Abstract
Autologous dendritic cells (DCs) loaded with tumor-associated antigens (TAAs) are a promising immunological tool for cancer therapy. These stimulate the antitumor response and immunological memory generation. Nevertheless, many patients remain refractory to DC approaches. Antigen (Ag) delivery to DCs is relevant to vaccine success, and antigen peptides, tumor-associated proteins, tumor cells, autologous tumor lysates, and tumor-derived mRNA have been tested as Ag sources. Recently, DCs loaded with allogeneic tumor cell lysates were used to induce a potent immunological response. This strategy provides a reproducible pool of almost all potential Ags suitable for patient use, independent of MHC haplotypes or autologous tumor tissue availability. However, optimizing autologous tumor cell lysate preparation is crucial to enhancing efficacy. This review considers the role of cancer cell-derived lysates as a relevant source of antigens and as an activating factor for ex vivo therapeutic DCs capable of responding to neoplastic cells. These promising therapies are associated with the prolonged survival of advanced cancer patients.
Abbreviations
| TRIMEL | = | Allogeneic melanoma cell lysate |
| TRIPRO | = | Allogeneic prostate cell lysate |
| Ags | = | Antigens |
| CRT | = | Calreticulin |
| CDAMs | = | Cell death-associated molecules |
| AM | = | Cytokine-activated monocytes |
| CTLs | = | Cytotoxic T lymphocytes |
| DAMPs | = | Damage-associated molecular patterns |
| DTH | = | Delayed-type IV hypersensitivity |
| DCs | = | Dendritic cells |
| GM-CSF | = | Granulocyte and macrophage colony stimulating factor |
| HSPs | = | Heat shock proteins |
| HMGB1 | = | High-mobility group box 1 protein |
| ICD | = | Immunogenic cell death |
| MHC | = | Major histocompatibility complex |
| MM | = | Malignant melanoma |
| MAAs | = | Melanoma-associated antigens |
| NKT | = | Natural killer T cell |
| PAMPs | = | Pathogen-associated molecular patterns |
| PRRs | = | Pattern recognition receptors |
| PBMCs | = | Peripheral blood mononuclear cells |
| PD1 | = | Programmed cell death protein 1 |
| PCCL | = | Prostate cancer cell lysate |
| PSA | = | Prostate specific antigen |
| RAGE | = | Receptor for advanced glycation endproducts |
| Tregs | = | Regulatory T lymphocytes |
| SNPs | = | Single nucleotide polymorphisms |
| TCRs | = | T cell receptors |
| TLRs | = | Toll-like receptors |
| TAPCells | = | Tumor antigen presenting cells |
| TNF | = | Tumor necrosis factor |
| TAAs | = | Tumor-associated antigens |
Introduction
Standard treatments for disseminated tumors, through the use of surgical or radio/chemotherapy procedures, provide limited results that rarely change the disease outcome. During the last 2 decades, several immunotherapy approaches have been tested as alternative treatments against solid and hematological malignancies.Citation1 The use of recombinant cytokines such as IL-2 and IFN-α, T cell-mediated adoptive therapies, monoclonal antibodies, and dendritic cells (DCs)-based vaccines, among others, have provided improvements in the control of tumor growth and patient survival.Citation1 However, despite the relative effectiveness of these treatments, disadvantages are still prevalent in a large proportion of cases in regards to the percentage of refractory patients and side effects.
Although the adoptive transfer of tumor-specific CD8+ T cells with or without systemic immune suppression has been used in different clinical trials and has resulted in significant reductions of tumor size, some relevant adverse reactions were observed in treated patients.Citation2-4 More recently, the re-infusion of ex vivo, genetically manipulated, autologous CD8+ T cells that express high-affinity T cell receptors (TCRs) for melanoma-specific antigens has shown promising results in relation to tumor regression.Citation5 In this context, the identification of new therapeutic targets in melanoma and immune cells has enabled the development of a wide range of monoclonal antibodies aimed at modulating the antitumor T cell-mediated immune response and at eradicating tumors.Citation6,7 In 2011, the United States Food and Drug Administration and the European Medicines Agency approved Ipilimumab, an anti-CTLA-4 monoclonal antibody, for the treatment of patients with advanced melanoma.Citation8 Furthermore, an anti-PD1 monoclonal antibody which targets a co-inhibitory molecule expressed in activated T lymphocytes has shown promising results in phase I and II clinical trials.Citation9-11 Immunotherapy based on monoclonal antibodies (mAbs) is effective in up to 30% of treated patients. However, this type of treatment is frequently limited in duration. Additionally, some types of cancers are not responsive to mAb-based immunotherapy. Currently, little is known about the mechanisms underlying the differential clinical responses of patients to mAb-based immunotherapy.Citation12 An additional strategy for cancer immunotherapy is the use of DCs as cell-based vaccines. DC-based vaccination strategies are likely to be safe and, most importantly, capable of providing long-lasting protective immunity.Citation13, 14 Nevertheless, although this strategy has been applied with success in clinical trials, an important percentage of treated patients remain refractory to these new approaches.
