Abstract
The Zagreb regimen has been used for 20 years in various countries. In China, until 2010, the Zagreb schedule was only approved for purified chick embryo cell vaccine (PCECV) and purified Vero cell rabies vaccines (PVRV). In this phase III clinical trial, we aimed to demonstrate the safety and immunogenic non-inferiority of the Zagreb regimen compared with the Essen regimen in healthy adult Chinese immunized with PCECV (Rabipur®). The study enrolled 825 subjects aged 18 to 50 years; serum samples were collected on Days 0, 7, 14, 42, and at 13 months to assess rabies virus neutralizing antibody (RVNA) concentrations. Solicited and unsolicited local and systemic reactions were recorded for 6 days following the day of vaccination, and collected throughout the entire study period (Day 1 until Month 13). The Zagreb regimen was non-inferior to the Essen regimen with regard to RVNA concentrations after 7, 14, and 42 days, and 13 months of immunization. The non-inferiority of seroconversion was established at Days 14 and 42. The incidence of local and systemic reactions was similar between groups, and mostly of mild or moderate severity. Vaccine-related adverse events occurred more frequently in the Essen group than in the Zagreb group. Vaccination with PCECV under a 2-1-1 regimen is as safe and immunogenic as under the traditional 5-dose Essen regimen for rabies post-exposure prophylaxis, and is a more cost-effective option, has a more practical vaccination schedule, and can potentially increase compliance.
Abbreviations
| AE | = | adverse event |
| CCEEV | = | cell culture or embryonated egg-based rabies vaccines |
| CI | = | confidence interval |
| FDA | = | Food and Drug Administration |
| GMC | = | geometric mean concentration |
| HDCV | = | human diploid cell rabies vaccine |
| PCECV | = | purified chick embryo cell rabies vaccine |
| PDEV | = | purified duck embryo cell vaccine |
| PEP | = | post-exposure prophylaxis |
| PPS | = | per protocol set |
| PrEP | = | pre-exposure prophylaxis |
| PVRV | = | purified Vero cell rabies vaccine |
| RFFIT | = | rapid fluorescent focus inhibition test |
| RVNA | = | rabies virus neutralizing antibody |
| SAE | = | serious adverse event |
| WHO | = | World Health Organization |
Trial Registration:
www.clinicaltrials.gov: NCT00825305, NCT01067079
Introduction
Rabies is a viral zoonosis that continues to be a major health problem throughout the world. It is estimated to cause more than 60,000 deaths every year, and is considered to be endemic in more than 150 countries and territories.Citation1 In China, 85%–95% of human rabies cases are dog-mediated rabies, and rabies is among the 3 leading causes of death due to infection.Citation2 Since 2000, a new epidemic outbreak has led to a rapid increase in the number of notified cases,Citation3 with over 3,300 clinically diagnosed deaths due to rabies recorded in 2007,Citation4 and an associated mortality rate that increased by an average of 26% per year from 1999 to 2008.Citation3-5 Since then, the incidence has started to decline, with 2,048 reported cases in 2010, a decrease of about 7.5% compared with 2009.Citation6
After an incubation period of approximately 1–3 months, the virus leads to a progressive encephalomyelitis that almost always results in cardiorespiratory arrest and death within a few days of the onset of symptoms.Citation7 However, and contrary to other human infections, timely rabies immunization can prevent the development of clinical symptoms even after exposure to the virus, and prophylaxis by vaccination is therefore a key component in the reduction of deaths due to the disease. The World Health Organization (WHO) recommends pre-exposure prophylaxis (PrEP), namely vaccination with cell culture or embryonated egg-based rabies vaccines (CCEEVs), for individuals at continual, frequent or increased risk of exposure to the virus, travelers in high-risk areas, and children living in or visiting rabies-affected areas.Citation1 After suspected or confirmed exposure to the virus, post-exposure prophylaxis (PEP) recommendations include immediate, proper wound cleaning, prompt vaccination with CCEEV, and simultaneous passive immunization with rabies immune globulin, if indicated.Citation1
