Abstract
The development of anti-staphylococcal vaccines is nowadays a priority to prevent surgical site infections (SSI). The objective of the present study was to identify a potential target population by assessing surveillance data on surgery patients for possible anti-staphylococcal vaccine administration. Individuals at high risk of SSI by Staphylococcus aureus (SA) were targeted by the French SSI Surveillance Network in south-eastern France between 2008 and 2011. Among 238,470 patients, those undergoing primary total hip replacement appeared to be an interesting and healthy enough population for anti-staphylococcal vaccine testing. These male patients, subjected to multiple procedures and with American Society of Anesthesiologists score >2, had a probability of SA SSI about 21 times higher than females with no severe systemic disease and no multiple procedures. Our study indicates that surveillance data on SSI might be an interesting epidemiological source for planning vaccine trials to prevent nosocomial infections.
Introduction
Vaccines have been proposed as promising preventive interventions against hospital-acquired infections by bacteria such as Staphylococcus aureusCitation1 (SA) or Clostridium difficile,Citation2 and viruses, such as influenza virus.Citation3-5 Surgical site infections (SSI) are the third most frequent healthcare-associated events, and SA is the main etiological agent. Methicillin-resistant SA SSI, which increase length of hospital stay, costs and mortality, are now recognized as a public health imperative.Citation6,7
Anti-staphylococcal vaccines might be alternatives to antibiotics with no risk of antimicrobial resistance. Ongoing anti-staphylococcal vaccine development has not yet been successful in humans,Citation8 but potential causes of failure have been reported recently.Citation9,10 One stage of anti-staphylococcal vaccine development is the identification of appropriate target populations, especially surgical patients. In addition, to test vaccine efficacy, target populations must be at high risk of SA SSI, but healthy enough to mount effective immune responses. Patient populations hospitalized for planned surgery may have time to develop effective immune responses before surgery. They may be reliable populations for the demonstration of vaccine efficacy. Surveillance data are being explored regularly to identify independent risk factors of SSICitation11 as they provide opportunities to compute cumulative risks and distinguish patients at high risk.
The objective of the present study was to identify a target population for testing anti-staphylococcal vaccine efficacy through a large SSI surveillance network in France.
Results
Among 290,121 patients under surveillance, 18,051 with missing data, 32,029 subjected to emergency procedures, 132 with American Society of Anesthesiologists (ASA) score of 5 and 1,439 SSI by agent other than SA were excluded (). A total of 238,470 were analyzed. These patients were allocated to 647 participating wards for 65 surgical procedures. Patient characteristics are summarized in . Median patient age at admission was 60.8 years, with 130,317 (54.6%) being female. In the analyzed dataset, 426 SA SSI (0.2%) occurred.
Table 1. Patient and surgical procedure characteristics with SA SSI ORs of risk factors (fixed effects of mixed multivariate logistic regression)
Figure 1. Flow chart of hospitalizations in the French SSI Surveillance Network during the period 2008–2011. Abbreviation: SA SSI: surgical site infections by Staphylococcus aureus. The right side of the figure reports successively excluded observations, and the left side, the remaining numbers of SA SSI.
reports the odds ratios (ORs) of SA SSI according to different risk factors. Factors carrying the most risk were duration of surgery, wound contamination class, ASA score, preoperative hospital stay, and gender. Because of adjustment for all risk factors, the effect of age was weak and non-significant (P = 0.29).
gives the predicted adjusted risk of SA SSI by surgical procedure independently of surgical ward (reference level for each covariate). Among 9 surgical procedures with higher risk (predicted adjusted incidence > 0.5 per 1,000 patients), 7 benefited from priority surveillance according to national objectives: 3 orthopedic surgical procedures for hip replacement, 2 procedures related to gynecology and 2 neurosurgeries. Orthopedic operations (23,455/50,146) were primarily retained because they were the most frequent procedures (47%) included in priority surveillance. Hip replacement revision was excluded because patients’ previous status was unknown. The remaining procedures were undertaken for primary total or partial hip prostheses. Their crude SA SSI incidence was 3.5 and 8.5 per 1,000 patients, respectively.
