Abstract
The glutathione S-transferase (GST)-L1 multiplex serology assay has favorable properties for use in clinical trials and epidemiologic studies, including low cost, high throughput capacity, and low serum volume requirement. Therefore, we evaluated the GST-L1 assay as a measure of HPV16/18 vaccine immunogenicity. Our study population included 65 women selected from the Costa Rica Vaccine Trial who received the bivalent HPV16/18 virus-like particle (VLP) vaccine at the recommended 0/1/6-month schedule. We tested replicate serum samples from months 0/1/12 (i.e., after 0/1/3 doses) by GST-L1 and 3 other commonly used serology assays, VLP-ELISA, SEAP-NA, and cLIA. We calculated the percentage of women seropositive by GST-L1 by time point and HPV type (14 HPV types), and compared GST-L1 to other assays using Spearman rank correlation coefficients. After 1 vaccine dose, seropositivity by GST-L1 was 40% each for HPV16 and HPV18, increasing to 100% and 98%, respectively, after 3 doses. Seropositivity after 3 doses ranged from 32% to 69% for HPV types 31/33/45, for which partial vaccine efficacy is reported, though increases also occurred for types with no evidence for cross-protection (e.g., HPV77). GST-L1 correlated best after 3 doses with VLP-ELISA (HPV16 and HPV18 each ρ = 0.72) and SEAP-NA (HPV16 ρ = 0.65, HPV18 ρ = 0.71) (all P < 0.001); correlation was lower with cLIA. The GST-L1 is suitable for evaluating HPV16/18 vaccine immunogenicity after 3 vaccine doses, although in contrast to other assays it may classify some samples as HPV16/18 seronegative. The assay's utility is limited for lower antibody levels such as after receipt of 1 dose.
Abbreviations
| BKV | = | BK virus |
| cLIA | = | competitive Luminex immunoassay |
| CV | = | coefficient of variation |
| CVT | = | Costa Rica Vaccine Trial |
| EU/mL | = | ELISA units per milliliter |
| GST-L1 | = | glutathione S-transferase L1 multiplex serology assay |
| HPV | = | human papillomavirus |
| ICC | = | intraclass correlation coefficient |
| JCV | = | JC virus |
| LLOD | = | lower limit of detection |
| MFI | = | median fluorescence units |
| mMU/mL | = | milli-Merck units per milliliter |
| OD | = | optical density |
| SEAP-NA | = | secreted alkaline phosphatase neutralization assay |
| VLP | = | virus-like particle |
| VLP-ELISA | = | virus-like particle-based enzyme linked immunosorbent assay |
Introduction
Two human papillomavirus (HPV) L1 virus-like particle (VLP) vaccines are licensed to prevent cervical cancer and related lesions. The bivalent vaccine contains VLPs of HPV types 16 and 18, which together cause 70% of cervical cancers,Citation1 and the quadrivalent vaccine additionally contains VLPs of HPV6 and 11, which cause the majority of genital warts. Both vaccines are highly efficacious for prevention of high-grade lesions related to HPV16 and 18,Citation2-4 and cross-protective efficacy of the HPV16/18 vaccine has been demonstrated against persistent infection by some non-vaccine α types, including HPV31/33/45Citation5,6 and potentially HPV6/11.Citation7
The presumed mechanism of protection of these vaccines, based on animal studies, is generation of serum neutralizing antibodies after vaccination.Citation8,9 Accordingly, vaccine immunogenicity is assessed by using serologic assays to measure antibodies to HPV L1 proteins in post-vaccination serum samples.Citation10 Though vaccine licensure was originally based on the results of clinical trials using endpoints related to development of cervical lesions, guidelines have been and may continue to be changed on the basis of immunogenicity data. For example, immunogenicity data were used to extend licensure to younger women among whom vaccine efficacy could not be evaluatedCitation11,12 and were of central importance in the approval of vaccine schedules of 2 rather than 3 doses in the European Union.Citation13
Typically, the VLP-based enzyme-linked immunosorbent assay (VLP-ELISA), competitive Luminex immunoassay (cLIA), and secreted alkaline phosphatase neutralization assay (SEAP-NA) have been used to assess HPV vaccine immunogenicity. A newer assay, the glutathione S-transferase multiplex serology assay (GST-L1), has recently become commonly used in seroepidemiological studies. In a natural infection setting, our group recently showed that the GST-L1 is a good measure of cumulative natural infection but not naturally acquired immune protection,Citation14 though another study suggested that GST-L1 seropositivity may indicate protection from more stringently defined outcomes such as persistent HPV16 infection.Citation15 The GST-L1 assay may be well-suited for vaccine immunogenicity studies, as it simultaneously measures responses to 14 HPV-L1 types, requires a small volume of serum (50 μL), is high-throughput, and offers a low cost alternative to other assays.Citation16 As more HPV types are combined in a single vaccine,Citation17 use of the GST-L1 as an immunogenicity measure will become more attractive. However, few data exist describing the assay as a measure of vaccine-induced immune responses.Citation18,19
In this study, we evaluate the GST-L1 for use in assessing HPV16/18 vaccine immunogenicity. In addition to HPV16 and 18, we present pre- and post-vaccination antibody levels for 12 non-vaccine HPV L1 types, including those which show cross-protection from the HPV16/18 vaccine.Citation5-7 We also evaluate antibodies to 5 control antigens and GST fusions of the HPV16/18 oncoproteins E6 and E7, which are analyzed within the assay and are not expected to be affected by L1 VLP vaccination.
Results
GST-L1 geometric means, seropositivity, and patterns over time
Prior to vaccination, geometric mean antibody levels measured by the GST-L1 assay were low (<200 median fluorescence intensity [MFI]) for all HPV-L1 types with the exception of HPV6, which had a geometric mean of 899 MFI (). Geometric means increased with each vaccine dose for all HPV-L1 types, but most strongly for the vaccine types HPV16 and 18.