After vaccination with CCEEV, e.g. human diploid cell vaccine (HDCV), purified duck embryo vaccine (PDEV), purified chick embryo cell (PCECV) or purified Vero cell rabies vaccine (PVRV), virus-neutralizing antibodies (RVNA) are usually present by Day 14 post first vaccination.Citation8-12 The immunogenicity, efficacy and safety of PCECV have been well established for both PrEP and PEP in previous clinical trials conducted in children and adults.Citation6, Citation11-15 Moreover, PCECV may to elicit long-lasting immunity even after 14 years in subjects receiving a single booster dose 2 years following primary 3-dose immunization.Citation16
The intramuscular PEP recommended by the WHO includes 2 alternative immunization regimens: the 5-dose “Essen” (1-1-1-1-1), with doses administered on each of Days 0, 3, 7, 14, and 28; and the abbreviated 4-dose “Zagreb” regimen (2-1-1), with 2 injections administered on Day 0, followed by one further dose on each Days 7, and 21. The Zagreb schedule has been widely adopted in many countries for a number of years, but in China, where about 12 million doses of rabies vaccine are administered annually,Citation4,17,18 this vaccination program has been recently approved for PVRV and PCECV. The vaccination given under the Zagreb regimen has several advantages over the Essen regimen through potentially achieving higher titers on Day 7, being more economical, and having an expectation of higher compliance due to fewer visits within a shorter time period.Citation19-21
Here we present the results of a randomized, single-center, open-label clinical trial, conducted in healthy Chinese adults to evaluate the immunogenicity and safety of 2 different post-exposure immunization schedules with PCECV – the Zagreb regimen compared with the Essen regimen – and to assess the long-term antibody response 13 months post-immunization.
Results
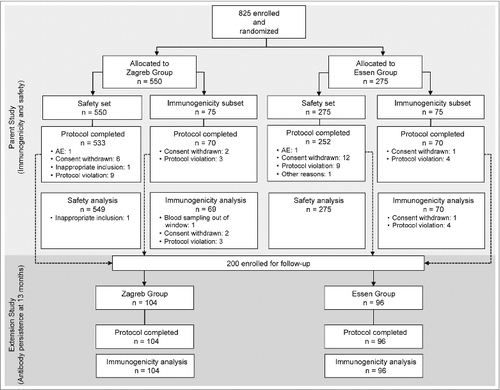
Of a total of 825 adult subjects assigned to the Zagreb and Essen PCECV regimen safety set, 549 and 275 subjects, respectively, were available in the parent study up to Day 42, and 69 subjects in the Zagreb group, and 70 in the Essen group were available for the immunogenicity analysis (). In the extension study, all enrolled subjects (104 in the Zagreb group, and 96 in the Essen group) completed the study and were available to assess persistence of neutralization antibodies 13 months after the primary vaccination. The baseline demographic characteristics of enrolled subjects are shown in . There were no statistically significant differences in age, gender or ethnicity between the regimen vaccination groups in the overall population, the subjects included in the immunogenicity subset up to Day 42 or in the subjects in the extension study (13-months follow-up).
Table 1. Baseline Demographic Characteristics of the Study Population
Immunogenicity
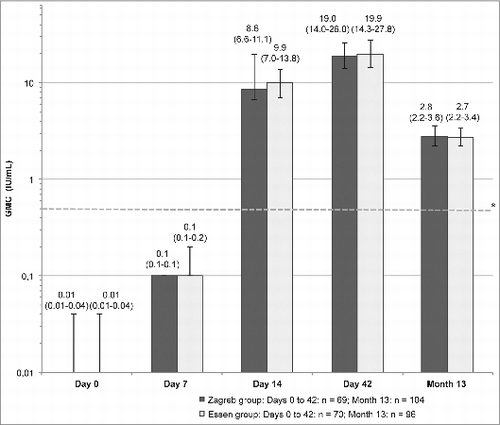
Analysis of antibody responses to the study vaccine under both regimens using the rapid fluorescent focus inhibition test (RFFIT) is shown in . At Day 7 (i.e., 4 or 7 days after 2 doses of PCECV), the mean neutralizing antibody concentrations were lower than 0.12 IU/mL regardless of the vaccine regimen. One week after the third vaccine dose (Day 14), subjects under both regimens reached adequate RVNA concentrations (geometric mean concentration [GMC] 8.6 IU/mL for the Zagreb regimen, and 9.86 IU/mL for the Essen regimen). The lower limit of the confidence interval (CI) of the difference between regimens was > −1.5, showing non-inferiority of the Zagreb regimen over the Essen regimen. At Day 42–2 weeks after the last injection post-Essen and 3 weeks post-Zagreb – the RVNA GMC increased for both groups (19.0 IU/mL in the Zagreb group, and 19.9 IU/mL in the Essen group), showing the Zagreb regimen was again non-inferior to the Essen regimen. One year after the first dose (Month 13), RVNA concentrations had significantly declined in both regimens (GMC 2.8 IU/mL and 2.7 IU/mL in Zagreb and Essen groups, respectively), but the Zagreb regimen was still non-inferior. Finally, the repeated measurement analysis showed that neutralizing antibody levels increased significantly in both groups at Days 7, 14 and 42 in comparison with baseline (p < 0.05).