Figure 2. Predicted Staphylococcus aureus surgical site infection (SA SSI) rate according to number of interventions per operative procedure category. Each plot represents an operating procedure. SA SSI rates are expressed in terms of SA SSI per 1,000 patients. Triangles represent procedures with the highest SSI rate (>0.5 per 1,000 patients), orthopedic surgeries are in gray [NTHR: non-total hip replacement; HRR: hip replacement revision; THR: total hip replacement; BRES: breast surgery; CSEC: cesarean section; LAM: laminectomy; DISH: lumbar disc hernia surgery; CABG: coronary artery bypass graft; ABLA: ablation of ostheosynthesis materials].
![Figure 2. Predicted Staphylococcus aureus surgical site infection (SA SSI) rate according to number of interventions per operative procedure category. Each plot represents an operating procedure. SA SSI rates are expressed in terms of SA SSI per 1,000 patients. Triangles represent procedures with the highest SSI rate (>0.5 per 1,000 patients), orthopedic surgeries are in gray [NTHR: non-total hip replacement; HRR: hip replacement revision; THR: total hip replacement; BRES: breast surgery; CSEC: cesarean section; LAM: laminectomy; DISH: lumbar disc hernia surgery; CABG: coronary artery bypass graft; ABLA: ablation of ostheosynthesis materials].](/cms/asset/de0a551c-8a0f-4d36-89c7-c88cb7c3dfc9/khvi_a_979625_f0002_b.gif)
Among the 22,113 patients retained, those most at risk of SA SSI were i) men: 44% of total hip replacements (OR = 2.3; 95% confidence interval (CI): 1.3–4.0) and 29% of partial hip replacements (OR = 2.1; 95% CI: 1.0–4.5), ii) patients with ASA score > 2: 25% of total hip replacements (OR = 2.6; 95% CI: 1.5–4.4), and iii) patients undergoing multiple procedures: 2% of total hip replacements (OR = 3.8; 95% CI: 1.3–11) and 4% of partial hip replacements (OR = 5.6; 95% CI: 2.0–16).
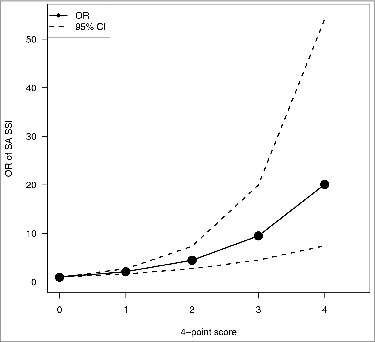
Four-point additive scores were computed for each patient according to gender (female = 0 / male = 1), ASA score (“1” = 0, “2” = 1, “> 2” = 2), and multiple procedures (absence = 0, presence = 1). depicts the association between score and the risk of SA SSI in patients undergoing primary hip replacement. The probability of SA SSI was about 2.1 times higher when scores increased by 1 point. Patients with a score of 4 had SA SSI probability about 21 times higher than patients with a score of 0.
Discussion
The objective of our study was to identify patients who could be a potential target population for anti-staphylococcal vaccination before planned surgery. Appropriate statistical modeling of surveillance data led to select primary hip prostheses implantation surgery as being of high risk for SA SSI among priority surgical procedures. Our results confirmed the impact of underlying diseases and multiple surgical procedures on the probability of infection. Among preoperative risk factors, age appeared to have no effect on infection risk, likely because of its association with other covariates and its small variability in the retained population. Estimation of a 4-point score with patient gender, ASA score and the presence or absence of multiple procedures was a convenient way of selecting patients most at risk at hospital admission and contributed to surveillance data evaluation. Patients who cumulate these characteristics may represent a population with the most to gain from anti-staphylococcal vaccination.