DCs are professional antigen presenting cells (APCs) that, upon encountering antigens (Ags), efficiently trigger adaptive immunity against pathogens and/or tumors,Citation13,15-19 thus establishing a link between the innate and adaptive arms of the immune system.Citation20 In humans, DCs that are found in blood, lymph nodes, and tonsils can be subdivided into plasmacytoid DCs and resident BDCA1+ DCs and BDCA3+ DCs.Citation21 The latter of these share similarities with CD8α+ DCs that are found in mice and that excel at antigen presentation to CD8α+ T cells.Citation22 In the skin, Langerhans cells, CD1a+ dermal DCs, and CD14+ dermal DCs can be found, with these having the ability to migrate to skin-draining lymph nodes.Citation21 In addition, DCs residing in the periphery may also originate from peripheral blood monocytes when they are recruited to these tissues by pro-inflammatory signals.Citation23 In fact, human DCs can be differentiated in vitro from several cellular sources, including bone marrow, umbilical cord blood, and peripheral blood mononuclear cells (PBMCs), by using a variety of cytokines and activating factors such as the granulocyte and macrophage colony stimulating factor (GM-CSF) and IL-4.Citation24-26
With respect to the application of DC-based immunotherapy, our group has performed a series of clinical trials in advanced malignant melanoma (MM) and prostate cancer patients using ex vivo-generated DC-like tumor antigen presenting cells (referred to as TAPCells). TAPCells are generated from autologous cytokine-activated monocytes (AM) using allogeneic cell lysates (referred to as TRIMEL and TRIPRO) derived from 3 melanoma and prostate cancer cell lines, respectively.Citation14,27-30 In these studies, we reported a correlation between the positive immune response induced by DC-vaccination, as established by a patient tumor-specific delayed-type IV hypersensitivity (DTH) reaction, and improved long-term patient survival in late-stage MM, which is an excellent predictor for clinical response.Citation14,27,28 Additionally, in prostate cancer patients, TAPCell-based immunotherapy is a safe approach capable of inducing memory T lymphocytes, which might be associated with clinical responses, including decreased serum Prostate Specific Antigen (PSA) levels and increased PSA doubling time.Citation31 Despite these positive outcomes, a large proportion of treated patients (∼40%) do not respond to the therapy and present the same survival rate as non-treated patients.Citation14,27,Citation28 This lack of response could be explained, at least in part, by absence of sufficient immunogenic danger signals, either during DCs ex vivo generation or immunization and/or to deficiencies in antigen processing and presentation by injected DCs, which might also be related to a deficient delivery of danger signals to DCs.Citation28
Several factors impact the efficacy of vaccination protocols using ex vivo-generated DCs. One of the most relevant aspects includes the expression and biological properties of specific receptors activated during DCs stimulation, mainly pattern recognition receptors (PRRs), in addition to the subsequent process of DCs maturation and activation in response to PRR triggering. Given this context, cancer vaccine approaches need to include a strategy for efficiently activating ex vivo-produced DCsCitation32 and, more importantly, for ensuring that this process results in a clinically effective and reproducible anticancer response in vivo. Taken together, these constraints support the imminent necessity to develop more tolerable, less expensive, and more effective therapeutic approaches that could particularly help patients with advanced metastatic disease.
The present report discusses the role of stressed, cancer cell-derived lysates as a strong immunological stimulus for therapeutically used ex vivo-generated DCs, in addition to exploring the potential contribution of these in developing more efficient DC-based immunotherapies against cancer.