Figure 2. Rabies virus neutralizing antibody concentrations (GMC) in the Zagreb and Essen groups (PP set) on Days 7, 14, 42, and Month 13. Error bars and values in parenthesis represent 95% CI. *Dotted reference line: the WHO recommends a specific antibody level of 0.5 IU/ml as being indicative of an adequate immune response after vaccination.
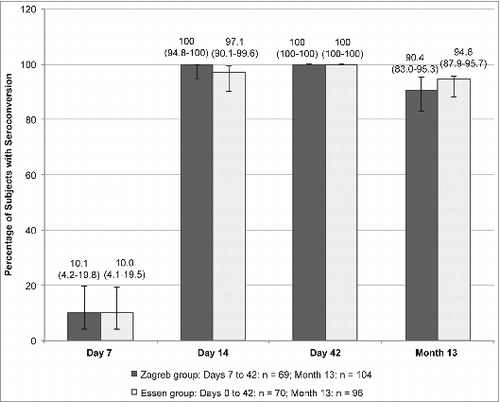
The proportion of subjects achieving an adequate immune response rate (RVNA concentration ≥ 0.5 IU/mL)Citation1 at Day 7 was above 10% for both regimens (). The rate of seroconversion significantly increased at Day 14 (100% for the Zagreb group and 97.1% for the Essen group), was 100% for both regimens at Day 42, and decreased, but was still high, at Month 13 (90.4% and 94.8% for the Zagreb and Essen regimens, respectively). The lower limit of the CI of the rate difference between regimens was >−3.0% at Days 14 and 42, demonstrating the non-inferiority of the 2-1-1 Zagreb regimen over the 5-dose Essen regimen at these 2 time points.
Safety
Overall, local and systemic reactions to the first vaccination of PCECV and during the whole trial were similar for both the regimens, and showed no statistical differences (). The incidence of any local reactions was 87.9% in the Zagreb regimen, and 87.6% in the Essen regimen. The most common solicited local reaction following administration of both PCECV vaccines was pain at the injection site, which was of mild or moderate intensity in all cases except for one case of severe pain in the Essen group. The total occurrence of systemic reactions was 32.2% and 37.4% for Zagreb and Essen regimens, respectively. The most common solicited systemic reactions were headache, followed by malaise and fatigue, in most cases of mild intensity. During the whole course of the study, there was 1 case of severe fever, 2 cases of severe shivering, and 1 case of severe myalgia in the Zagreb group, while no cases of severe systemic AEs were reported in the Essen group.
Table 2. Local and systemic reactions occurring in the Zagreb and Essen groups after the first vaccination and during the whole trial duration
The total frequency of spontaneously reported adverse events, recorded on Day 1 to Day 42 following vaccination (), was similar for both the groups (18.6% in the Zagreb regimen, and 18.5% in the Essen regimen). Vaccine-related AEs occurred more frequently in the Essen group than in the Zagreb group (p = 0.03), and were of mild intensity in all but one case, which was considered of moderate severity and occurred in the Zagreb group; pain at the injection site was the most frequently related AE, reported by 2 subjects in the Zagreb group, and 5 in Essen group. One subject in each group discontinued vaccination due to an AE considered possibly related to the vaccine; in one case this consisted of fever of moderate severity in a subject in the Zagreb group, and in the other one of blurred vision and bulbar conjunctive hyperemia of mild severity in a subject in the Essen group; both subjects recovered completely. One subject in the Essen group experienced a Serious Adverse Event (SAE), consisting on soft tissue injury in left knee and left shoulder; this SAE was judged as not related to the vaccine and of mild severity; the subject was completely recovered after hospital therapy, and did not lead to study discontinuation. No deaths were reported during the study.