Table 1. Geometric means and seropositive percentages by the GST-L1 assay among HPV16/18-vaccinated women at months 0, 1, and 12
After 1 vaccine dose, 40% of women were seropositive for each vaccine type, increasing to 100% and 98% after 3 doses for HPV16 and 18, respectively (). HPV6 seropositivity was high prior to vaccination (60%) and increased to 75% after 3 doses. For the other non-vaccine α L1 types, seropositivity after 3 doses ranged from 25% (HPV58) to 69% (HPV45), while for the non-α types seropositivity ranged from 23% (HPV1) to 31% (HPV4) (). For the oncoproteins HPV16-E6, HPV18-E6, and HPV18-E7, seropositivity was 3% or less at all time points. Seropositivity was higher for HPV16-E7 (11% prior to vaccination) but did not increase over time. Geometric means and seropositivity for the control antigens were stable over time ().
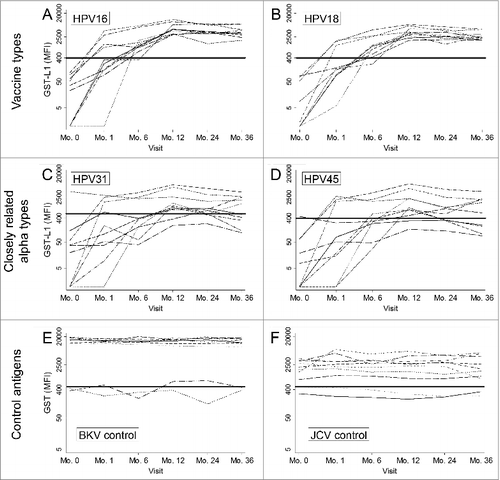
For HPV16 and 18, individual GST-L1 antibody levels increased steeply after vaccination then declined slightly from month 12 to month 36 (). Levels for HPV31 and HPV45 showed a similar overall pattern (), but were lower and showed more inter-individual variation. All 10 women showed some response to HPV31 and 45 beginning after 2 doses, though some did not achieve seropositivity at any time point. For the BKV and JCV control antigens (), individual antibody levels were stable over time.
Figure 1. Individual antibody levels over time as measured by the GST-L1 assay among HPV16/18 vaccinated women, separately for HPV vaccine types, 2 HPV α types closely related to vaccine types, and 2 control antigens. Sample includes 10 women who received doses of the HPV16/18 vaccine at months 0, 1, and 6. Bold lines represent assay seropositivity cutoffs. BKV, BK virus; JCV, JC virus.
Pairwise comparison of GST-L1 to other assays after 1 vaccine dose
Pairwise discordance (i.e., simultaneous seropositivity by one assay and seronegativity by another) between GST-L1 and other assays after vaccination occurred almost exclusively after 1 vaccine dose and was nearly always due to GST-L1-seronegative samples (). After 1 dose, for HPV16, 59% of women were seronegative by GST-L1 but seropositive by VLP-ELISA; the remaining 41% were seropositive by both assays (). Discordance after 1 dose was similar for HPV18 GST-L1/VLP-ELISA (61% of women were seronegative by GST-L1, ), HPV16 GST-L1/SEAP-NA (60%, ), and HPV18 GST-L1/SEAP-NA (61%, ). Comparing GST-L1 and cLIA after one dose, for HPV16, 40% of women were seropositive by both assays, 5% were seronegative by both assays, and 55% were seronegative by GST-L1 but seropositive by cLIA (). For HPV18, GST-L1/cLIA pairwise discordance after one dose was due both to GST-L1-seronegative/cLIA-seropositive samples (37%) and to GST-L1-seropositive/cLIA-seronegative samples (3%) (). For each comparison, GST-L1 gave a significantly lower proportion of seropositives than the comparison assay (all McNemar's P < 0.001).
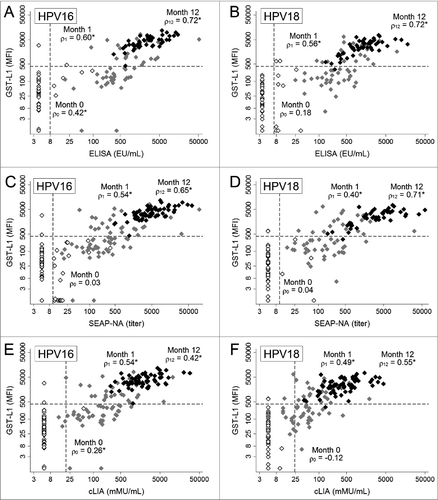
Figure 2. Correlation between HPV16/18 GST-L1 and VLP-ELISA, SEAP-NA, and cLIA among HPV16/18-vaccinated women at months 0, 1, and 12. HPV16/18 vaccine was administered at months 0, 1, and 6. Sample includes 51 women for panels A, B, and D, and 65 women for panels C, E, and F. White markers are pre-vaccination (month 0), gray markers are after 1 dose (month 1), and black markers are after 3 doses (month 12). Asterisks indicate statistical significance (P < 0.05), and dashed lines represent assay seropositivity cutoffs.
Continuous comparison of GST-L1 to other assays
We evaluated Spearman rank correlation coefficients between GST-L1 and other assays for HPV16 and 18 at each time point. Correlation generally increased at higher antibody levels (i.e., after each vaccine dose), and was typically highest for GST-L1/VLP-ELISA and lowest for GST-L1/cLIA (). For HPV16, correlation between GST-L1 and VLP-ELISA after 1 dose was moderate (ρ = 0.60, ), and increased after 3 doses (ρ = 0.72). For HPV16 GST-L1 and SEAP-NA, correlation was similarly moderate after one dose (ρ = 0.54, ) and increased after 3 doses (ρ = 0.65). For HPV16 GST-L1 and cLIA, correlation was highest after one dose (ρ = 0.54, ) and marginally lower after 3 doses (ρ = 0.42) (all P < 0.001). Results were generally similar for HPV18 (). In each of the comparisons after 3 doses, the GST-L1 reached its upper limit of linearity at the 1:100 sample dilution, i.e., showed a plateauing of antibody levels in its uppermost range (, y-axes). The other assays did not show a plateau (, x-axes).