Allogeneic tumor cell lysates as a source of antigens for loading of DCs
An effective antigen presentation from DCs for initiating specific anticancer cellular immunity requires optimal activation and migration to secondary draining lymphoid organsCitation13 where they can engage Ag-specific, naïve CD4+ and CD8+ T cells. This process results in T-cell activation, proliferation, and mobilization to peripheral tissues where these cells carryout effector functions.Citation13,27,Citation33-35
Remarkably, DCs are the main cell type able to present exogenous peptides loaded onto major histocompatibility complex (MHC) class I molecules to naïve CD8+ T cells in a process denoted cross-presentation.Citation36,37 The cross-presentation process has proved essential for the generation of cytotoxic T lymphocytes (CTLs) against viruses, transplanted cells, and tumor cells.Citation38,39 During an antitumor immune response, DC-mediated cross-presentation subsequent to the uptake and processing of material derived from apoptotic, necrotic, or even live cancer cells constitutes a relevant natural mode of TAA-presentation, thereby contributing to an efficient triggering of immunity against tumors.Citation40
Optimal delivery of tumor Ags is also a crucial aspect in DC-based immunotherapy success. Several methods for Ags preparation/delivery to DCs have been developed to improve capturing and presentation of Ags by DCs.Citation41 These methods include the use of RNA and DNA derived from tumor cells; TAA-derived synthetic peptides; the generation of recombinant proteins; tumor-derived apoptotic bodies; and transfection of DCs with vectors codifying for TAAs, among others. Synthetic peptide-based vaccines require knowledge of the patient's haplotypes and specific epitopes suitable for binding the MHC. Most clinical trials using synthetic peptide-induced immunological responses mediated by CD4+ and CD8+ T-cells have failed to produce objective clinical responses or improvements in patient survival.Citation42-45 One possible explanation may be due to the probable induction of tolerance through high affinity peptides, the limited persistence of peptide-MHC complexes on DCs, tumor escape by clones lacking antigen expression, or the absence of immunological danger signals associated with Ags.Citation28 The combined results of these studies suggest that peptide-loaded DC immunization could be a highly specific strategy, but further efforts are required to produce significant therapeutic effects.Citation42-44,46 Related to this, longer peptides (28–35 amino acids long) have been recently tested in cervical cancer patients and have been shown safe and immunogenic.Citation47-49 These peptides are unable to directly bind MHC molecules, and must be internalized and processed by DCs for presentation.Citation50 To date, one of the most successful immunotherapeutic approaches has been sipuleucel-T (Provenge®, Dendreon), which is the first FDA-approved cell-based therapy for the treatment of hormone-refractory prostate cancer patients.Citation51 Sipuleucel-T consists of autologous PBMCs, including DCs, B cells, monocytes, and natural killer (NK) cells, that have been activated ex vivo with a recombinant fusion protein containing prostatic acid phosphatase, a prostate cancer associated antigen, fused to GM-CSF. Sipuleucel-T has shown overall, prolonged survival with moderate side effects among men with metastatic castration-resistant prostate cancer.Citation51
Transfection of DCs with tumor-derived cDNA or mRNA appears to be an interesting approach for TAAs delivery. Furthermore, the mRNA coding for co-stimulatory molecules could be co-transfected to ensure the induction of a mature phenotype on DCs. Nevertheless, this technique allows the delivery of a limited amount of antigens due to a damaged integrity and viability of DCs.Citation52
Another tested method consists in loading DCs with attenuated pathogenic particles (derived from bacteria or viruses) containing genes encoding for TAAs with the purpose of inducing their expression coupled with pathogen associated molecular patterns (PAMPs).Citation52 Despite being an interesting concept, it is important to evaluate the immune response developed against DNA or proteins from the vector, which could limit the clinical efficacy of this approach.
Additionally, autologous tumor cell lysates, whole tumor cells, and tumor-derived mRNA have also been tested as antigen providers for DCs.Citation53-56 When fused or loaded with autologous tumor cells or tumor lysates, these cells induce a stronger and more extensive immunological response against tumors.Citation53-59 Still, these therapies are limited to a reduced proportion of patients that have tumor masses at surgically accessible sites, therefore ensuring the possibility of obtaining the amount of biological material required.