Table 3. Incidence of all adverse events occurring in the Zagreb and Essen groups during the trial
Discussion
Intramuscular rabies PEP has been established for several decades, and the traditional 5-dose Essen regimen recommended by the WHO has been widely adopted in China, where approximately 12 million PEP vaccinations are given every year.Citation1,18 Although this regimen gives reliable post-exposure immunization, it is associated with well-known drawbacks.Citation22,23 Firstly, more than 90% of human rabies cases occur in rural areas where people have lower income levels, and many patients still cannot afford immunization.Citation22,23 Secondly, studies investigating the compliance with the full vaccination schedule have shown that it is high for the first 3 doses, but drops significantly after thereafter.Citation24 Finally, the higher the number of visits required to complete the vaccination schedule, the higher is the impact on the considerable indirect costs associated with travel and/or accommodation to the health centers.Citation25 In order to reduce costs and offer more simple and economical immunization regimens, WHO started to recommend a reduced 2-1-1 vaccine schedule in 1992.Citation26 This Zagreb scheme has been intensively investigated, with favorable data from experimental animal models, from clinical studies showing that human subjects produce early virus-neutralizing antibodies by Day 14, epidemiologic surveillance studies demonstrating that it is safe, and health economics studies showing the reduced costs and increased compliance with the omission of the 5th dose.Citation20,21,24
In China, until recently, the only licensed rabies vaccine under the 2-1-1 regimen was PCECV and PVRV that was manufactured locally.Citation19 In this study, we have demonstrated the immunogenic non-inferiority of a PCECV given under a post-exposure 4-dose prophylaxis regimen compared with the traditional 5-dose schedule in healthy Chinese adult volunteers.
The Zagreb regimen induced adequate neutralizing antibody concentrations after 3 doses (Day 14), and remained constant until Day 42. The 2-1-1 regimen was found to be non-inferior to the Essen regimen at Day 7-, 14- and 42-Day time-points, results which concur with previous studies comparing the PCECV under 2-1-1 regimen with the PVRV under both regimens.Citation19 Moreover, all subjects in both PEP regimens achieved adequate RVNA concentrations ≥ 0.5 IU/mL as defined by the WHO at Day 14, which is in agreement with previous trials conducted in children and adults, which have shown that adequate antibody levels are achieved at Day 14 after 3 or 4 doses of PEP vaccination, and that titers do not increase, or increase only slightly, after the fifth dose.Citation19,21 Although in our study antibody levels decreased 1 year (13 months) after PEP, subjects on both regimens still had adequate RNVA concentrations, and the Zagreb regimen was again non-inferior to the Essen regimen. Our results are in line with studies examining the antibody longevity after PEP vaccination with PCECV, HDCV and PVRV, which showed that antibodies persist and remain above the ≥ 0.5 IU/mL level regardless the number of doses received.Citation21 In addition, our results are in line with other studies conducted with PVRV and PCECV reporting long-term immunity against rabies virus following pre-exposure immunization with 3 doses and a single booster dose after one or 2 years.Citation16,27 Regarding rates of seroconversion, we did not observe significant differences between the groups, the Zagreb regimen was non-inferior to the Essen regimen at Days 14 and 42. Seroconversion rates at the 1-year (13-months) time point showed a significantly lower response in both groups, which is also in accordance with previous studies conducted in subjects vaccinated with PCECV and PVRV.Citation13,21,27
The safety profile of PCECV, in terms of the reactogenicity of the Zagreb regimen, was similar to that of the Essen regimen, indicating similar tolerability. The incidence of adverse events (AEs) resulting in premature withdrawal from the study was low, and of similar frequency with both regimens, but vaccine-related AEs occurred more frequently in the Essen group than in the Zagreb group.
