Abstract
Upon analysis of archived primary tumors of 482 patients with triple negative breast cancer (TNBC) enrolled in two randomized Phase III adjuvant chemotherapy trials, we have found that tumor infiltrating lymphocytes (TILs), as assessed and quantified by hematoxylin and eosin (H&E) staining are a robust and independent predictor of disease-free survival (DFS), distant recurrence-free interval (DRFI) and overall survival (OS).Citation1 Our findings provide confirmation of results observed in TNBC in a European adjuvant chemotherapy dataset and therefore elevate TILs as prognostic biomarker for operable TNBC to level I evidence.
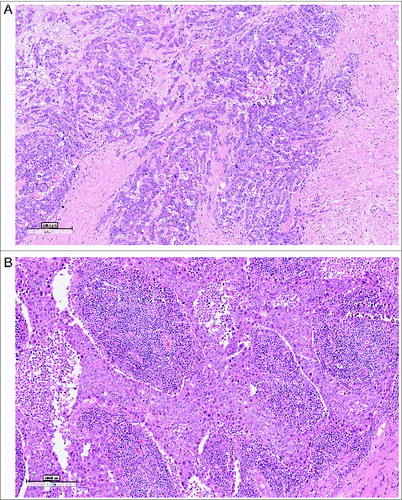
Evidence of the complex and intricate crosstalk of breast cancer with the host immune system is increasing and subsets of breast cancers have been recently identified in which TILs carry prognostic implications. The level of evidence for TILs as prognostic biomarkers was recently elevated by examining TILs in the context of high quality clinical trials with annotated specimens.Citation2,3 We evaluated TILs in archived triple negative breast tumors from 500 women enrolled in two randomized Phase III clinical trials of adjuvant anthracycline-containing chemotherapy.Citation1 The study was conducted according to a predefined analysis plan and followed standard criteria for biomarker reporting. TILs were assessed in the tumor bed (both within the stroma and within tumor cell nests) on H&E stained sections and graded by deciles, reflecting the percentage of an area occupied by lymphocytes within the tumor area. The majority of cancers (80%) had TILs (mostly stromal) and approximately 5% of the cases exhibited more TILs than tumor cells (so-called “lymphocyte-predominant breast cancer” (Fig. 1).Citation2,3 At the median follow-up of 10.6 y, higher stromal TIL scores were associated with better prognosis: for every 10% increase in lymphocytes there was a risk reduction of 14% for recurrence or death (p = 0.02), 18% for distant recurrence (p = 0.04) and 19% for death (p = 0.01). Multivariate analysis confirmed stromal TILs to be an independent prognostic marker of DFS, DRFI, and OS. Interestingly, the likelihood of TILs in the primary tumor increased with nodal involvement.
Figure 1. Tumor infiltrating lymphocytes, for quantification on H&E-stained sections. Examples of a TIL low (A) and TIL rich (B) primary triple negative breast cancer (magnification 10 ×).
Analysis of infiltrating lymphocyte subsets in breast cancers by immunohistochemistry, gene expression profiling and flow cytometry, have shown a heterogeneous composition including B cells and T cells with effector and regulatory function.Citation4 It is therefore intriguing that a simple quantification of TILs performed on H&E-stained tumor sections provides a highly reproducible and nearly identical result in predicting prognosis in TNBC across studies.Citation3
While the reproducible prognostic significance of TIL scoring supports efforts to move toward implementation of this simple method in clinical practice,Citation5 several intriguing questions need to be addressed. First, why do TILs have prognostic value in TNBC but not hormone receptor-positive breast cancer?