One of the simplest and most promising sources of tumor Ags is the preparation of allogeneic cancer cell lysates.Citation14,28-30,60-62 An advantage of this strategy is that it provides a standardized, applicable source of tumor-specific Ags, which is also useful in high-risk, tumor-free patients. Furthermore, allogeneic cancer cell lysates constitute a valuable alternative for obtaining immunogenic DCs ().Citation28,60,63,64
Table 1. Comparison of different strategies for antigen-delivery to DCs
Tumor cell lysates are excellent sources for delivering of a wide variety of Ags associated with MHC class I/II molecules, inducing a more integral immune response. Importantly, the method for inducing cell death or chemical protein modifications during whole tumor lysate preparation could impact the immunogenicity and efficacy of the therapy. Studies in murine models using DCs pulsed with tumor lysates have shown significant results in the induction of potent immune responses, as evidenced by the generation of specific CTLs against tumor Ags and a significant reduction of tumors in these animals.Citation65-67 Moreover, several studies using DCs loaded with mainly autologous, but also allogeneic, tumor lysates have been performed with positive results in mice and humans.Citation68-71 Likewise, positive results using allogeneic lysates from a variety of human tumor cell lines have been obtained in several clinical trials for the treatment of different types of cancer.Citation14,28, Citation72,73 However, a potential problem lays in the fact that some cancer cells are able to secrete immunoregulatory cytokines such as IL-10 and TGF-β during the cell culture process, thus inducing a more tolerogenic phenotype on DCs. Related to this, the majority of protocols designed to produce tumor cell lysates for DC loading include several washing steps, which result in a minimal amount of these cytokines present in preparations.Citation27 Taken together, these studies show that DCs pulsed with tumor lysates or apoptotic bodies are safe and capable of inducing immune responses in a broad vaccinated population, thus representing a promising approach for future studies. In this same line, TRIMEL, a tumor lysate generated from a mixture of 3 established metastatic melanoma cell lines, contains the majority of the currently described MAAs ().Citation28 In fact, we have previously shown that TAPCells induced IFN-γ release by an HLAA2+-restricted/MART-1–specific CD8+ T-cell clone (epitope MART-127-35), therefore highlighting the ability of TAPCells to cross-present exogenous MAAs in the context of MHC class I to melanoma-specific CD8+ T lymphocytes.Citation28 Tumor-associated danger signals derived from the lysate may be responsible for an efficient Ag cross-presentation process mediated by TAPCells.Citation28 Related to this, it is important to distinguish between the capacity of cancer cell lysates to provide a wide spectrum of TAAs from their capacity to provide danger signals that trigger the optimal maturation and activation of APCs.
Table 2. MAA expression of the 3 melanoma cell lines that compose the TRIMEL lysate.
Tumor cell lysates as immune activators in DC-based vaccines: Interplay of damage-associated molecular patterns (DAMPs), PRRs, and tumor immunogenic cell death (ICD)
Recent studies suggest that DCs, through their PRRs, interpret signals from peripheral physiological or pathological microenvironments. Depending on the nature, amount, and combinations of these stimuli, DCs acquire different functional capabilities.Citation26,74
Several endogenous factors are translocated to the cell membrane or are released into the extracellular milieu by dying, stressed, or injured cells. These signals can alert the immune system and initiate repair and remodeling mechanisms in damaged tissues.Citation75 These so-called damage-associated molecular patterns (DAMPs) can function as either as adjuvants or danger signals for immune cells or can also play an important role in homeostatic mechanisms. A remarkable characteristic of DAMPs is that the majority of these molecules have completely distinct, non-immunological related functions under normal physiological conditions.Citation76
DAMPs are normally absent or found in very low concentrations in the extracellular matrix of any given tissue, but in conditions of tissue damage, these molecules are either exposed or secreted and can interact with almost all types of immune cells, such as DCs, through PRRs on their surface. This interaction is primarily mediated by Toll-like receptors (TLRs), which compose a family of membrane-spanning proteins that recognize structurally conserved self- and pathogen-related molecules.Citation77 To date, 10 different TLRs have been described in humans that recognize different DAMPsCitation78 and PAMPs.Citation79 These receptors mediate the interaction between immune system cells and pathogens and play a central role in the innate immune response.Citation80 The signals mediated by different TLRs also have a crucial impact on the induction and regulation of effective adaptive immune responses against pathogens and tumors.Citation29, 81-83 In addition to TLRs, several PRRs have been found involved in sensing DAMPs. These include receptors belonging to the family of C-type lectins, such as CLEC9a and Mincle,Citation84 and cytosolic PRRs, such as DAI, AIM, RIG-I, MDA-5, and NLRP3.Citation85
In fact, the events associated with DCs maturation and migration are partly the result of PRR activation expressed on the cell surface,Citation86 the synthesis of which is regulated by the environment where these cells reside.Citation87,88 Due to this aspect, it is important to recognize that adequate PRRs stimulation during the ex vivo-generation of DCs can minimize the possibility of obtaining a tolerogenic phenotype, ensuring instead an immunogenic DC phenotype with effective priming CD4+ and CD8+ T lymphocytes that could trigger an in vivo cellular response against neoplastic cells.