To our knowledge, this is the largest trial assessing the PEP Zagreb regimen with PCECV conducted in the Chinese population. The present study, however, may have some limitations. First, as with any other clinical trial, the sample size is not big enough to detect rare adverse events, even if the safety population of the trial exceeded the immunogenicity population. The safety profile however, is not expected to be different in the 2 PEP regimens tested and new safety concerns are not expected based on the results of previous studies using the Zagreb post-exposure regimen with PCECVCitation19. Second, the objective of this study was limited to directly compare the 2 PEP regimens (Zagreb vs. Essen regimen). Any other comparison, e.g., with different types of cell culture rabies vaccine like PVRV or HDCV were not part of this investigation.Citation19
In conclusion, vaccination with PCECV under the 2-1-1 Zagreb regimen is as safe and immunogenic as under the 5-dose Essen regimen for PEP against the rabies virus, but is also a more cost-effective option, has a more practical vaccination schedule, and can potentially increase compliance.
Materials and Methods
Study design and objectives
This phase III, randomized and open-label study was conducted at a single site in Jizhou City, Hebei Province, China between November 2008 and July 2010 (Clinicaltrials.gov identifier: NCT00825305, NCT01067079). The protocol was approved by the Investigational Review Board of Hebei Province Center for Disease Prevention and Control, and the study was conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice guidelines. Before enrolment, written informed consent was obtained from each participant.
Subject disposition and study design are illustrated in . The study consisted of 2 phases: a first phase or parental study was performed to evaluate the immunogenicity and safety of the vaccine at Days 7, 14 and 42 after initial vaccination; and an extension phase took place to evaluate the safety and persistence of neutralizing antibodies 13 months post- vaccination.
The primary objectives of the study were to determine the non-inferiority of the 2-1-1 Zagreb regimen compared with the conventional Essen regimen with regard to geometric mean RVNA concentrations on Day 14, and to compare the rates of seroconversion, defined as RVNA concentrations ≥0.5 IU/mL,Citation1 13 months after immunization (extension study). Secondary objectives included comparison of seroconversion rates between the 2 regimens on Days 7, 14, and 42, to assessment of the immunization response kinetics at Days 7, 42, and at 13 months (extension study), and evaluation of the vaccine safety.
The participants were randomized into 2 study groups in a 2:1 ratio to receive the abbreviated PEP Zagreb (2-1-1) regimen or the conventional Essen (1-1-1-1-1) regimen. Random group codes were generated with SAS 9.1 statistical software by means of randomized blocking; the size of the block was set as variable, with the length of the smallest block being 6. The first 75 subjects randomized to each group were used for the immunogenicity assessment in the parent study. In the extension study, all subjects in the immunogenicity subgroup of the parent study were to be included, and other subjects from the safety set were also recruited so that 100 subjects could be recruited for each schedule; this was also done randomly, until the number of 100 per group was reached.
Subjects
Healthy adult volunteers were enrolled, aged 18–50 years, of both genders, and having not previously undergone vaccination against rabies. The subjects were in good health as determined by medical history, physical examination and the clinical judgment of the investigator. The main exclusion criteria were a history of rabies vaccination; a current, significant acute or chronic infectious disease which may impact the subject's safety and /or immunogenicity at the time of enrollment; a fever higher than 38°C (axillary) and / or a significant acute or chronic infectious disease requiring a systemic antibiotic or anti-viral therapy within 7 days of enrollment; receiving treatment with corticosteroids, immunosuppressive or antimalarial drugs within 2 months of enrollment; receipt of any vaccine; diagnosis of a known / suspected immunodeficiency, or autoimmune disease, or any immunologic disorder; a history of allergy to egg protein or known hypersensitivity to any vaccine component; and treatment with a parenteral immunoglobulin preparation, blood products and /or plasma derivatives within the past 3 months. Moreover, individuals who suffered an animal bite (dog or other wild animal) or who received an additional dose of rabies vaccine after their vaccination in the parental study were excluded from the extension phase.
Vaccines
One mL of PCECV (Rabipur®; Novartis Vaccines, Lot no. 1495 and 1554; manufactured in India and provided directly by the manufacturers), and containing lyophilized, inactivated rabies virus (Flury LEP strain) with a potency ≥2.5 IU/mL, was administered intramuscularly based on one of the following schemes: 2 doses on Day 0, and one dose both on Days 7 and 21 for subjects in the Zagreb group, and one dose on Days 0, 3, 7, 14, and 28 for subjects in the Essen group.