Anti-staphylococcal vaccination
Anti-staphylococcal vaccine development could represent valuable progress in preventing hospital-acquired infections with high attack rates and related antimicrobial resistance. Such advances remain challenging for many reasons, including the choice of antigens,Citation12-14 and target populations for vaccine efficacy and effectiveness calculation. Large clinical trials implemented in high-risk populations, namely hemodialysis patients and patients undergoing cardiac surgery, failed to find vaccine efficacy which may have been partially linked to immune dysfunction and/or population selection.Citation9 A worthwhile population for anti-staphylococcal vaccine testing would be healthy enough and immuno-competent. Fortunately, based on our results, many patients undergoing primary total hip replacement exhibited one of these characteristics: 75% had no severe systemic disease, and very few (0.3%) were exposed to cancer surgery that would likely have disclosed immune deficiency. To the best of our knowledge, anti-staphylococcal vaccines have never been tested in patients undergoing total hip replacement, and no results have yet been published.
Use of surveillance data in clinical vaccine studies/projects
The advantage of surveillance data is their timeliness, geographic coverage and large number of patients sampled. However, they provide limited covariates for etiological analysis. They fail to systematically report patients’ co-morbidities (diabetes, immune status, cigarette smoking, etc.) that could impact immune responses to vaccination. The dearth of data on preoperative and postoperative antibiotic administration is a limitation of our study. Moreover, no information on SA nasal carriage was obtained. While the literature acknowledges that SA nasal carriage is a major SSI risk, the efficacy of nasal decontamination in orthopedic surgery remains controversial.Citation15-17 To date, the utility of anti-staphylococcal vaccines against nasal carriage has not been demonstrated.Citation18 Vaccination would be of great interest independently of nasal carrier status. Moreover, the criteria applied to surveillance data to detect SSI cases underestimate SSI in part because they do not account for SSI occurring more than 1 month after surgery. This underestimation is highlighted in the literature.Citation19-21 Interesting facets of surveillance data in planned clinical vaccine studies are the capacity: i) to ascertain accurate incidence of SSI, ii) to facilitate sample size calculation for randomized clinical trials, and iii) to provide information for clinical or economic impact evaluation by modeling.Citation1 Anti-staphylococcal vaccine outcomes could be estimated from large surveillance databases according to expected or simulated efficacy or effectiveness. In addition, vaccine impact could be evaluated not only on SA incidence but also on other variables, such as mortality, length of hospital stay, and costs.
Vaccine approaches are being explored to control other hospital-acquired infections, such as Clostridium difficile, Pseudomonas aeruginosa as well as influenza. Our results indicate that surveillance data on surgical infections could be a valuable tool to identify target populations for clinical vaccine studies on SA.
Methods
The study population consisted of patients hospitalized for surgery in wards participating in the national SSI Surveillance Network in south-eastern France (Center de Coordination de la Lutte contre les Infections Nosocomiales [CClin] Sud-Est Network) between 2008 and 2011 [http://cclin-sudest.chu-lyon.fr/Reseaux/accueil.html]. This population and the surveillance network have already been described in detail elsewhere.Citation22
The outcome was SA SSI occurrence. SA SSI were defined as infections involving only skin and subcutaneous tissue at incision site within 30 d after surgical procedures, with SA isolated from aseptically-obtained fluid or tissue cultures from superficial incisions. Complete case analysis was performed. Data were supposed to be missing completely at random and we checked for no association between outcome and missing data to verify that list-wise deletion implied no potential bias. Elective surgical procedures were included. Cases with ASA score of 5 and classified as moribund were excluded, as were cases infected by agent other than SA.
First, the surgical procedures were ranked by decreasing SA SSI probability, using a multilevel, multivariate logistic regression model adjusted for: i) preoperative risk factors, depending on patients (age, gender, ASA score) and surgical procedures (wound class, preoperative hospital stay), ii) presence or absence of multiple surgeries, and iii) intraoperative risk factors (duration of operation). Random effects were considered for: i) surgical procedures and, independently, ii) surgical procedures nested in the surgical ward, leading to a hierarchical model with 3 levels: patient, ward and procedure. Interventions carrying the highest risk were then selected. Finally, for patients undergoing selected, pooled procedures, a 4-point preoperative scoring system was built on the basis of significant predictive risk factors to estimate populations with cumulated risk factors. SA SSI risk was estimated by ORs according to scores reported as dose-response curves.