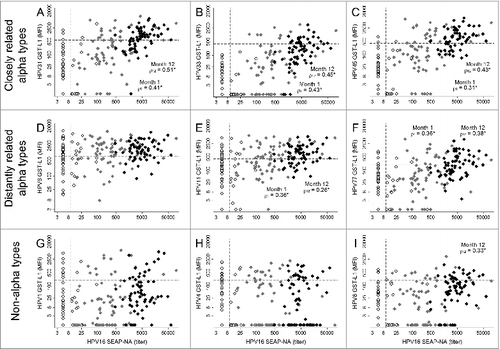
To characterize the GST-L1-measured antibody response to non-vaccine α and non-α types, we examined correlation with neutralizing responses as measured by HPV16 SEAP-NA. Correlation with HPV16 SEAP-NA was highest for the GST-L1 α types closely related to vaccine types for which definitive cross-protection has been shown, i.e., HPV31 (ρ = 0.51 after 3 doses, ), HPV33 (ρ = 0.45, ), and HPV45 (ρ = 0.43, ) (all P < 0.005). Among the more distantly related α types, we observed weak correlation with HPV16 SEAP-NA for HPV11 (ρ = 0.26 after 3 doses, p = 0.037, ) and HPV77 (ρ = 0.38 after 3 doses, p = 0.002, ), but not for HPV6 (ρ = 0.09, p = 0.456, ). Among the non-α types, HPV16 SEAP-NA did not correlate with HPV1 or 4 (), but showed weak correlation with HPV8 after 3 doses (ρ = 0.33, p = 0.007, ).
Figure 3. Correlation between HPV16 SEAP-NA and selected GST-L1 α and non-α types among HPV16/18-vaccinated women at months 0, 1, and 12. Sample includes 65 women who received HPV16/18 vaccine at months 0, 1, and 6. White markers are pre-vaccination (month 0), gray markers are after 1 dose (month 1), and black markers are after 3 doses (month 12). Dashed lines represent assay seropositivity cutoffs. “Closely” and “distantly” refer to degree of relatedness to vaccine types (HPV16/18). In each panel, a GST-L1 HPV type is compared with HPV16 SEAP-NA. The GST-L1 types are as follows: (A) HPV31; (B) 33; (C) 45; (D) 6; (E) 11; (F) 77; (G) 1; (H) 4; (I) 8. Correlation coefficients are displayed only where statistically significant (P < 0.05).
Discussion
Evaluations of HPV vaccine immunogenicity, which rely on serologic measurements, have been used to extend vaccine licensure to new groups and lend support to a vaccine schedule of 2 doses.Citation11-13 We evaluated the GST-L1 multiplex assay, which measures antibodies to many antigens at low cost and high throughput, as a measure of HPV16/18 vaccine immunogenicity.
For the vaccine types, HPV16 and 18, GST-L1-measured antibody levels increased strongly after vaccination and followed a pattern similar to other assays.Citation20 After 3 vaccine doses, HPV16/18 seropositivity is generally 100% by VLP-ELISA, SEAP-NA, and cLIA.Citation20 In our study, seropositivity by GST-L1 was 100% for HPV16 as well, though one sample's MFI was only slightly above the seropositivity cutoff at 431 MFI. For HPV18, one observation was marginally below the cutoff at 308 MFI, giving 98% seropositivity. Our study's power is limited for examining immune responses after 2 vaccine doses, as our sample includes only 10 women at month 6. However, these data show a similar pattern, with one out of 10 women marginally below the seropositivity cutoff for both HPV16 (334 MFI) and HPV18 (232 MFI).
Though 3 vaccine doses are currently recommended in the United States, vaccine immunogenicity after only 1 dose is also of interest.Citation21 After 1 vaccine dose, seropositivity is typically 100% by VLP-ELISA and SEAP-NA for HPV16/18, approaches 100% by cLIA for HPV16, and is approximately 70% by cLIA for HPV18.Citation20 In our study, by GST-L1, seropositivity after 1 dose was lower at 40% for each of HPV16 and 18. As a sensitivity analysis, we calculated the seropositive percentages using a lowered 200 MFI cutoff, which has been used previously for GST-L1.Citation22 At this cutoff, seropositivity increased marginally to 58% (95% CI 46-71%) for HPV16 and 57% (95% CI 44-69%) for HPV18. Notably, some subjects had a very low or no HPV16/18 response (i.e., close to 0 MFI) by GST-L1 after one dose, despite seropositivity by VLP-ELISA, cLIA, and SEAP-NA. Therefore, regardless of the seropositivity cutoff used for GST-L1, it may not be possible to distinguish low responses from true non-responses in settings where antibody levels are lower. Lower levels are observed after 1 vaccine dose, but may also occur many years after receipt of 2 or 3 doses.
For the vaccine types, GST-L1 correlated only moderately with cLIA, which measures antibodies to single neutralizing epitopes and therefore differs most from GST-L1. Generally, correlation between GST-L1 and other assays after 1 dose was only moderate, which may relate to non-specific background within the GST-L1 at lower antibody levels.Citation24 In contrast, GST-L1-measured responses correlated well with SEAP-NA and VLP-ELISA, particularly at higher levels. A priori, we expected correlation to be highest with VLP-ELISA, because both assays measure neutralizing and non-neutralizing responsesCitation23 and VLP-ELISA shows the best agreement with GST-L1 in a natural infection context.Citation14 However, even these rank correlations were not perfect (ρ = 0.72 each for HPV16 and 18 after 3 doses). This is likely because GST-L1 reaches its upper limit of linearity for very high antibody levels using a 1:100 sample dilution (i.e., shows a plateau, ). Correlation after 3 doses would likely improve with use of a higher dilution (e.g., 1:1000). However, higher dilutions might decrease analytical sensitivity for samples with lower antibody levels (e.g., fewer than 3 doses).