Immunogenicity assessment
During randomization, 5-mL samples of venous blood were obtained from the first 150 subjects (75 subjects in each vaccination regimen) for immunogenicity analyses. Serum samples from each volunteer were obtained prior to vaccination, and on Days 7, 14, 42, and Month 13. Determination of RVNA using the RFFIT Citation28 with CVS-11 as the challenge virus for the assay was carried out at the National Institute for the Control of Pharmaceutical and Biological Products (NICPBP) in Beijing. Based on WHO criteria, an RVNA concentration ≥0.5 IU/mL was considered as adequate based on WHO criteria.Citation1
Reactogenicity assessment
Vaccinated subjects were observed for approximately 30 minutes after each immunization to monitor for immediate adverse reactions. The frequency and severity of all solicited or unsolicited AEs were recorded for 6 days following the day of vaccination, and collected throughout the entire study period (Day 1 until Month 13). Solicited local reactions were pain at the site of injection, erythema, and swelling. Solicited systemic reactions were fever, malaise, myalgia, arthralgia, headache, nausea, and fatigue. The investigator used a standard scale to grade adverse events, in which symptoms were defined as mild, moderate or severe if they resulted in no limitation of, some limitation of, or inability to perform the normal daily activities, respectively.
Statistical analysis
For the immunogenicity objectives, each regimen group required 75 subjects (taking into account a 15% drop out rate) in the parent study to have a 90% power to support the hypothesis that the Zagreb regimen was non-inferior to the Essen regimen in terms of Day 14 antibody titers, and assuming no difference between the 2 regimens and an inferiority limit of −1.5 titers levels (log2). Due to an anticipated drop out of subjects, the extension study had to be bigger than the immunogenicity subset of the initial study. The sample size of the extension study was based on the largest number of subjects included in the immunogenicity subgroup of the parent study (n = 150) plus the number of subjects possibly lost to follow-up in the study in the following year (n = 50). As for the safety objectives, and taking into account a drop rate of about 9%, there was a probability higher than 75% to observe and adverse event (with a true/underlying rate of 3 per million or more) in a sample size of 500 subjects in the Zagreb group and 250 in the Essen group.
To assess the non-inferiority of the PCECV immune response under the Zagreb or Essen regimen, the per-protocol population (PP) was used, defined as all trial participants who received relevant doses of the study vaccination correctly, who provided all relevant RVNA determinations, and had no major protocol violations. Safety was analyzed for all subjects exposed to PCECV. In the extension study, the full analysis set (FAS), defined as all participants who provided at least one evaluable serum specimen and underwent random analysis in the parent study, was used for immunogenicity assessments. Summary statistics of immune response variables were determined for the different vaccine groups, stratified by age, ethnicity, and gender. Log2-transformed GMC of RVNA and the corresponding 2-sided 95% CIs were determined and compared between the 2 groups using the generalized linear model (GLM). The criterion for non-inferiority of the Zagreb group vs. the Essen group was demonstrated if the lower limit of the 2-sided 95% CI for the log2 GMC ratio was higher than −1.5. The non-inferiority test for the rate of seroconversion was defined as the lower limit of the 2-sided 95% CI difference between groups higher than −3%. Safety data were summarized by regimen group, providing the frequency and proportion of participants reporting an event.
Disclosure of Potential Conflicts of Interest
Dr. Jingchen Ma, Dr. Hongchang Wang, Dr Jun Li, Dr. Likuan Chang, Dr. Yun Xie, Dr. Zhonglin Liu, and Dr. Yuliang Zhao declare no conflicts of interest. Dr. Claudius Malerczyk is an employee of Novartis Vaccines, manufacturer of the vaccine used in this study.
Acknowledgments
The authors wish to thank Dr. Hari Sai Priya Baddela (Novartis Vaccines and Diagnostics, Hyderabad, India) and Dr. Mònica Gratacòs (CHC-Europe) for editorial assistance in the preparation of this manuscript.
Funding
The study was funded by Novartis Vaccines, Inc..
References
- WHO Expert Consultation on Rabies. Second report. World Health Organ Tech Rep Ser 2013:1-139, back cover.