Statistical analyses were performed with R language version 3.0.2 available at http://cran.r-project.org with the lme4 and Epi R packages. Known risk factors of subsequent SA SSI were assessed by monovariate logistic regression (data not reported). ORs of logistic models were estimated with 95% CI. All covariates, whatever the significance of bivariate testing, were entered into multivariate regression models, and 10% was retained as level of significance. Anonymous use of databases has been approved under French national regulations and does not warrant research ethics approval.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Authors’ Contribution
Marie-Paule Gustin analyzed-interpreted the data and drafted the article under supervision by Philippe Vanhems. Thomas Bénet and Philippe Vanhems conceived the study, participated in drafting the manuscript and revised it critically. Marine Giard was responsible for study design and data acquisition. All authors approved the final version of the article.
Acknowledgments
The authors thank Dr Anne Savey and the Scientific Committee of the CClin ISO Sud-Est Network for their close cooperation. Ovid Da Silva edited this manuscript.
Funding
The parent study and the current analysis were sponsored and funded by Merck.
References
- Song Y, Tai JH, Bartsch SM, Zimmerman RK, Muder RR, Lee BY. The potential economic value of a Staphylococcus aureus vaccine among hemodialysis patients. Vaccine 2012; 30(24):3675-82; PMID:22464963; http://dx.doi.org/10.1016/j.vaccine.2012.03.031
- Rebeaud F, Bachmann MF. Immunization strategies for clostridium difficile infections. Expert Rev Vaccines 2012; 11(4):469-79; PMID:22551032; http://dx.doi.org/10.1586/erv.12.18
- Voirin N, Barret B, Metzger MH, Vanhems P. Hospital-acquired influenza: a synthesis using the outbreak reports and intervention studies of nosocomial infection (ORION) statement. J Hosp Infect 2009; 71(1):1-14; PMID:18952319; http://dx.doi.org/10.1016/j.jhin.2008.08.013
- Vanhems P, Voirin N, Roche S, Escuret V, Regis C, Gorain C, Pires-Cronenberger S, Giard M, Lina B, Najioullah F, et al. Risk of influenza-like illness in an acute health care setting during community influenza epidemics in 2004-2005, 2005-2006, and 2006-2007: a prospective study. Arch Intern Med 2011; 171:151-7; PMID:21263105; http://dx.doi.org/10.1001/archinternmed.2010.500
- Bénet T, Régis C, Voirin N, Robert O, Lina B, Cronenberger S, Comte B, Coppéré B, Vanhems P. Influenza vaccination of healthcare workers in acute-care hospitals: a case-control study of its effect on hospital-acquired influenza among patients. BMC Infect Dis 2012; 12:30; http://dx.doi.org/10.1186/1471-2334-12-30
- Fry DE. The continued challenge of Staphylococcus aureus in the surgical patient. Am Surg 2013; 79(1):1-10; PMID:23317583
- Anderson DJ, Sexton DJ, Kanafani ZA, Auten G, Kaye KS. Severe surgical site infection in community hospitals: epidemiology, key procedures, and the changing prevalence of methicillin-resistant Staphylococcus aureus. Infect Control Hosp Epidemiol 2007; 28(9):1047-53; PMID:17932825; http://dx.doi.org/10.1086/520731
- Fowler VG, Allen KB, Moreira ED, Moustafa M, Isgro F, Boucher HW, Corey GR, Carmeli Y, Betts R, Hartzel JS, et al. Effect of an investigational vaccine for preventing Staphylococcus aureus infections after cardiothoracic surgery: a randomized trial. JAMA 2013; 309(13):1368-78; PMID:23549582; http://dx.doi.org/10.1001/jama.2013.3010
- Jansen KU, Girgenti DQ, Scully IL, Anderson AS. Vaccine review: “Staphylococcus aureus vaccines: problems and prospects.” Vaccine 2013; 31(25):2723-30; PMID:23624095; http://dx.doi.org/10.1016/j.vaccine.2013.04.002
- Kobayashi SD, DeLeo FR. Staphylococcus aureus protein A promotes immune suppression. MBio 2013; 4(5):e00764-13; PMID:24085782; http://dx.doi.org/10.1128/mBio.00764-13
- Jarvis W.R. (Editor)J, Bennett and Brachman's Hospital infections., Lippincott Williams & Wilkins, Philadelphia, 2007.