Recent evidence suggests that the way in which tumor cells die could be a key factor in triggering an appropriate anti-tumor immune response.Citation89,90 It is therefore relevant to identify danger signals induced during the cell death process that could be involved in both antigenicity and adjuvanticity. Recent studies have suggested that radiation and certain chemotherapeutic agents are able to induce a variety of stress signals, such as Heat Shock Proteins (HSPs), the translocation of calreticulin (CRT, a well described “eat-me” signal), and the chromatin-associated protein high-mobility group box 1 (HMGB1) that can act as an adjuvants in Ag delivery.Citation65,91-95
Immunogenic cell death (ICD) of cancer cells is a novel concept that has emerged during the last decade, and it underlines the fundamental role of the immune system in cancer biology in regards to the identification of DAMPs released by tumor cells during ICD.Citation75 In an attempt to differentiate the specific origin of DAMPs between distinct mechanisms, specific danger signals exposed or released during ICD have been referred as to cell death-associated molecules (CDAMs).Citation85 Several studies have reported that cancer cell lines treated ex vivo with chemotherapeutic drugs, photodynamic therapy, or gamma-irradiation and implanted subcutaneously into syngeneic immunocompetent mice work as a cancer vaccine, even in the absence of any adjuvant.Citation92,96-98 Additionally, a proportion of these mice are protected against subsequent challenges with untreated live cancer cell lines. Moreover, tumor cell death caused by radiotherapy also promotes cross-presentation.Citation83,99,100 In this context, specific DAMPs such as surface exposed CRT, secreted ATP, and passively released HMGB1 and subsequent interactions with phagocytosis receptors, purinergic receptors, and PRRs, respectively, are required for ICD, and this ultimately leads to the activation of potent anticancer immunity ().Citation85,96,101-105
Table 3. Danger signals (DAMPs) associated with cancer cell death and their immune effects.
Additionally, radio- and chemotherapy ICD are 2 well-described and important factors in patient response to treatment. In other words, the release of danger signals during cancer cell death, as induced by these anticancer therapies, is relevant to increase patient survival.Citation83 Interestingly, ICD of cancer cells appears to not only play a role in the in vivo response to gold standard anticancer therapies, but it is also a fundamental mechanism that can be exploited during DC-based immunotherapy.
Tumor ICD depends, at least in part, on the specific DAMPs released by dying/stressed cells and the danger signaling response triggered by these on immune cells.Citation75 By far, most of the studied ICD inducers are chemotherapeutical agents. Enhanced immunogenicity of tumor cell death depends, at least in part, on the death-initiating stimulus. In this context, some but not all cancer cell death inducers cause exposure of danger signals on the cell surface (such as ecto-CRT and ecto-HSP70) or release into the extracellular space (such as HMGB1 or ATP).Citation106 In fact, Gamma and UVC-irradiation are able to trigger CRT exposure, an “eat me” signal for APCs, on apoptotic cancer cells.Citation92,107 Recently, the use of hypochlorous acid before lysate purification was examined as a way to improve the immunogenicity of ovarian cancer lysates through oxidation.Citation108,Citation73
Interestingly, heat shock is another strong stimulator of ICD. In an in vitro study, it was shown that human DCs loaded with melanoma cells that were heat-treated at 42°C prior to cell lysis were more efficient at cross-priming naïve human CD8+ T cells than DCs loaded with unheated, killed melanoma cells.Citation109 These heat-treated melanoma cells expressed enhanced amounts of HSP70, and the enhanced cross-priming could also be reproduced by overexpressing HSP70. In the lysate generation protocol used by our group, the ICD of cancer cells was induced in vitro by heat shock at 42°C for one hour followed by an additional 37°C for 2 hours.Citation14 Importantly, heat-shock conditioning of cancer cells increased their CRT plasma membrane translocation and induced the release of HMGB1 protein.Citation28 CRT and HMGB1 mobilization were associated with enhanced maturation of DCs and efficient Ag cross-presentation capacity, respectively.Citation28
Additionally, HMGB1 co-localizes with TLR4 in monocytes, therefore the blockage of TLR4 inhibits the expression of maturation-associated markers, pro-inflammatory cytokines, and the CCR7 chemokine receptor induced by tumor cell lysates.Citation29 Interestingly, TLR4 gene-specific single nucleotide polymorphisms (SNPs) are thought to be related with a diminished immune response in TAPCell-vaccinated MM patients and in breast cancer patients treated with radiotherapy and chemotherapy.Citation29,83 The ability of DCs to migrate in vivo to draining lymph nodes, a relevant pre-requisite for its clinical efficacy, is also increased through tumor cell lysate stimulation.Citation110 In summary, accumulated evidence supports the notion that heat conditioning is able to induce ICD in cancer cells and, in turn, generate cancer cell-derived lysates that establish proper conditions for ex vivo-generated antitumor-DCs.