The HPV16/18 vaccine has cross-protective efficacy for HPV31, 33, and 45Citation5,6 and possibly for HPV6 and 11.Citation7 For these types, we observed increases in GST-L1-measured geometric means and seropositivity across vaccination time points. Seropositivity after 3 doses was comparable to SEAP-NA for HPV31 (62% by GST-L1 compared to 74% by SEAP-NA) and HPV45 (69% by GST-L1 compared to 61% by SEAP-NA) ().Citation25 Seropositivity by GST-L1 was lower (32%) for HPV33; reported cross-protective vaccine efficacy is also lower for HPV33 than for HPV31 and 45.Citation5 Among these cross-protection types, with the exception of HPV6, we further observed correlation with neutralizing responses as measured by HPV16 SEAP-NA. It is possible that GST-L1-measured responses to cross-protection types may indicate clinically relevant protection for those types, but to our knowledge this has only been shown directly for HPV31 SEAP-NA.Citation26
Pre-vaccination seropositivity for HPV6 was very high (60%), despite use of a high seropositivity cutoff (800 MFI). In other populations, GST-L1 has been shown a valid marker of HPV6/11 exposure.Citation27 However, as none of the women in our study population had a cervical HPV6 infection at baseline, the high reactivity to HPV6 in our study may indicate cross-reactivity within the assay or reactivity of the HPV6-L1 antigen with non-HPV antibodies. The HPV16/18 vaccine may confer cross-protection against HPV6,Citation7 but the high baseline HPV6 seropositivity by GST-L1 complicates interpretation of the marginal (15%) increase in seropositivity after vaccination. Further, though our study used the HPV16/18 vaccine, future studies might use the GST-L1 to evaluate immunogenicity for the HPV6/11/16/18 vaccine. If seroconversion is an immunogenicity endpoint, then the GST-L1's utility for the quadrivalent vaccine might be limited by artifactually high HPV6 seropositivity among non-vaccinees.
We observed increases after vaccination for some HPV-L1 types in the GST-L1 assay for which there is little or no evidence of cross-protection. This occurred for the non-α type HPV8 and the α types HPV35, 52, 58, and 77. For HPV52 and 58, this pattern has also been observed with SEAP-NACitation25 and cLIA.Citation28 Seropositivity by SEAP-NA after 3 doses for HPV52 and 58 has been reported as 44% and 33% Citation25; we obtained similar estimates by GST-L1 of 54% and 25%. For GST-L1, as well as other assays, the implication of increases after vaccination for L1 types for which cross protection has not been shown is unclear. It could indicate, for example, cross-reactivity with antibodies to vaccine types within the assay, or type-specific antibodies that occur at low levels and/or are non-neutralizing. For the GST-L1, reactivity to these types could relate to non-specific background of the assay,Citation24 which might be improved by optimizations that increase sensitivity at low levels.
Finally, for the oncoproteins HPV16/18-E6 and -E7, consistent specificity of the GST-L1 assay was demonstrated by stable antibody levels and seropositivity between pre- and post-vaccination samples. This suggests that the potential utility of the E6 protein as a cancer biomarkerCitation29 would not be affected by population-level HPV vaccination.
The key strength of our study is testing of an informative set of replicate samples by multiple assays, which allows direct comparison of different assays. Additionally, our study population was sampled from a well-characterized vaccine trial with complete follow-up extending to 36 months for a subset of individuals. One limitation is a relatively small sample size; a larger sample would allow more precise estimation of seropositive percentages, particularly after 2 doses. Second, while we conducted a sensitivity analysis using a lowered, empirically determined GST-L1 cutoff, we recognize that formal assay cutoffs capturing assay variability and sensitivity/specificity at the lower detection limit of the assay must be determined to confirm the precision and validity of empirically determined cutoffs.
In conclusion, GST-L1-measured antibody responses to the HPV16/18 vaccine show similar patterns to and correlate best at higher levels with other, more established assays. Among women receiving 2 or 3 vaccine doses, examination of absolute GST-L1-measured antibody levels is expected to be sufficient for distinguishing lower responses from true non-responses. However, because of lower analytical sensitivity, the assay's utility for evaluating vaccine immunogenicity may be more limited in settings where antibody levels are more modest, such as after receipt of 1 vaccine dose or many years after 2 or 3 doses.
Methods
Study population
Our study population was sampled from participants in the HPV vaccination arm of the Costa Rica Vaccine Trial (CVT),Citation30 who received HPV16/18 vaccine at the recommended 0, 1, and 6 month schedule. The CVT protocol was approved by the institutional review boards of the U.S. National Cancer Institute and the Costa Rican INCIENSA, and all participants signed IRB-approved informed consent forms.
Our sample included 65 women who were selected for previous serological studies to evaluate immune responses to vaccination.Citation20,25,32 These studies selected (after exclusion of 12 women who received fewer than 3 vaccine doses, had missing assay data, or had results indicating technical or sample retrieval errors) 41 women randomlyCitation25,31 and separate groups of 10Citation32 and 14 women who were HPV DNA negative at the cervix at baseline. The group of 10 was required to be HPV-negative for HPV16, 18, 31, 45, and 58,Citation32 and the group of 14 was required to be negative for all 25 types included in the SPF10-LiPA HPV DNA detection assay.
Antibody testing using GST-L1, VLP-ELISA, SEAP-NA, and cLIA was performed using samples collected from each woman at 0-, 1-, and 12-month time points. The 4 assays were performed on the combined study population (N = 65 women), with the exception of HPV16/18 VLP-ELISA and HPV18 SEAP-NA, which were not performed for the subset of 14 women. This gave a sample of 65 women for most analyses, and a sample of 51 women for analyses involving HPV16/18 VLP-ELISA or HPV18 SEAP-NA. To evaluate longer term longitudinal antibody levels, the subset of 10 women was additionally tested using all assays at months 6 (i.e., after 2 doses), 24, and 36.
Serologic assays
GST-L1 multiplex serology
The glutathione S-transferase (GST)-L1 multiplex serology assay was performed at the German Cancer Research Center (DKFZ) as previously describedCitation16,24 to detect antibodies to 14 HPV-L1 proteins, 4 HPV oncoproteins, and 5 control antigens (see for details). Briefly, sets of beads carrying each antigen were individually washed, combined (N = 3,000 beads each), mixed with 50 μL of a single 1:100 dilution of serum in a single well-format, and incubated. Biotinylated anti-human secondary antibody and fluorescent detection conjugate were used to detect antibodies bound to beads. Reporter fluorescence of individual beads was determined using a Luminex analyzer, and the MFI for each antigen was calculated based on at least 100 beads per set. To obtain the final MFI, the background fluorescence as determined by a negative control (i.e., no serum) and the MFI of GST-tag (i.e., the fusion proteins without viral antigen) were subtracted from the raw MFI.