Clinically, TNBC are the most aggressive tumors and exhibit a high rate of cancer cell proliferation. This is likely correlated with increased genomic instability and antigenicity. A recent study using mathematical models in estimating single cell mutation frequencies showed that TNBCs have a 13.3 × fold higher mutation rates over normal or hormone receptor positive breast cancer cells.Citation6 This likely leads to generation of mutated neo-antigens that have been recently suggested to be strong targets for antitumor T cell responses.Citation7 However, the variability in the degree of TILs in different patients with TNBC may depend on the genetic makeup of the host, presence of somatic genetic or epigenetic aberrations in the cancer cells, or environmental factors including metabolism and the microbiome, all of which interact to influence the cancer immune phenotype (“immune contexture”Citation8)
Second, does tumor immune microenvironment have implications for therapy? Tumors without TILs may require modalities which induce an antitumor immune response, overcome physical or functional barriers to lymphocyte recruitment or inhibit immune suppressive oncogene pathways. TIL rich tumors may be indicative of an endogenous immune response, carry a better prognosis, and may benefit from therapies targeting counter-regulatory immune suppressive mechanisms (such as PD-1 and PD-L1 expressionCitation9) which can arise in response to an activated immune response. Strategies may include inhibitors of PD-L1/PD-1 interactions and IDO, T cell-intrinsic anergy, and T regulatory cells. Therapeutic interventions aiming to overcome many of these potential barriers have been validated in preclinical models and are being investigated clinically.Citation10
TIL assessment should be included in clinical trials for TNBC, and efforts to develop guidelines for this purpose have been recently undertaken by an international team of pathologists, immunologists and breast cancer medical oncologists of which we are part.Citation5
In Summary
Work by us and othersCitation1,3 provides further evidence for the role of preexisting host antitumor immunity in the survival of patients with TNBC, raising optimism that therapeutics harnessing the immune system can lead to survival improvements for this disease.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Adams S, Gray RJ, Demaria S, Goldstein L, Perez EA, Shulman LN, Martino S, Wang M, Jones VE, Saphner TJ et al. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol 2014; 32(27):2959-66; PMID:25071121
- Denkert C. Diagnostic and therapeutic implications of tumor-infiltrating lymphocytes in breast cancer. J Clin Oncol 2013; 31:836-7; PMID:23341523; http://dx.doi.org/10.1200/JCO.2012.47.1698
- Loi S, Sirtaine N, Piette F, Salgado R, Viale G, Van Eenoo F, Rouas G, Francis P, Crown JP, Hitre E et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol 2013; 31:860-7; PMID:23341518; http://dx.doi.org/10.1200/JCO.2011.41.0902
- Ruffell B, Au A, Rugo HS, Esserman LJ, Hwang ES, Coussens LM. Leukocyte composition of human breast cancer. Proc Natl Acad Sci U S A 2012; 109:2796-801; PMID:21825174; http://dx.doi.org/10.1073/pnas.1104303108
- Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, Wienert S, Van den Eynden G, Baehner FL, Penault-Llorca F et al. Harmonization of the evaluation of tumor infiltrating lymphocytes (TILs) in breast cancer: recommendations by an international TILs-working group 2014. Ann Oncol 2015; 26(2): 259–271.
- Wang Y, Waters J, Leung ML, Unruh A, Roh W, Shi X, Chen K, Scheet P, Vattathil S, Liang H et al. Clonal evolution in breast cancer revealed by single nucleus genome sequencing. Nature 2014; 512:155-60; PMID:25079324; http://dx.doi.org/10.1038/nature13600
- Robbins PF, Lu YC, El-Gamil M, Li YF, Gross C, Gartner J, Lin JC, Teer JK, Cliften P, Tycksen E et al. Mining exomic sequencing data to identify mutated antigens recognized by adoptively transferred tumor-reactive T cells. Nat Med 2013; 19:747-52; PMID:23644516; http://dx.doi.org/10.1038/nm.3161
- Galon J, Angell HK, Bedognetti D, Marincola FM. The continuum of cancer immunosurveillance: prognostic, predictive, and mechanistic signatures. Immunity 2013; 39:11-26; PMID:23890060; http://dx.doi.org/10.1016/j.immuni.2013.07.008
- Mittendorf EA, Philips AV, Meric-Bernstam F, Qiao N, Wu Y, Harrington S, Su X, Wang Y, Gonzalez-Angulo AM, Akcakanat A et al. PD-L1 expression in triple-negative breast cancer. Cancer Immunol Res 2014; 2:361-70; PMID:24764583; http://dx.doi.org/10.1158/2326-6066.CIR-13-0127
- Gajewski TF, Woo SR, Zha Y, Spaapen R, Zheng Y, Corrales L, Spranger S. Cancer immunotherapy strategies based on overcoming barriers within the tumor microenvironment. Curr Opin Immunol 2013 25:268-76; PMID:23579075; http://dx.doi.org/10.1016/j.coi.2013.02.009