Despite currently significant research in this field, there is no consensus about whether the immunomodulatory effects of DAMPs on APCs can be categorized based on their timing. In fact, DAMP-mediated effects in the early stage, such as chemotaxis, phagocytosis, and pro-inflammatory cytokine production, have not yet been defined in relation to late stage effects, such as DCs migration to draining lymph nodes, the proper expression of co-stimulatory molecules, and TAAs cross-presentation to naïve CD8+ T lymphocytes.
Furthermore, there is still debate in defining which ICD inducers are more suitable for ex vivo and/or in vivo immune system stimulation, or whether specific combinations of these are more efficient in triggering an adaptive anticancer immune response.
Concluding remarks
The biology of DCs and their interaction with other immune cells is not completely understood. Several aspects of DCs generation and vaccination require optimization, including adequate stimulation using specific signals through specific receptors for DCs activation and maturation, correct antigen loading of DCs, and an adequate delivery of DCs to ensure appropriate migration to T lymphocyte areas in draining lymphoid tissues. The generation of cancer cell-derived lysates, after ICD induction, seems to combine TAAs and proper danger signal to ensure a committed phenotype of ex vivo-generated DCs. This process results in an efficient polarization of T lymphocytes in an anticancer-Th1/Th17 immune response, resulting in objective clinical benefits for cancer patients (). Immunotherapeutic approaches have been applied in a variety of tumors with different clinical and immunological results. Implementing combination therapies that target distinct arms of antitumor immunity, including immunization and checkpoint antibody therapy, might be synergistic and may result in improved clinical benefits that could accomplish stronger, more sustained responses and long-lasting tumor destruction, therefore leading to better survival and quality of life for patients.
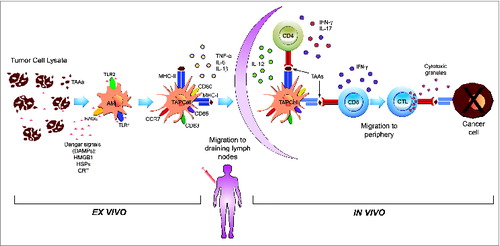
Figure 1. Summary of the effects of stressed cancer cell-derived lysates on the phenotypic and functional characteristics of ex vivo-generated DCs. The ex vivo stimulation of therapeutic TAPCells with 2 different cancer cell-derived lysates (TRIMEL and TRIPRO) induced rapid (24–48 hours) and committed maturation to Th1/Th17 polarizing DCs. This is achieved through the engagement of different cancer cell-expressed DAMPs (e.g. CRT and HMGB1), and by induction through a previous heat conditioning of cancer cells, with different innate immune-receptors (e.g., TLR2, TLR4, and RAGE) expressed on the surface of AM (an immature DC phenotype). These functional characteristics include the secretion of pro-inflammatory cytokines (TNF-α, IL-6, and IL-1β), the migration capacity to draining lymph nodes, Th1 and Th17 polarizing cytokines (IL-12, IFN-α, and IL-17) and the presentation and cross-presentation of TAAs to naïve CD4+ and CD8+ T lymphocytes, respectively, which can explain, at least in part, the in vivo responses observed in vaccinated MM and prostate cancer patients.Citation14,27–29,31,110
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors thank Mr. Eugenio Rivas and Mr. Cristián Pereda for artwork assistance, and Mr. Dagoberto Donoso for technical assistance.
Funding
This work was funded by grants from the Millennium Science Initiative of the Ministry of Economy, Development and Tourism (P09/016-F, F.S.-O.); the Chilean National Fund for Scientific and Technological Development (FONDECYT 1130320 F.S-O., 11130607 F.E.G., and 1130324 M.L.); Funding for the Promotion of Scientific and Technological Development (FONDEF D11I1036 F.S.-O. and ML); and the National Commission of Scientific and Technological Research (CONICYT, Advanced Human Capital Program, F.E.G., CONICYT Programa de Atracción de Capital Humano Avanzado, PAI 82130031, F.O.).
References
- Klebanoff CA, Acquavella N, Yu Z, Restifo NP. Therapeutic cancer vaccines: are we there yet? Immunol Rev 2011; 239:27-44; PMID:21198663; http://dx.doi.org/10.1111/j.1600-065X.2010.00979.x
- Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP, Royal RE, Kammula U, White DE, Mavroukakis SA, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol 2005; 23:2346-57; PMID:15800326; http://dx.doi.org/10.1200/JCO.2005.00.240
- Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer 2008; 8:299-308; PMID:18354418; http://dx.doi.org/10.1038/nrc2355
- Mackensen A, Meidenbauer N, Vogl S, Laumer M, Berger J, Andreesen R. Phase I study of adoptive T-cell therapy using antigen-specific CD8+ T cells for the treatment of patients with metastatic melanoma. J Clin Oncol 2006; 24:5060-9; PMID:17075125; http://dx.doi.org/10.1200/JCO.2006.07.1100
- Robbins PF, Morgan RA, Feldman SA, Yang JC, Sherry RM, Dudley ME, Wunderlich JR, Nahvi AV, Helman LJ, Mackall CL, et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J Clin Oncol 2011; 29:917-24; PMID:21282551; http://dx.doi.org/10.1200/JCO.2010.32.2537
- Guida M, Pisconte S, Colucci G. Metastatic melanoma: the new era of targeted therapy. Expert Opin Ther Targets 2012; 16 Suppl 2:S61-70; http://dx.doi.org/10.1517/14728222.2011.645807
- Amaria RN, Lewis KD, Gonzalez R. Therapeutic options in cutaneous melanoma: latest developments. Ther Adv Med Oncol 2011; 3:245-51; PMID:21957431; http://dx.doi.org/10.1177/1758834011415308
- Farolfi A, Ridolfi L, Guidoboni M, Nicoletti SV, Piciucchi S, Valmorri L, Costantini M, Scarpi E, Amadori D, Ridolfi R. Ipilimumab in advanced melanoma: reports of long-lasting responses. Melanoma Res 2012; 22:263-70; PMID:22516968; http://dx.doi.org/10.1097/CMR.0b013e328353e65c
- Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, Stankevich E, Pons A, Salay TM, McMiller TL, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol 2010; 28:3167-75; PMID:20516446; http://dx.doi.org/10.1200/JCO.2009.26.7609
- Simeone E, Ascierto PA. Immunomodulating antibodies in the treatment of metastatic melanoma: the experience with anti-CTLA-4, anti-CD137, and anti-PD1. J Immunotoxicol 2012; 9:241-7; PMID:22524673; http://dx.doi.org/10.3109/1547691X.2012.678021
- Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med 2013; 369:134-44; PMID:23724846; http://dx.doi.org/10.1056/NEJMoa1305133
- Kirkwood JM, Butterfield LH, Tarhini AA, Zarour H, Kalinski P, Ferrone S. Immunotherapy of cancer in 2012. CA Cancer J Clin 2012; 62:309-35; PMID:22576456
- Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer 2012; 12:265-77; PMID:22437871; http://dx.doi.org/10.1038/nrc3258
- Lopez MN, Pereda C, Segal G, Munoz L, Aguilera R, Gonzalez FE, Escobar A, Ginesta A, Reyes D, Gonzalez R, et al. Prolonged survival of dendritic cell-vaccinated melanoma patients correlates with tumor-specific delayed type IV hypersensitivity response and reduction of tumor growth factor β-expressing T cells. J Clin Oncol 2009; 27:945-52; PMID:19139436; http://dx.doi.org/10.1200/JCO.2008.18.0794
- Schuler G, Schuler-Thurner B, Steinman RM. The use of dendritic cells in cancer immunotherapy. Curr Opin Immunol 2003; 15:138-47; PMID:12633662; http://dx.doi.org/10.1016/S0952-7915(03)00015-3
- Banchereau J, Paczesny S, Blanco P, Bennett L, Pascual V, Fay J, Palucka AK. Dendritic cells: controllers of the immune system and a new promise for immunotherapy. Ann N Y Acad Sci 2003; 987:180-7; PMID:12727638; http://dx.doi.org/10.1111/j.1749-6632.2003.tb06047.x
- Schmitt N, Morita R, Bourdery L, Bentebibel SE, Zurawski SM, Banchereau J, Ueno H. Human dendritic cells induce the differentiation of interleukin-21-producing T follicular helper-like cells through interleukin-12. Immunity 2009; 31:158-69; PMID:19592276; http://dx.doi.org/10.1016/j.immuni.2009.04.016