Seropositivity cutoff values were determined by a distribution based method,Citation22,33 i.e., visual inspection of percentile plots of combined samples from this and anotherCitation14 study, and are shown in . For HPV16, 18, 31, 33, 45, and 52 L1, this method gave very similar results compared to cutoffs calculated as 5 standard deviations above the mean antibody level among 371 HPV DNA-negative, Korean female self-reported virgins, after iterative exclusion of outliers.Citation34
For GST-L1, we used results from 15 blinded duplicate sample pairs to calculate 2 measures of assay reproducibility, the coefficient of variation (CV) and the intraclass correlation coefficient (ICC). We calculated CVs and ICCs for each HPV type among pairs where at least one result was seropositive. CVs for HPV16 and 18 were 18.8% and 10.5% respectively, and ICCs were 83.3% and 94.3%. Among the non-vaccine HPV-L1 types, where calculable, CVs ranged from 0.1% (HPV35) to 24.0% (HPV33), and ICCs ranged from 67.3% (HPV31) to 97.5% (HPV77).
VLP-ELISA
The HPV16/18 virus-like particle-based enzyme linked immunosorbent assay (VLP-ELISA) was performed at GlaxoSmithKline Biologicals as previously described.Citation35 Briefly, serial dilutions of serum samples and standards were added to HPV VLP-coated ELISA microtiter plates. A peroxidase-conjugated anti-human polyclonal antibody, enzyme substrate, and chromogen were added; reactions were then stopped. Optical density (OD) at 620 nm (background) was read and subtracted from OD at 450 nm. Antibody levels in ELISA units (EU)/mL were calculated by interpolating OD values from the standard curve, and results reflect the mean of between 1 and 5 runs per sample. Seropositivity cutoffs were calculated as 3 standard deviations above the geometric mean titers from HPV-negative individuals, giving 8 EU/mL and 7 EU/mL for HPV16 and 18, respectively.
For VLP-ELISA, extensive reproducibility studies were previously conducted among a subset of our initial study population. Mean CVs were 11.4% and 12.5% for HPV16 and 18 respectively, and ICCs were 99.4% and 99.2%.Citation31
SEAP-NA
The HPV16/18 secreted alkaline phosphatase-based pseudovirus neutralization assay (SEAP-NA) was performed at the HPV Immunology Laboratory of the National Cancer Institute as previously described.Citation31,36 Briefly, 293TT cells were expanded, cultured, seeded in 96-well plates, and incubated. Serial dilutions of serum samples and controls were incubated with HPV VLPs; serum and controls were then added to 293TT cells. The Great EscAPe SEAP assay kit was used according to the manufacturer's protocol (BD Biosciences-Clontech Laboratories Inc..). Neutralization titers were calculated by linear interpolation and defined as the reciprocal of the dilution that caused 50% reduction in SEAP activity compared to control wells. Reported titers reflect the mean of between 2 and 15 runs for each sample. Titers above 10 (i.e., the lowest dilution) were considered seropositive.Citation25
Reproducibility studies for SEAP-NA were previously performed among a subset of our study population, giving CVs of 39.7% and 33.9% for HPV16 and 18, respectively.Citation31 Higher variability is expected for SEAP-NA, which is a biological assay requiring a high degree of specimen handling and manipulation. ICCs for SEAP-NA in the reproducibility study were 96.5% (HPV16) and 97.0% (HPV18).Citation31
cLIA
The competitive Luminex immunoassay (cLIA) was performed at PPD Vaccines and Biologicals as previously described.Citation37 Briefly, sera at 1:4, 1:40, 1:400, and 1:4,000 dilutions were incubated in a multiplex system with phycoerythrin-labeled, HPV type-specific monoclonal antibodies that bind to conformational, neutralizing epitopes on VLPs. VLPs conjugated to Luminex microspheres were added, and incubation plates were washed with PBS and 1% TX100. Mean fluorescence intensities were measured and converted to arbitrary milli-Merck unit (mMU)/mL values using standard curves. Seropositivity cutoff values were determined using a clinical sensitivity/specificity algorithm to maximize the distinction between “likely positive” and “likely negative” samples,Citation38 giving 20 and 24 mMU/mL for HPV16 and 18, respectively.
For cLIA, we assessed reproducibility using 3 internal control samples, which spanned a range of seropositive antibody levels (low, medium, high) and were placed on each plate of each batch. CVs calculated individually by sample, separately within and across batch, did not exceed 17.1%. Mean CVs among the 3 samples, calculated across all plates and batches, were 7.2% and 8.7% for HPV16 and 18 respectively, and ICCs were 98.9% and 98.6%.
Statistical analysis
For each assay, we assigned results below the lower limit of detection (LLOD) a value of ½(LLOD). At each time point and for each GST-L1 antigen, including HPV-L1 types, HPV oncoproteins, and control antigens, we calculated geometric means among all subjects, the percentage of seropositive samples, and geometric means among seropositive samples only. We examined individual antibody levels from month 0 to month 36 using the subset of 10 women for whom 6 time points were available, and we present these patterns graphically for the HPV vaccine types (HPV16 and 18), 2 closely related HPV α types for which there is evidence of cross-protection (HPV31 and 45), and 2 control antigens (BK virus (BKV) and JC virus (JCV)).
We compared the GST-L1 assay to VLP-ELISA, SEAP-NA, and cLIA for HPV16 and 18, using the pairwise McNemar's test to examine serostatus discordance. On a continuous scale, we compared GST-L1 to other assays using Spearman rank correlation coefficients calculated separately for each time point. To explore whether GST-L1-measured responses to non-vaccine HPV types might be indicative of cross-protective neutralizing responses, we further calculated correlation coefficients between HPV16 SEAP-NA and GST-L1 responses for HPV31, 33, and 45 (closely related α types); HPV 6, 11, and 77 (distantly related α types); and HPV 1, 4, and 8 (non-α types). We used HPV16 SEAP-NA for this comparison because it measures neutralizing antibodies, which are believed to be the mechanism by which the vaccine induces protection. A significance level of α = 0.05 was used to assess statistical significance of correlation coefficients.
Investigators in the Costa Rica Vaccine Trial (CVT) group
Proyecto Epidemiológico Guanacaste, Fundación INCIENSA, San José, Costa Rica
Mario Alfaro (cytopathologist), Manuel Barrantes (field supervisor), M. Concepción Bratti (co-investigator), Fernando Cárdenas (general field supervisor), Bernal Cortés (specimen and repository manager), Albert Espinoza (head, coding and data entry), Yenory Estrada (pharmacist), Paula González (co-investigator), Diego Guillén (pathologist), Roland Herrero (co-principal investigator), Silvia E. Jiménez (trial coordinator), Jorge Morales (colposcopist), Luis Villegas (colposcopist), Lidia Ana Morera (head study nurse), Elmer Pérez (field supervisor), Carolina Porras (co-investigator), Ana Cecilia Rodríguez (co-investigator), Libia Rivas (clinical coordinator).
University of Costa Rica, San José, Costa Rica
Enrique Freer (director, HPV diagnostics laboratory), José Bonilla (head, HPV immunology laboratory), Alfanso García-Piñeres (immunologist), Sandra Silva (head microbiologist, HPV diagnostics laboratory), Ivannia Atmella (microbiologist, immunology laboratory), Margarita Ramírez (microbiologist, immunology laboratory).
United States National Cancer Institute, Bethesda, MD, USA
Allan Hildesheim (co-principal investigator & NCI co-project officer), Hormuzd Katki (stastitician), Aimée R. Kreimer (co-investigator), Douglas R. Lowy (HPV virologist), Nora Macklin (trial coordinator), Mark Schiffman (medical monitor & NCI co-project officer), John T. Schiller (HPV virologist), Mark Sherman (QC pathologist), Diane Solomon (medical monitor & QC pathologist), Sholom Wacholder (statistician).
SAIC, NCI-Frederick, Frederick, MD, UDA
Ligia Pinto (head, HPV immunology laboratory), Troy Kemp (immunologist).
Women's and Infants’ Hospital, Providence, RI, USA
Claire Eklund (QC cytology), Martha Hutchinson (QC cytology).
Georgetown University, Washington, DC, USA
Mary Sidawy (histopathologist),
DDL Diagnostic Laboratory, Netherlands
Wim Quint (virologist, HPV DNA testing), Leen-Jan van Doorn (HPV DNA testing).
Information Management Services
Brian Befano (data management programmer)
Disclosure of Potential Conflicts of Interest
One or more of the authors is employed by a commercial company. These include: Mark Esser, MedImmune; Katie Matys, PPD Vaccines and Biologics; Sylviane Poncelet, GlaxoSmithKline Biologicals; Wim Quint and Leen-Jan van Doorn, DDL Diagnostic Laboratory. John Schiller and Douglas Lowy report that they are named inventors on US. Government-owned HPV vaccine patents that are licensed to GlaxoSmithKline and Merck and for which the National Cancer Institute receives licensing fees. They are entitled to limited royalties as specified by federal law. The other authors declare that they have no conflicts of interest.
Funding
The Costa Rica HPV Vaccine Trial is a long-standing collaboration between investigators in Costa Rica and the National Cancer Institute (NCI). The trial is sponsored and funded by the NCI (contract N01-CP-11005), with funding support from the National Institutes of Health Office of Research on Women's Health, and done with the support from the Ministry of Health of Costa Rica. Vaccine was provided for our trial by GlaxoSmithKline Biologicals, under a Clinical Trials Agreement with the NCI. GlaxoSmithKline also provided support for aspects of the trial associated with regulatory submission needs of the company under US Food and Drug Administration BB-IND 7920.
References
- Lowy DR, Schiller JT. Reducing HPV-associated cancer globally. Cancer Prev Res (Phila) 2012; 5(1):18-23; PMID:22219162; http://dx.doi.org/10.1158/1940-6207.CAPR-11-0542
- Villa LL, Costa RL, Petta CA, Andrade RP, Paavonen J, Iversen OE, Olsson SE, Hoye J, Steinwall M, Riis-Johannessen G, et al. High sustained efficacy of a prophylactic quadrivalent human papillomavirus types 6/11/16/18 L1 virus-like particle vaccine through 5 years of follow-up. Br J Cancer 2006; 95(11):1459-66; PMID:17117182; http://dx.doi.org/10.1038/sj.bjc.6603469
- Lehtinen M, Paavonen J, Wheeler CM, Jaisamrarn U, Garland SM, Castellsague X, Skinner SR, Apter D, Naud P, Salmeron J, et al. Overall efficacy of HPV-16/18 AS04-adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol 2012; 13(1):89-99; PMID:22075171; http://dx.doi.org/10.1016/S1470-2045(11)70286-8
- International Agency for Research on Cancer. IARC Working Group Reports: Primary End-points for Prophylactic HPV Vaccine Trials. Chapter 1. Lyon, France; 2014. Report No.: 7.