- Yu J, Li H, Tang Q, Rayner S, Han N, Guo Z, Liu H, Adams J, Fang W, Tao X, et al. The spatial and temporal dynamics of rabies in China. PLoS Negl Trop Dis 2012; 6:e1640; PMID:22563518; http://dx.doi.org/10.1371/journal.pntd.0001640
- Zhang L, Wilson DP. Trends in notifiable infectious diseases in China: implications for surveillance and population health policy. PLoS One 2012; 7:e31076; PMID:22359565; http://dx.doi.org/10.1371/journal.pone.0031076
- Hu R, Tang Q, Tang J, Fooks AR. Rabies in China: an update. Vector Borne Zoonotic Dis 2009; 9:1-12; PMID:18803503; http://dx.doi.org/10.1089/vbz.2008.0046
- Si H, Guo ZM, Hao YT, Liu YG, Zhang DM, Rao SQ, Lu JH. Rabies trend in China (1990-2007) and post-exposure prophylaxis in the Guangdong province. BMC Infect Dis 2008; 8:113; PMID:18717989; http://dx.doi.org/10.1186/1471-2334-8-113
- Yin CP, Zhou H, Wu H, Shen XX, Wang LH, Yin WW, Wang SM, Tang Q. [Epidemiological analysis of rabies in 2010, China]. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi 2011; 25:434-6; PMID:22734228;
- Hemachudha T, Wacharapluesadee S, Mitrabhakdi E, Wilde H, Morimoto K, Lewis RA. Pathophysiology of human paralytic rabies. J Neurovirol 2005; 11:93-100; PMID:15804967; http://dx.doi.org/10.1080/13550280590900409
- Chutivongse S, Wilde H, Fishbein DB, Baer GM, Hemachudha T. One-year study of the 2-1-1 intramuscular postexposure rabies vaccine regimen in 100 severely exposed Thai patients using rabies immune globulin and Vero cell rabies vaccine. Vaccine 1991; 9:573-6; PMID:1771970; http://dx.doi.org/10.1016/0264-410X(91)90244-Z
- Colnot F, Sureau P, Alexandre JL, Arnaudo JP, Hesse JY, Jeanmaire H. Post-exposure antirabies vaccination. Early serological response to vaccine cultivated on VERO cells using a reduced 2-1-1 schedule. Presse Med 1994; 23:1609-12; PMID:7831241;
- Briggs DJ, Banzhoff A, Nicolay U, Sirikwin S, Dumavibhat B, Tongswas S, Wasi C. Antibody response of patients after postexposure rabies vaccination with small intradermal doses of purified chick embryo cell vaccine or purified Vero cell rabies vaccine. Bull World Health Organ 2000; 78:693-8; PMID:10859864;
- Scheiermann N, Baer J, Hilfenhaus J, Marcus I, Zoulek G. Reactogenicity and immunogenicity of the newly developed purified chick embryo cell (PCEC)-rabies vaccine in man. Zentralbl Bakteriol Mikrobiol Hyg A 1987; 265:439-50; PMID:2445127;
- Vodopija I, Sureau P, Lafon M, Baklaic Z, Ljubicic M, Svjetlicic M, Smerdel S. An evaluation of second generation tissue culture rabies vaccines for use in man: a four-vaccine comparative immunogenicity study using a pre-exposure vaccination schedule and an abbreviated 2-1-1 postexposure schedule. Vaccine 1986; 4:245-8; PMID:3541428; http://dx.doi.org/10.1016/0264-410X(86)90138-6
- Kamoltham T, Thinyounyong W, Phongchamnaphai P, Phraisuwan P, Khawplod P, Banzhoff A, Malerczyk C. Pre-exposure rabies vaccination using purified chick embryo cell rabies vaccine intradermally is immunogenic and safe. J Pediatr 2007; 151:173-7; PMID:17643772; http://dx.doi.org/10.1016/j.jpeds.2007.02.044
- Quiambao BP, Dimaano EM, Ambas C, Davis R, Banzhoff A, Malerczyk C. Reducing the cost of post-exposure rabies prophylaxis: efficacy of 0.1 ml PCEC rabies vaccine administered intradermally using the Thai Red Cross post-exposure regimen in patients severely exposed to laboratory-confirmed rabid animals. Vaccine 2005; 23:1709-14; PMID:15705476; http://dx.doi.org/10.1016/j.vaccine.2004.09.027
- Dobardzic A, Izurieta H, Woo EJ, Iskander J, Shadomy S, Rupprecht C, Ball R, Braun MM. Safety review of the purified chick embryo cell rabies vaccine: Data from the Vaccine Adverse Event Reporting System (VAERS), 1997-2005. Vaccine 2007; 25:4244-51; PMID:17382435; http://dx.doi.org/10.1016/j.vaccine.2007.02.075
- Malerczyk C, Briggs DJ, Dreesen DW, Banzhoff A. Duration of immunity: an anamnestic response 14 years after rabies vaccination with purified chick embryo cell rabies vaccine. J Travel Med 2007; 14:63-4; PMID:17241256; http://dx.doi.org/10.1111/j.1708-8305.2006.00097.x
- WHO Expert Consultation on rabies. World Health Organ Tech Rep Ser 2005; 931:1-88, back cover; PMID:16485446;
- Ministry of Health, People's Republic of China. Situation of rabies prevention and control in China. http://www.moh.gov.cn, 2009.;
- Liu H, Huang G, Tang Q, Li J, Cao S, Fu C, Cao Q, Liu B, Pan H, Wang M. The immunogenicity and safety of vaccination with purified Vero cell rabies vaccine (PVRV) in China under a 2-1-1 regimen. Hum Vaccin 2011; 7:220-4; PMID:21311216; http://dx.doi.org/10.4161/hv.7.2.14003
- Rupprecht CE, Briggs D, Brown CM, Franka R, Katz SL, Kerr HD, Lett SM, Levis R, Meltzer MI, Schaffner W, et al. Use of a reduced (4-dose) vaccine schedule for postexposure prophylaxis to prevent human rabies: recommendations of the advisory committee on immunization practices. MMWR Recomm Rep 2010; 59:1-9; PMID:20300058
- Rupprecht CE, Briggs D, Brown CM, Franka R, Katz SL, Kerr HD, Lett S, Levis R, Meltzer MI, Schaffner W, et al. Evidence for a 4-dose vaccine schedule for human rabies post-exposure prophylaxis in previously non-vaccinated individuals. Vaccine 2009; 27:7141-8; PMID:19925944; http://dx.doi.org/10.1016/j.vaccine.2009.09.029
- Song M, Tang Q, Wang DM, Mo ZJ, Guo SH, Li H, Tao XY, Rupprecht CE, Feng ZJ, Liang GD. Epidemiological investigations of human rabies in China. BMC Infect Dis 2009; 9:210; PMID:20025742; http://dx.doi.org/10.1186/1471-2334-9-210
- Hampson K, Cleaveland S, Briggs D. Evaluation of cost-effective strategies for rabies post-exposure vaccination in low-income countries. PLoS Negl Trop Dis 2011; 5:e982; PMID:21408121; http://dx.doi.org/10.1371/journal.pntd.0000982
- Wang CL, Zhang XW, Yu YX. Study on the compliance and economic cost of rabies vaccination. Zhongguo Yi Miao He Mian Yi 2010; 16:254-7; PMID:20726270;
- Knobel DL, Cleaveland S, Coleman PG, Fevre EM, Meltzer MI, Miranda ME, Shaw A, Zinsstag J, Meslin FX. Re-evaluating the burden of rabies in Africa and Asia. Bull World Health Organ 2005; 83:360-8; PMID:15976877;
- WHO expert committee on rabies. Guide for post-exposure treatment. 8th report. WHO tecnical report 824. Geneva, Switzerland: World Health Organization; 1992
- Suwansrinon K, Wilde H, Benjavongkulchai M, Banjongkasaena U, Lertjarutorn S, Boonchang S, Suttisri R, Khowplod P, Daviratanasilpa S, Sitprija V. Survival of neutralizing antibody in previously rabies vaccinated subjects: a prospective study showing long lasting immunity. Vaccine 2006; 24:3878-80; PMID:16530893; http://dx.doi.org/10.1016/j.vaccine.2006.02.027
- Zalan E, Wilson C, Pukitis D. A microtest for the quantitation of rabies virus neutralizing antibodies. J Biol Stand 1979; 7:213-20; PMID: 387798