- Daum RS, Spellberg B. Progress toward a Staphylococcus aureus vaccine. Clin Infect Dis 2012; 54(4):560-7; PMID:22186773; http://dx.doi.org/10.1093/cid/cir828
- Fritz SA, Tiemann KM, Hogan PG, Epplin EK, Rodriguez M, Al-Zubeidi DN, Wardenburg JB. A serologic correlate of protective immunity against community-onset Staphylococcus aureus infection. Clin Infect Dis 2013; 56(11):1554-61; PMID:23446627; http://dx.doi.org/10.1093/cid/cit123
- Botelho-Nevers E, Verhoeven P, Paul S, Grattard F, Pozzetto B, Berthelot P, Lucht F. Staphylococcal vaccine development: review of past failures and plea for a future evaluation of vaccine efficacy not only on staphylococcal infections but also on mucosal carriage. Expert Rev Vaccines 2013; 12(11):1249-59; PMID:24111513; http://dx.doi.org/10.1586/14760584.2013.840091
- Levy PY, Ollivier M, Drancourt M, Raoult D, Argenson JN. Relation between nasal carriage of Staphylococcus aureus and surgical site infection in orthopedic surgery: the role of nasal contamination. A systematic literature review and meta-analysis. Orthop Traumatol Surg Res 2013; 99(6):645-51; PMID:23992764; http://dx.doi.org/10.1016/j.otsr.2013.03.030
- Chen AF, Wessel CB, Rao N. Staphylococcus aureus screening and decolonization in orthopaedic surgery and reduction of surgical site infections. Clin Orthop Relat Res 2013; 471:2383-99; PMID:23463284; http://dx.doi.org/10.1007/s11999-013-2875-0
- Verhoeven PO, Berthelot P, Chapelle C, Gagnaire J, Grattard F, Pozzetto B, Farizon F, Lucht F, Botelho-Nevers E. Letter to the editor: Staphylococcus aureus screening and decolonization in orthopaedic surgery and reduction of surgical site infections. Clin Orthop Relat Res 2013; 471:3709-11; PMID:24014267; http://dx.doi.org/10.1007/s11999-013-3254-6
- Creech CB 2nd, Johnson BG, Alsentzer AR, Hohenboken M, Edwards KM, Talbot TR 3rd. Vaccination as infection control: a pilot study to determine the impact of Staphylococcus aureus vaccination on nasal carriage. Vaccine 2009; 28(1):256-60; PMID:19799842; http://dx.doi.org/10.1016/j.vaccine.2009.09.088
- Atchley KD, Pappas JM, Kennedy AT, Coffin SE, Gerber JS, Fuller SM, Spray TL, McCardle K, Gaynor JW. Use of administrative data for surgical site infection surveillance after congenital cardiac surgery results in inaccurate reporting of surgical site infection rates. Ann Thorac Surg 2014; 97(2):651-7; PMID:24365216; http://dx.doi.org/10.1016/j.athoracsur.2013.08.076
- Leaper D, Tanner J, Kiernan M. Surveillance of surgical site infection: more accurate definitions and intensive recording needed. J Hosp Infect 2013; 83(2):83-6; PMID:23332350; http://dx.doi.org/10.1016/j.jhin.2012.11.013
- Rosenthal VD, Richtmann R, Singh S, Apisarnthanarak A, Kübler A, Viet-Hung N, Ramírez-Wong FM, Portillo-Gallo JH, Toscani J, Gikas A, et al. International nosocomial infection control consortium. surgical site infections, international nosocomial infection control consortium (INICC) report, data summary of 30 countries, 2005-2010. Infect Control Hosp Epidemiol 2013; 34(6):597-604; PMID:23651890; http://dx.doi.org/10.1086/670626
- Couris CM, Rabilloud M, Ecochard R, Metzger MH, Caillat-Vallet E, Savey A, Fabry J, Vanhems P. Nine-year downward trends in surgical site infection rate in southeast France (1995-2003). J Hosp Infect 2007; 67(2):127-34; PMID:17900755; http://dx.doi.org/10.1016/j.jhin.2007.07.013