- Paavonen J, Naud P, Salmeron J, Wheeler CM, Chow SN, Apter D, Kitchener H, Castellsague X, Teixeira JC, Skinner SR, et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet 2009; 374(9686):301-14; PMID:19586656; http://dx.doi.org/10.1016/S0140-6736(09)61248-4
- Herrero R, Wacholder S, Rodriguez AC, Solomon D, Gonzalez P, Kreimer AR, Porras C, Schussler J, Jimenez S, Sherman ME, et al. Prevention of persistent human papillomavirus infection by an HPV16/18 vaccine: a community-based randomized clinical trial in Guanacaste, Costa Rica. Cancer Discov 2011; 1(5):408-19; PMID:22586631; http://dx.doi.org/10.1158/2159-8290.CD-11-0131
- Szarewski A, Skinner SR, Garland SM, Romanowski B, Schwarz TF, Apter D, Chow SN, Paavonen J, Del Rosario-Raymundo MR, Teixeira JC, et al. Efficacy of the HPV-16/18 AS04-adjuvanted vaccine against low-risk HPV types (PATRICIA randomized trial): an unexpected observation. J Infect Dis 2013; 208(9):1391-6; PMID:24092907; http://dx.doi.org/10.1093/infdis/jit360
- Breitburd F, Kirnbauer R, Hubbert NL, Nonnenmacher B, Trin-Dinh-Desmarquet C, Orth G, Schiller JT, Lowy DR. Immunization with viruslike particles from cottontail rabbit papillomavirus (CRPV) can protect against experimental CRPV infection. J Virol 1995; 69(6):3959-63; PMID:7745754
- Suzich JA, Ghim SJ, Palmer-Hill FJ, White WI, Tamura JK, Bell JA, Newsome JA, Jenson AB, Schlegel R. Systemic immunization with papillomavirus L1 protein completely prevents the development of viral mucosal papillomas. Proc Natl Acad Sci U S A 1995; 92(25):11553-7; PMID:8524802; http://dx.doi.org/10.1073/pnas.92.25.11553
- Schiller JT, Lowy DR. Immunogenicity testing in human papillomavirus virus-like-particle vaccine trials. J Infect Dis 2009; 200(2):166-71; PMID:19519255; http://dx.doi.org/10.1086/599988
- Pedersen C, Petaja T, Strauss G, Rumke HC, Poder A, Richardus JH, Spiessens B, Descamps D, Hardt K, Lehtinen M, et al. Immunization of early adolescent females with human papillomavirus type 16 and 18 L1 virus-like particle vaccine containing AS04 adjuvant. J Adolesc Health 2007; 40(6):564-71; PMID:17531764; http://dx.doi.org/10.1016/j.jadohealth.2007.02.015
- Block SL, Nolan T, Sattler C, Barr E, Giacoletti KE, Marchant CD, Castellsague X, Rusche SA, Lukac S, Bryan JT, et al. Comparison of the immunogenicity and reactogenicity of a prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in male and female adolescents and young adult women. Pediatrics 2006; 118(5):2135-45; PMID:17079588; http://dx.doi.org/10.1542/peds.2006-0461
- Lyseng-Williamson KA. Human Papillomavirus-16/18 AS04-Adjuvanted Vaccine (Cervarix): A Guide to Its Two-Dose Schedule in Girls Aged 9-14 Years in the EU. Paediatr Drugs 2014; 16(3):247–53; PMID:24659468
- Robbins HA, Li Y, Porras C, Pawlita M, Ghosh A, Rodriguez AC, Schiffman M, Wacholder S, Kemp TJ, Gonzalez P, et al. Glutathione S-transferase L1 multiplex serology as a measure of cumulative infection with human papillomavirus. BMC Infect Dis 2014; 14:120; PMID:24588945; http://dx.doi.org/10.1186/1471-2334-14-120
- Wilson L, Pawlita M, Castle PE, Waterboer T, Sahasrabuddhe V, Gravitt PE, Schiffman M, Wentzensen N. Seroprevalence of 8 oncogenic human papillomavirus genotypes and acquired immunity against reinfection. J Infect Dis 2014; 210(3):448-55; PMID:24569064; http://dx.doi.org/10.1093/infdis/jiu104
- Waterboer T, Sehr P, Michael KM, Franceschi S, Nieland JD, Joos TO, Templin MF, Pawlita M. Multiplex human papillomavirus serology based on in situ-purified glutathione s-transferase fusion proteins. Clin Chem 2005; 51(10):1845-53; PMID:16099939; http://dx.doi.org/10.1373/clinchem.2005.052381
- Luxembourg A, the V503 Program Team. An overview of the 9-valent HPV L1 virus-like particle vaccine clinical development program. Florence, Italy: EUROGIN; 2013; 101
- Kaufmann AM, Nieland JD, Jochmus I, Baur S, Friese K, Gabelsberger J, Gieseking F, Gissmann L, Glasschroder B, Grubert T, et al. Vaccination trial with HPV16 L1E7 chimeric virus-like particles in women suffering from high grade cervical intraepithelial neoplasia (CIN 2/3). Int J Cancer 2007; 121(12):2794-800; PMID:17721997; http://dx.doi.org/10.1002/ijc.23022
- Sehr P, Rubio I, Seitz H, Putzker K, Ribeiro-Muller L, Pawlita M, Muller M. High-throughput pseudovirion-based neutralization assay for analysis of natural and vaccine-induced antibodies against human papillomaviruses. PLoS One 2013; 8(10):e75677; PMID:24124504; http://dx.doi.org/10.1371/journal.pone.0075677
- Robbins HA, Kemp TJ, Porras C, Rodriguez AC, Schiffman M, Wacholder S, Gonzalez P, Schiller J, Lowy D, Poncelet S, et al. Comparison of antibody responses to human papillomavirus vaccination as measured by three assays. Front Oncol 2014; 3:328; PMID:24455487; http://dx.doi.org/10.3389/fonc.2013.00328
- Safaeian M, Porras C, Pan Y, Kreimer A, Schiller JT, Gonzalez P, Lowy DR, Wacholder S, Schiffman M, Rodriguez AC, et al. Durable antibody responses following one dose of the bivalent human papillomavirus L1 virus-like particle vaccine in the Costa Rica Vaccine Trial. Cancer Prev Res (Phila) 2013; 6(11):1242-50; PMID:24189371; http://dx.doi.org/10.1158/1940-6207.CAPR-13-0203
- Michael KM, Waterboer T, Sehr P, Rother A, Reidel U, Boeing H, Bravo IG, Schlehofer J, Gartner BC, Pawlita M. Seroprevalence of 34 human papillomavirus types in the German general population. PLoS Pathog 2008; 4(6):e1000091; PMID:18566657; http://dx.doi.org/10.1371/journal.ppat.1000091
- Rizk RZ, Christensen ND, Michael KM, Muller M, Sehr P, Waterboer T, Pawlita M. Reactivity pattern of 92 monoclonal antibodies with 15 human papillomavirus types. J Gen Virol 2008; 89(Pt 1):117-29; PMID:18089735; http://dx.doi.org/10.1099/vir.0.83145-0
- Waterboer T, Sehr P, Pawlita M. Suppression of non-specific binding in serological Luminex assays. J Immunol Methods 2006; 309(1-2):200-4; PMID:16406059; http://dx.doi.org/10.1016/j.jim.2005.11.008
- Kemp TJ, Hildesheim A, Safaeian M, Dauner JG, Pan Y, Porras C, Schiller JT, Lowy DR, Herrero R, Pinto LA. HPV16/18 L1 VLP vaccine induces cross-neutralizing antibodies that may mediate cross-protection. Vaccine 2011; 29(11):2011-4; PMID:21241731; http://dx.doi.org/10.1016/j.vaccine.2011.01.001
- Safaeian M, Kemp TJ, Pan DY, Porras C, Rodriguez AC, Schiffman M, Cortes B, Katki H, Wacholder S, Schiller JT, et al. Cross-protective vaccine efficacy of the bivalent HPV vaccine against HPV31 is associated with humoral immune responses: results from the Costa Rica Vaccine Trial. Hum Vaccin Immunother 2013; 9(7):1399-406; PMID:23571174; http://dx.doi.org/10.4161/hv.24340
- Poynten IM, Waterboer T, Jin F, Templeton DJ, Prestage G, Donovan B, Pawlita M, Fairley CK, Garland SM, Grulich AE. Human papillomavirus types 6 and 11 seropositivity: risk factors and association with ano-genital warts among homosexual men. J Infect 2013; 66(6):503-11; PMID:23528181; http://dx.doi.org/10.1016/j.jinf.2013.03.005
- Opalka D, Matys K, Bojczuk P, Green T, Gesser R, Saah A, Haupt R, Dutko F, Esser MT. Multiplexed serologic assay for nine anogenital human papillomavirus types. Clin Vaccine Immunol 2010; 17(5):818-27; PMID:20237197; http://dx.doi.org/10.1128/CVI.00348-09
- Kreimer AR, Johansson M, Waterboer T, Kaaks R, Chang-Claude J, Drogen D, Tjonneland A, Overvad K, Quiros JR, Gonzalez CA, et al. Evaluation of human papillomavirus antibodies and risk of subsequent head and neck cancer. J Clin Oncol 2013; 31(21):2708-15; PMID:23775966; http://dx.doi.org/10.1200/JCO.2012.47.2738
- Herrero R, Hildesheim A, Rodriguez AC, Wacholder S, Bratti C, Solomon D, Gonzalez P, Porras C, Jimenez S, Guillen D, et al. Rationale and design of a community-based double-blind randomized clinical trial of an HPV 16 and 18 vaccine in Guanacaste, Costa Rica. Vaccine 2008; 26(37):4795-808; PMID:18640170; http://dx.doi.org/10.1016/j.vaccine.2008.07.002
- Kemp TJ, Garcia-Pineres A, Falk RT, Poncelet S, Dessy F, Giannini SL, Rodriguez AC, Porras C, Herrero R, Hildesheim A, et al. Evaluation of systemic and mucosal anti-HPV16 and anti-HPV18 antibody responses from vaccinated women. Vaccine 2008; 26(29-30):3608-16; PMID:18541349; http://dx.doi.org/10.1016/j.vaccine.2008.04.074
- Kemp TJ, Safaeian M, Hildesheim A, Pan Y, Penrose KJ, Porras C, Schiller JT, Lowy DR, Herrero R, Pinto LA. Kinetic and HPV infection effects on cross-type neutralizing antibody and avidity responses induced by Cervarix((R)). Vaccine 2012; 31(1):165-70; PMID:23123024; http://dx.doi.org/10.1016/j.vaccine.2012.10.067
- Carter JJ, Paulson KG, Wipf GC, Miranda D, Madeleine MM, Johnson LG, Lemos BD, Lee S, Warcola AH, Iyer JG, et al. Association of Merkel cell polyomavirus-specific antibodies with Merkel cell carcinoma. J Natl Cancer Inst 2009; 101(21):1510-22; PMID:19776382; http://dx.doi.org/10.1093/jnci/djp332
- Clifford GM, Shin HR, Oh JK, Waterboer T, Ju YH, Vaccarella S, Quint W, Pawlita M, Franceschi S. Serologic response to oncogenic human papillomavirus types in male and female university students in Busan, South Korea. Cancer Epidemiol Biomarkers Prev 2007; 16(9):1874-9; PMID:17855708; http://dx.doi.org/10.1158/1055-9965.EPI-07-0349
- Dessy FJ, Giannini SL, Bougelet CA, Kemp TJ, David MP, Poncelet SM, Pinto LA, Wettendorff MA. Correlation between direct ELISA, single epitope-based inhibition ELISA and pseudovirion-based neutralization assay for measuring anti-HPV-16 and anti-HPV-18 antibody response after vaccination with the AS04-adjuvanted HPV-16/18 cervical cancer vaccine. Hum Vaccin 2008; 4(6):425-34; PMID:18948732; http://dx.doi.org/10.4161/hv.4.6.6912
- Pastrana DV, Buck CB, Pang YY, Thompson CD, Castle PE, FitzGerald PC, Kruger KS, Lowy DR, Schiller JT. Reactivity of human sera in a sensitive, high-throughput pseudovirus-based papillomavirus neutralization assay for HPV16 and HPV18. Virology 2004; 321(2):205-16; PMID:15051381; http://dx.doi.org/10.1016/j.virol.2003.12.027
- Opalka D, Lachman CE, MacMullen SA, Jansen KU, Smith JF, Chirmule N, Esser MT. Simultaneous quantitation of antibodies to neutralizing epitopes on virus-like particles for human papillomavirus types 6, 11, 16, and 18 by a multiplexed luminex assay. Clin Diagn Lab Immunol 2003; 10(1):108-15; PMID:12522048
- Dias D, Van DJ, Schlottmann S, Kelly S, Puchalski D, Ruiz W, Boerckel P, Kessler J, Antonello JM, Green T, et al. Optimization and validation of a multiplexed luminex assay to quantify antibodies to neutralizing epitopes on human papillomaviruses 6, 11, 16, and 18. Clin Diagn Lab Immunol 2005; 12(8):959-69; PMID:16085914