- Mayordomo JI, Zorina T, Storkus WJ, Zitvogel L, Celluzzi C, Falo LD, Melief CJ, Ildstad ST, Kast WM, Deleo AB, et al. Bone marrow-derived dendritic cells pulsed with synthetic tumour peptides elicit protective and therapeutic antitumour immunity. Nat Med 1995; 1:1297-302; PMID:7489412; http://dx.doi.org/10.1038/nm1295-1297
- Ferrantini M, Capone I, Belardelli F. Dendritic cells and cytokines in immune rejection of cancer. Cytokine Growth Factor Rev 2008; 19:93-107; PMID:18054517; http://dx.doi.org/10.1016/j.cytogfr.2007.10.003
- Hoebe K, Janssen E, Beutler B. The interface between innate and adaptive immunity. Nat Immunol 2004; 5:971-4; PMID:15454919; http://dx.doi.org/10.1038/ni1004-971
- Segura E, Valladeau-Guilemond J, Donnadieu MH, Sastre-Garau X, Soumelis V, Amigorena S. Characterization of resident and migratory dendritic cells in human lymph nodes. J Exp Med 2012; 209:653-60; PMID:22430490; http://dx.doi.org/10.1084/jem.20111457
- Poulin LF, Salio M, Griessinger E, Anjos-Afonso F, Craciun L, Chen JL, Keller AM, Joffre O, Zelenay S, Nye E, et al. Characterization of human DNGR-1+ BDCA3+ leukocytes as putative equivalents of mouse CD8alpha+ dendritic cells. J Exp Med 2010; 207:1261-71; PMID:20479117; http://dx.doi.org/10.1084/jem.20092618
- Steinman RM. Some interfaces of dendritic cell biology. Apmis 2003; 111:675-97; PMID:12974772
- Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor α. J Exp Med 1994; 179:1109-18; PMID:8145033; http://dx.doi.org/10.1084/jem.179.4.1109
- Bonasio R, von Andrian UH. Generation, migration and function of circulating dendritic cells. Curr Opin Immunol 2006; 18:503-11; PMID:16777395; http://dx.doi.org/10.1016/j.coi.2006.05.011
- Lin CL, Suri RM, Rahdon RA, Austyn JM, Roake JA. Dendritic cell chemotaxis and transendothelial migration are induced by distinct chemokines and are regulated on maturation. Eur J Immunol 1998; 28:4114-22; PMID:9862347; http://dx.doi.org/10.1002/(SICI)1521-4141(199812)28:12%3c4114::AID-IMMU4114%3e3.0.CO;2-C
- Escobar A, Lopez M, Serrano A, Ramirez M, Perez C, Aguirre A, Gonzalez R, Alfaro J, Larrondo M, Fodor M, et al. Dendritic cell immunizations alone or combined with low doses of interleukin-2 induce specific immune responses in melanoma patients. Clin Exp Immunol 2005; 142:555-68; PMID:16297169
- Aguilera R, Saffie C, Tittarelli A, Gonzalez FE, Ramirez M, Reyes D, Pereda C, Hevia D, Garcia T, Salazar L, et al. Heat-shock induction of tumor-derived danger signals mediates rapid monocyte differentiation into clinically effective dendritic cells. Clin Cancer Res 2011; 17:2474-83; PMID:21292818; http://dx.doi.org/10.1158/1078-0432.CCR-10-2384
- Tittarelli A, Gonzalez FE, Pereda C, Mora G, Munoz L, Saffie C, Garcia T, Diaz D, Falcon C, Hermoso M, et al. Toll-like receptor 4 gene polymorphism influences dendritic cell in vitro function and clinical outcomes in vaccinated melanoma patients. Cancer Immunol Immunother 2012; 61:2067-77; PMID:22552381; http://dx.doi.org/10.1007/s00262-012-1268-7
- Duran-Aniotz C, Segal G, Salazar L, Pereda C, Falcon C, Tempio F, Aguilera R, Gonzalez R, Perez C, Tittarelli A, et al. The immunological response and post-treatment survival of DC-vaccinated melanoma patients are associated with increased Th1/Th17 and reduced Th3 cytokine responses. Cancer Immunol Immunother 2013; 62:761-72; PMID:23242374; http://dx.doi.org/10.1007/s00262-012-1377-3
- Reyes D, Salazar L, Espinoza E, Pereda C, Castellon E, Valdevenito R, Huidobro C, Ines Becker M, Lladser A, Lopez MN, et al. Tumour cell lysate-loaded dendritic cell vaccine induces biochemical and memory immune response in castration-resistant prostate cancer patients. Br J Cancer 2013; 109:1488-97; PMID:23989944; http://dx.doi.org/10.1038/bjc.2013.494
- Tacken PJ, de Vries IJ, Torensma R, Figdor CG. Dendritic-cell immunotherapy: from ex vivo loading to in vivo targeting. