim of this review was to identify the correlations between a high amount of IL-17 protein or high number of Th17 cells in human cancer and patient survival. Following an extensive electronic database search, publications were manually selected without format or language restrictions. Survival analyses were studied in the full article if any analysis regarding prognosis was mentioned in the abstract, minimizing the risk of publication bias. Although the risk of bias in included studies was limited, all studies used different cut-off levels to divide IL-17 or Th17 expression in a high and low expression group due to a lack of established cut-off levels. This study limitation makes it difficult to compare different studies directly.
The sample type studied proved to be crucial for the correlation with clinical outcome. This may partly be explained by a difference in cell source. Some tumor microenvironments may be more favorable for Th17 cells, while others may be more readily infiltrated by IL-17 producing neutrophils. Additionally, the method used determines whether Th17 cells, IL-17 protein, or all IL-17 producing cells are measured. A high amount of IL-17 protein, predominantly produced by neutrophils and mast cells in cancerCitation37-39 and measured in serum, FFPE tissue and tumor-associated fluids, was over three times more frequently correlated with poor than with improved prognosis. A meta-analysis could only be performed for IL-17 in FFPE tissue due to the limited number of studies on the other sample types. The forest plots clearly showed that a high number of IL-17+ cells was correlated with an increased hazard ratio, despite the use of a range of cut-off values, which might depend on the type of cancer and analysis. In contrast, a high number of Th17 cells measured in FFPE tissue, peripheral blood or tumor-associated fluids was four times more frequently correlated with improved than with poor prognosis. Since, IL-17 RNA can generally not be quantified in neutrophilsCitation37,72 the data obtained by RT-PCR analyses most likely represent IL-17 produced by Th17 cells. The PCR measurements in tumor-associated fluids and fresh frozen tissue are thus regarded as an indicator of the Th17 cell frequency. Because of limited data available, we could not conclude on an association between IL-17 RNA expression and survival.
Th17 cells seemed to primarily have a tumor-suppressing effect, whereas IL-17 was generally associated with poor outcome. IL-17 has been shown to be produced by only a small Th17 cell population.Citation37-39 The tumor-promoting function can be explained by the role of IL-17 in inducing angiogenesisCitation73 and recruiting neutrophils.Citation74 Neutrophils have been reported to convert to a tumor promoting phenotype and to induce angiogenesis.Citation75 The immune cells capable of producing IL-17 include neutrophils as well as other cell types,Citation7-9 which may determine an important part of the clinical outcome. The tumor suppression by Th17 cells is probably due to different properties than the secretion of IL-17. Th17 cells might stimulate the Th1 and cytotoxic T cell tumor targeting immune responses.Citation76 Additionally, Th17 cells have been shown to have memory stem cell-like properties and the ability to differentiate to Th1/Th17 cells that produce interferon-gamma.Citation77 Th17 cells may thus either directly or indirectly suppress tumorigenesis.
The type of IL-17 response is thus likely to be context dependent. Liver cancer, colorectal cancer, and non-small cell lung cancer seem to be correlated with an unfavorable IL-17 response.Citation15,16,18–20,24-26,40-46 Leukemia on the other hand might provide an environment favorable for Th17 cells to suppress tumor growth.Citation32,38 Similarly, ovarian cancer might attract a tumor suppressing IL-17 response, as the majority of studies found a correlation with a favorable outcome.Citation28,69 A possible explanation might be that different microenvironments favor infiltration of or differentiation toward more or less tumor-promoting immune cell phenotypes. Not only Th17 cells, but also innate cell types capable of producing IL-17 may be correlated with improved prognosis, as we and others have shown for mast cells.Citation37,39 Although we cannot discriminate whether the cancer type or method used is more important for the correlations found, it is likely that both are important for the cell source studied and thus clinical outcome.
Based on the findings described in the current review, cancer patients with high total IL-17 protein levels might benefit from anti-IL-17 treatment, blocking the tumor promoting response. Adoptive transfer of Th17 cells might be another promising treatment. The feasibility of both approaches needs to be investigated further. Human Th17 cells can be induced by a combination of IL-1β, IL-6, and IL-23, although the exact conditions required are still under debate.Citation78 The functions of differentially obtained Th17 cell populations should be determined by functional studies. Animal models may be helpful to clarify this, as the induction of Th17 cells by IL-6 and TGF-β is clearer in mice than in humans, although the described effects on survival are still contradictory.Citation12,79
We conclude that while IL-17 primarily promotes tumorigenesis, the subpopulation of IL-17 producing Th17 cells seems to have a tumor-suppressing effect. Future research should use methodology that makes a distinction between soluble IL-17 protein, Th17 cells, and other IL-17+ cells. This will help to determine whether IL-17 and Th17 cells should be targeted or used in a clinical setting.
Methods
Study design
A systematic search in the NCBI PubMed, Ovid Embase, and Web of Science bibliographic databases was conducted without language restriction in collaboration with information specialist JML following the PRISMA guidelines.Citation80 Since IL-17 is frequently studied in autoimmune diseases and together with tumor necrosis factor, these terms were excluded as major topic. The full search terms are provided in Table S9.
Selection criteria
All original research studies reporting on an IL-17A or Th17 cell measurement and OS or DFS in human cancer published until 10 September 2014 were included. Articles that did not meet all inclusion criteria (e.g., conference abstracts; studies on IL-17 producing γδTCR or CD8+ cytotoxic T cells or only describing remission or progression-free survival) were excluded. All relevant references in reviews were manually checked for presence in the systematic search.
Data collection and analysis
Survival analyses, generally Kaplan–Meier survival curves, a log rank test and Cox regression analyses, but in some cases a Spearman's rank correlation,Citation33,68 Wilcoxon signed-rank testCitation29 or Satterwaithe t-testCitation61 were obtained. All included articles were reviewed by both SP and ESJ. If extracted data did not match, the data were discussed until a consensus was reached. Cox regression hazard ratios and confidence intervals were used to perform a meta-analysis. The authors of all articles that did not provide sufficient information on study details, clinico-pathological data or Cox regression analyses were contacted via e-mail. Due to the number of studies with sufficient data per sample type studied, only the analyses of the number of IL-17+ cells in FFPE tissue were suitable for meta-analysis. To keep the data as comparable as possible, scores in the tumor center were used in the cases where data were reported on different scores (e.g., invasive margin, peritumor).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplementary_Tables.docx
Download MS Word (54.9 KB)984547_Supplementary_Materials.zip
Download Zip (55.7 KB)Acknowledgments
We thank Dr. J. Cao for extracting data from the Chinese articles. We also acknowledge Dr. I.T.A. Peters for providing critical feedback on the manuscript. We are very grateful for the data provided by authors via e-mail.
Funding
This work was supported by grant UL2010–4801 from the Dutch Cancer Society.
References
- Rouvier E, Luciani MF, Mattei MG, Denizot F, Golstein P. CTLA-8, cloned from an activated T cell, bearing AU-rich messenger RNA instability sequences, and homologous to a herpesvirus saimiri gene. J Immunol 1993; 150:5445-56; PMID:8390535.
- Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity 2004; 21:467-76; PMID:15485625; http://dx.doi.org/10.1016/j.immuni.2004.08.018
- Bettelli E, Korn T, Kuchroo VK. Th17: the third member of the effector T cell trilogy. Curr Opin Immunol 2007; 19:652-7; PMID:17766098; http://dx.doi.org/10.1016/j.coi.2007.07.020
- Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med 2005; 201:233-40; PMID:15657292; http://dx.doi.org/10.1084/jem.20041257
- Bettelli E, Korn T, Oukka M, Kuchroo VK. Induction and effector functions of T(H)17 cells. Nature 2008; 453:1051-7; PMID:18563156; http://dx.doi.org/10.1038/nature07036
- Miossec P, Kolls JK. Targeting IL-17 and TH17 cells in chronic inflammation. Nat Rev Drug Discov 2012; 11:763-76; PMID:23023676; http://dx.doi.org/10.1038/nrd3794
- Cua DJ, Tato CM. Innate IL-17-producing cells:the sentinels of the immune system. Nat Rev Immunol 2010; 10:479-89; PMID:20559326; http://dx.doi.org/10.1038/nri2800
- Hirota K, Ahlfors H, Duarte JH, Stockinger B. Regulation and function of innate and adaptive interleukin-17-producing cells. EMBO Rep 2012; 13:113-20; PMID:22193778; http://dx.doi.org/10.1038/embor.2011.248
- Moran EM, Heydrich R, Ng CT, Saber TP, McCormick J, Sieper J, Appel H, Fearon U, Veale DJ. IL-17A expression is localised to both mononuclear and polymorphonuclear synovial cell infiltrates. PLoS One 2011; 6:e24048; PMID:21887369; http://dx.doi.org/10.1371/journal.pone.0024048
- Khader SA, Gaffen SL, Kolls JK. Th17 cells at the crossroads of innate and adaptive immunity against infectious diseases at the mucosa. Mucosal Immunol 2009; 2:403-11; PMID:19587639; http://dx.doi.org/10.1038/mi.2009.100
- Ye J, Livergood RS, Peng G. The role and regulation of human Th17 cells in tumor immunity. Am J Pathol 2013; 182:10-20; PMID:23159950; http://dx.doi.org/10.1016/j.ajpath.2012.08.041
- Wilke CM, Kryczek I, Wei S, Zhao E, Wu K, Wang G, Zou W. Th17 cells in cancer:help or hindrance? Carcinogenesis 2011; 32:643-9; PMID:21304053; http://dx.doi.org/10.1093/carcin/bgr019
- Wu X, Zeng Z, Chen B, Yu J, Xue L, Hao Y, Chen M, Sung JJ, Hu P. Association between polymorphisms in interleukin-17A and interleukin-17F genes and risks of gastric cancer. Int J Cancer 2010; 127:86-92; PMID:19904747; http://dx.doi.org/10.1002/ijc.25027
- Braga WM, da Silva BR, de Carvalho AC, Maekawa YH, Bortoluzzo AB, Rizzatti EG, Atanackovic D, Colleoni GW. FOXP3 and CTLA4 overexpression in multiple myeloma bone marrow as a sign of accumulation of CD4 T regulatory cells. Cancer Immunol Immunother 2014; 63:1189-97; PMID:25099367; http://dx.doi.org/10.1007/s00262-014-1589-9
- Chen X, Wan J, Liu J, Xie W, Diao X, Xu J, Zhu B, Chen Z. Increased IL-17-producing cells correlate with poor survival and lymphangiogenesis in NSCLC patients. Lung Cancer 2010; 69:348-54; PMID:20022135; http://dx.doi.org/10.1016/j.lungcan.2009.11.013
- Liu J, Duan Y, Cheng X, Chen X, Xie W, Long H, Lin Z, Zhu B. IL-17 is associated with poor prognosis and promotes angiogenesis via stimulating VEGF production of cancer cells in colorectal carcinoma. Biochem Biophys Res Commun 2011; 407:348-54; PMID:21396350; http://dx.doi.org/10.1016/j.bbrc.2011.03.021
- Yamada Y, Saito H, Ikeguchi M. Prevalence and clinical relevance of Th17 cells in patients with gastric cancer. J Surg Res 2012; 178:685-91; PMID:22940035; http://dx.doi.org/10.1016/j.jss.2012.07.055
- Liao R, Sun J, Wu H, Yi Y, Wang JX, He HW, Cai XY, Zhou J, Cheng YF, Fan J et al. High expression of IL-17 and IL-17RE associate with poor prognosis of hepatocellular carcinoma. J Exp Clin Cancer Res 2013; 32:3; PMID:23305119; http://dx.doi.org/10.1186/1756-9966-32-3
- Zhang JP, Yan J, Xu J, Pang XH, Chen MS, Li L, Wu C, Li SP, Zheng L. Increased intratumoral IL-17-producing cells correlate with poor survival in hepatocellular carcinoma patients. J Hepatol 2009; 50:980-9; PMID:19329213; http://dx.doi.org/10.1016/j.jhep.2008.12.033
- Tosolini M, Kirilovsky A, Mlecnik B, Fredriksen T, Mauger S, Bindea G, Berger A, Bruneval P, Fridman WH, Pages F et al. Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancer. Cancer Res 2011; 71:1263-71; PMID:21303976; http://dx.doi.org/10.1158/0008-5472.CAN-10-2907
- Yu Q, Lou XM, He Y. Prediction of local recurrence in cervical cancer by a Cox model comprised of lymph node status, lymph-vascular space invasion, and intratumoral Th17 cell-infiltration. Med Oncol 2014; 31:795; PMID:24310812; http://dx.doi.org/10.1007/s12032-013-0795-1
- Lin SZ, Chen KJ, Xu ZY, Chen H, Zhou L, Xie HY, Zheng SS. Prediction of recurrence and survival in hepatocellular carcinoma based on two Cox models mainly determined by FoxP3+ regulatory T cells. Cancer Prev Res (Phila) 2013; 6:594-602; PMID:23599540; http://dx.doi.org/10.1158/1940-6207.CAPR-12-0379
- Lan C, Huang X, Lin S, Huang H, Cai Q, Lu J, Liu J. High density of IL-17-producing cells is associated with improved prognosis for advanced epithelial ovarian cancer. Cell Tissue Res 2013; 352:351-9; PMID:23397428; http://dx.doi.org/10.1007/s00441-013-1567-0
- Tseng JY, Yang CY, Liang SC, Liu RS, Yang SH, Lin JK, Chen YM, Wu YC, Jiang JK, Lin CH. Interleukin-17A modulates circulating tumor cells in tumor draining vein of colorectal cancers and affects metastases. Clin Cancer Res 2014; 20:2885-97; PMID:24677375; http://dx.doi.org/10.1158/1078-0432.CCR-13-2162
- Xu C, Hao K, Yu L, Zhang X. Serum interleukin-17 as a diagnostic and prognostic marker for non-small cell lung cancer. Biomarkers 2014; 19:287-90; PMID:24731052; http://dx.doi.org/10.3109/1354750X.2014.908954
- Wu J, Du J, Liu L, Li Q, Rong W, Wang L, Wang Y, Zang M, Wu Z, Zhang Y et al. Elevated pretherapy serum IL17 in primary hepatocellular carcinoma patients correlate to increased risk of early recurrence after curative hepatectomy. PLoS One 2012; 7:e50035; PMID:23227158; http://dx.doi.org/10.1371/journal.pone.0050035
- Xu C, Yu L, Zhan P, Zhang Y. Elevated pleural effusion IL-17 is a diagnostic marker and outcome predictor in lung cancer patients. Eur J Med Res 2014; 19:23; PMID:24887477; http://dx.doi.org/10.1186/2047-783X-19-23
- Droeser RA, Guth U, Eppenberger-Castori S, Stadlmann S, Hirt C, Terracciano L, Singer G. High IL-17-positive tumor immune cell infiltration is indicative for chemosensitivity of ovarian carcinoma. J Cancer Res Clin Oncol 2013; 139:1295-302; PMID:23624523; http://dx.doi.org/10.1007/s00432-013-1441-1
- Lutz ER, Wu AA, Bigelow E, Sharma R, Mo G, Soares K, Solt S, Dorman A, Wamwea A, Yager A et al. Immunotherapy converts nonimmunogenic pancreatic tumors into immunogenic foci of immune regulation. Cancer Immunol Res 2014; 2:616-31; PMID:24942756; http://dx.doi.org/10.1158/2326-6066.CIR-14-0027
- Chen WC, Lai YH, Chen HY, Guo HR, Su IJ, Chen HH. Interleukin-17-producing cell infiltration in the breast cancer tumour microenvironment is a poor prognostic factor. Histopathology 2013; 63:225-33; PMID:23738752; http://dx.doi.org/10.1111/his.12156
- Cho BS, Lim JY, Yahng SA, Lee SE, Eom KS, Kim YJ, Chung NG, Jeong DC, Lee S, Kim HJ et al. Circulating IL-17 levels during the peri-transplant period as a predictor for early leukemia relapse after myeloablative allogeneic stem cell transplantation. Ann Hematol 2012; 91:439-48; PMID:21894475; http://dx.doi.org/10.1007/s00277-011-1318-9
- Abousamra NK, Salah El-Din M, Helal R. Prognostic value of Th17 cells in acute leukemia. Med Oncol 2013; 30:732; PMID:24085544; http://dx.doi.org/10.1007/s12032-013-0732-3
- Hus I, Bojarska-Junak A, Chocholska S, Tomczak W, Wos J, Dmoszynska A, Rolinski J. Th17/IL-17A might play a protective role in chronic lymphocytic leukemia immunity. PLoS One 2013; 8:e78091; PMID:24223764; http://dx.doi.org/10.1371/journal.pone.0078091
- Yan XJ, Dozmorov I, Li W, Yancopoulos S, Sison C, Centola M, Jain P, Allen SL, Kolitz JE, Rai KR et al. Identification of outcome-correlated cytokine clusters in chronic lymphocytic leukemia. Blood 2011; 118:5201-10; PMID:21911837; http://dx.doi.org/10.1182/blood-2011-03-342436
- Song XN, Yang JZ, Sun LX, Meng JB, Zhang JQ, Lv HY, Kong LJ. Expression levels of IL-27 and IL-17 in multiple myeloma patients: a higher ratio of IL-27:IL-17 in bone marrow was associated with a superior progression-free survival. Leuk Res 2013; 37:1094-9; PMID:23849453; http://dx.doi.org/10.1016/j.leukres.2013.06.022
- Vizio B, Novarino A, Giacobino A, Cristiano C, Prati A, Ciuffreda L, Montrucchio G, Bellone G. Potential plasticity of T regulatory cells in pancreatic carcinoma in relation to disease progression and outcome. Exp Ther Med 2012; 4:70-78; PMID:23060925; http://dx.doi.org/10.3892/etm.2012.553
- Punt S, Fleuren GJ, Kritikou E, Lubberts E, Trimbos JB, Jordanova ES, Gorter A. Angels and demons:Th17 cells represent a beneficial response, while neutrophil IL-17 is associated with poor prognosis in squamous cervical cancer. OncoImmunology 2015; 4: e984539; http://dx.doi.org/10.4161/2162402X.2014.984539
- Jain P, Javdan M, Feger FK, Chiu PY, Sison C, Damle RN, Bhuiya TA, Sen F, Abruzzo LV, Burger JA et al. Th17 and non-Th17 interleukin-17-expressing cells in chronic lymphocytic leukemia: delineation, distribution, and clinical relevance. Haematologica 2012; 97:599-607; PMID:22058222; http://dx.doi.org/10.3324/haematol.2011.047316
- Wang B, Li L, Liao Y, Li J, Yu X, Zhang Y, Xu J, Rao H, Chen S, Zhang L et al. Mast cells expressing interleukin 17 in the muscularis propria predict a favorable prognosis in esophageal squamous cell carcinoma. Cancer Immunol Immunother 2013; 62:1575-85; PMID:23912243; http://dx.doi.org/10.1007/s00262-013-1460-4
- Gu FM, Li QL, Gao Q, Jiang JH, Zhu K, Huang XY, Pan JF, Yan J, Hu JH, Wang Z et al. IL-17 induces AKT-dependent IL-6/JAK2/STAT3 activation and tumor progression in hepatocellular carcinoma. Mol Cancer 2011; 10:150; PMID:22171994; http://dx.doi.org/10.1186/1476-4598-10-150
- Yan J, Liu XL, Xiao G, Li NL, Deng YN, Han LZ, Yin LC, Ling LJ, Liu LX. Prevalence and clinical relevance of T-helper cells, th17 and Th1, in hepatitis B virus-related hepatocellular carcinoma. PLoS One 2014; 9:e96080; PMID:24867183; http://dx.doi.org/10.1371/journal.pone.0096080
- Huang Y, Wang F, Wang Y, Zhu Z, Gao Y, Ma Z, Xu R, Du Z. Intrahepatic interleukin-17+ T cells and FoxP3+ regulatory T cells cooperate to promote development and affect the prognosis of hepatocellular carcinoma. J Gastroenterol Hepatol 2014; 29:851-9; PMID:24303990; http://dx.doi.org/10.1111/jgh.12418
- Li J, Lau GK, Chen L, Dong SS, Lan HY, Huang XR, Li Y, Luk JM, Yuan YF, Guan XY. Interleukin 17A promotes hepatocellular carcinoma metastasis via NF-kB induced matrix metalloproteinases 2 and 9 expression. PLoS One 2011; 6:e21816; PMID:21760911; http://dx.doi.org/10.1371/journal.pone.0021816
- Gu FM, Gao Q, Shi GM, Zhang X, Wang J, Jiang JH, Wang XY, Shi YH, Ding ZB, Fan J et al. Intratumoral IL-17(+) cells and neutrophils show strong prognostic significance in intrahepatic cholangiocarcinoma. Ann Surg Oncol 2012; 19:2506-14; PMID:22411204; http://dx.doi.org/10.1245/s10434-012-2268-8
- Chen J, Chen Z. The effect of immune microenvironment on the progression and prognosis of colorectal cancer. Med Oncol 2014; 31:82; PMID:25034363; http://dx.doi.org/10.1007/s12032-014-0082-9
- Zhang GQ, Han F, Fang XZ, Ma XM. CD4+, IL17 and Foxp3 expression in different pTNM stages of operable non-small cell lung cancer and effects on disease prognosis. Asian Pac J Cancer Prev 2012; 13:3955-60; PMID:23098499; http://dx.doi.org/10.7314/apjcp.2012.13.8.3955
- Zhang Y, Huang Y, Qin M. Tumour-infiltrating FoxP3+ and IL-17-producing T cells affect the progression and prognosis of gallbladder carcinoma after surgery. Scand J Immunol 2013; 78:516-22; PMID:24007242; http://dx.doi.org/10.1111/sji.12109
- He S, Fei M, Wu Y, Zheng D, Wan D, Wang L, Li D. Distribution and clinical significance of th17 cells in the tumor microenvironment and peripheral blood of pancreatic cancer patients. Int J Mol Sci 2011; 12:7424-37; PMID:22174607; http://dx.doi.org/10.3390/ijms12117424
- Wang Y, Yang J, Xu H. [Expression of IL-17 in laryngeal squamous cell carcinoma tissues and the clinical significance]. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2013; 27:779-83; PMID:24167997.
- Liu X, Jin H, Zhang G, Lin X, Chen C, Sun J, Zhang Y, Zhang Q, Yu J. Intratumor IL-17-Positive Mast Cells Are the Major Source of the IL-17 That Is Predictive of Survival in Gastric Cancer Patients. PLoS One 2014; 9:e106834; PMID:25197971; http://dx.doi.org/10.1371/journal.pone.0106834
- Chen JG, Xia JC, Liang XT, Pan K, Wang W, Lv L, Zhao JJ, Wang QJ, Li YQ, Chen SP et al. Intratumoral expression of IL-17 and its prognostic role in gastric adenocarcinoma patients. Int J Biol Sci 2011; 7:53-60; PMID:21234303; http://dx.doi.org/10.7150/ijbs.7.53
- Lv L, Pan K, Li XD, She KL, Zhao JJ, Wang W, Chen JG, Chen YB, Yun JP, Xia JC. The accumulation and prognosis value of tumor infiltrating IL-17 producing cells in esophageal squamous cell carcinoma. PLoS One 2011; 6:e18219; PMID:21483813; http://dx.doi.org/10.1371/journal.pone.0018219
- Zhang YL, Li J, Mo HY, Qiu F, Zheng LM, Qian CN, Zeng YX. Different subsets of tumor infiltrating lymphocytes correlate with NPC progression in different ways. Mol Cancer 2010; 9:4; PMID:20064222; http://dx.doi.org/10.1186/1476-4598-9-4
- Xu M, Song Z-G, Xu C-X, Rong G-H, Fan K-X, Chen J-Y, Zhang W, Jia J-P, Han G, Wang W et al. IL-17A stimulates the progression of giant cell tumors of bone. Clinical Cancer Research 2013; 19:4697-705; PMID:23857601; http://dx.doi.org/10.1158/1078-0432.CCR-13-0251
- Lin Y, Xu J, Su H, Zhong W, Yuan Y, Yu Z, Fang Y, Zhou H, Li C, Huang K. Interleukin-17 is a favorable prognostic marker for colorectal cancer. Clin Transl Oncol 2014; 17:50-6; PMID:25015721; http://dx.doi.org/10.1007/s12094-014-1197-3
- Cui X, Xu Z, Zhao Z, Sui D, Ren X, Huang Q, Qin J, Hao L, Wang Z, Shen L et al. Analysis of CD137l and IL-17 expression in tumor tissue as prognostic indicators for gliblastoma. Int J Biol Sci 2013; 9:134-41; PMID:23411595; http://dx.doi.org/10.7150/ijbs.4891
- Cui G, Yang H, Zhao J, Yuan A, Florholmen J. Elevated Proinflammatory Cytokine IL-17A in the Adjacent Tissues Along the Adenoma-Carcinoma Sequence. Pathol Oncol Res 2014; 21:139-46; PMID:24859972; http://dx.doi.org/10.1007/s12253-014-9799-1
- Kato T, Furumoto H, Ogura T, Onishi Y, Irahara M, Yamano S, Kamada M, Aono T. Expression of IL-17 mRNA in ovarian cancer. Biochem Biophys Res Commun 2001; 282:735-8; PMID:11401524; http://dx.doi.org/10.1006/bbrc.2001.4618
- Cunha LL, Morari EC, Guihen AC, Razolli D, Gerhard R, Nonogaki S, Soares FA, Vassallo J, Ward LS. Infiltration of a mixture of immune cells may be related to good prognosis in patients with differentiated thyroid carcinoma. Clin Endocrinol (Oxf) 2012; 77:918-25; PMID:22738343; http://dx.doi.org/10.1111/j.1365-2265.2012.04482.x
- Liao Y, Wang B, Huang ZL, Shi M, Yu XJ, Zheng L, Li S, Li L. Increased circulating Th17 cells after transarterial chemoembolization correlate with improved survival in stage III hepatocellular carcinoma:a prospective study. PLoS One 2013; 8:e60444; PMID:23565248; http://dx.doi.org/10.1371/journal.pone.0060444
- Sarnaik AA, Yu B, Yu D, Morelli D, Hall M, Bogle D, Yan L, Targan S, Solomon J, Nichol G et al. Extended dose ipilimumab with a peptide vaccine:immune correlates associated with clinical benefit in patients with resected high-risk stage IIIc/IV melanoma. Clin Cancer Res 2011; 17:896-906; PMID:21106722; http://dx.doi.org/10.1158/1078-0432.CCR-10-2463
- Liu T, Peng L, Yu P, Zhao Y, Shi Y, Mao X, Chen W, Cheng P, Wang T, Chen N et al. Increased circulating Th22 and Th17 cells are associated with tumor progression and patient survival in human gastric cancer. J Clin Immunol 2012; 32:1332-9; PMID:22760549; http://dx.doi.org/10.1007/s10875-012-9718-8
- Lee IC, Huang YH, Chau GY, Huo TI, Su CW, Wu JC, Lin HC. Serum interferon gamma level predicts recurrence in hepatocellular carcinoma patients after curative treatments. Int J Cancer 2013; 133:2895-902; PMID:23749461; http://dx.doi.org/10.1002/ijc.28060
- Wang HT, Zhao XY, Zhao XS, Han TT, Lv M, Chang YJ, Huang XJ. [Association of the ratio of regulatory and effector T cells with recurrence and chronic graft-versus-host disease after allogeneic hematopoietic stem cell transplantation]. Zhonghua Xue Ye Xue Za Zhi 2013; 34:679-84; PMID:23978019; http://dx.doi.org/10.3760/cma.j.issn.0253-2727.2013.08.008
- Zelba H, Weide B, Martens A, Derhovanessian E, Bailur JK, Kyzirakos C, Pflugfelder A, Eigentler TK, Di Giacomo AM, Maio M et al. Circulating CD4+ T cells that produce IL4 or IL17 when stimulated by melan-a but not by NY-ESO-1 have negative impacts on survival of patients with stage IV melanoma. Clin Cancer Res 2014; 20:4390-9; PMID:24938524; http://dx.doi.org/10.1158/1078-0432.CCR-14-1015
- Han Y, Ye A, Bi L, Wu J, Yu K, Zhang S. Th17 cells and IL-17 increase with poor prognosis in patients with acute myeloid leukemia. Cancer Sci 2014; 105:933-42; PMID:24890519; http://dx.doi.org/10.1111/cas.12459
- Ye ZJ, Zhou Q, Gu YY, Qin SM, Ma WL, Xin JB, Tao XN, Shi HZ. Generation and differentiation of IL-17-producing CD4+ T cells in malignant pleural effusion. J Immunol 2010; 185:6348-54; PMID:20952674; http://dx.doi.org/10.4049/jimmunol.1001728
- Gong Y, Chen SX, Gao BA, Yao RC, Guan L. Cell origins and significance of IL-17 in malignant pleural effusion. Clin Transl Oncol 2014; 16:807-13; PMID:24399072; http://dx.doi.org/10.1007/s12094-013-1152-8
- Kryczek I, Banerjee M, Cheng P, Vatan L, Szeliga W, Wei S, Huang E, Finlayson E, Simeone D, Welling TH et al. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood 2009; 114:1141-9; PMID:19470694; http://dx.doi.org/10.1182/blood-2009-03-208249
- Iida T, Iwahashi M, Katsuda M, Ishida K, Nakamori M, Nakamura M, Naka T, Ojima T, Ueda K, Hayata K et al. Prognostic significance of IL-17 mRNA expression in peritoneal lavage in gastric cancer patients who underwent curative resection. Oncol Rep 2014; 31:605-12; PMID:24337702; http://dx.doi.org/10.3892/or.2013.2911
- Fridman WH, Pages F, Sautes-Fridman C, Galon J. The immune contexture in human tumours:impact on clinical outcome. Nat Rev Cancer 2012; 12:298-306; PMID:22419253; http://dx.doi.org/10.1038/nrc3245
- Cowland JB, Borregaard N. The individual regulation of granule protein mRNA levels during neutrophil maturation explains the heterogeneity of neutrophil granules. J Leukoc Biol 1999; 66:989-95; PMID:10614782.
- Numasaki M, Fukushi J, Ono M, Narula SK, Zavodny PJ, Kudo T, Robbins PD, Tahara H, Lotze MT. Interleukin-17 promotes angiogenesis and tumor growth. Blood 2003; 101:2620-7; PMID:12411307; http://dx.doi.org/10.1182/blood-2002-05-1461
- Laan M, Cui ZH, Hoshino H, Lotvall J, Sjostrand M, Gruenert DC, Skoogh BE, Linden A. Neutrophil recruitment by human IL-17 via C-X-C chemokine release in the airways. J Immunol 1999; 162:2347-52; PMID:9973514.
- Gregory AD, Houghton AM. Tumor-associated neutrophils:new targets for cancer therapy. Cancer Res 2011; 71:2411-6; PMID:21427354; http://dx.doi.org/10.1158/0008-5472.CAN-10-2583
- Benchetrit F, Ciree A, Vives V, Warnier G, Gey A, Sautes-Fridman C, Fossiez F, Haicheur N, Fridman WH, Tartour E. Interleukin-17 inhibits tumor cell growth by means of a T-cell-dependent mechanism. Blood 2002; 99:2114-21; PMID:11877287; http://dx.doi.org/10.1182/blood.V99.6.2114
- Bailey SR, Nelson MH, Himes RA, Li Z, Mehrotra S, Paulos CM. Th17 cells in cancer:the ultimate identity crisis. Front Immunol 2014; 5:276; PMID:24987392; http://dx.doi.org/10.3389/fimmu.2014.00276
- Ji Y, Zhang W. Th17 cells:positive or negative role in tumor? Cancer Immunol Immunother 2010; 59:979-87; PMID:20352428; http://dx.doi.org/10.1007/s00262-010-0849-6
- De Simone V, Pallone F, Monteleone G, Stolfi C. Role of T17 cytokines in the control of colorectal cancer. Oncoimmunology 2013; 2:e26617; PMID:24498548; http://dx.doi.org/10.4161/onci.26617
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses:the PRISMA statement. Ann Intern Med 2009; 151:264-9, W64; PMID:19622511; http://dx.doi.org/10.7326/0003-4819-151-4-200908180-00135
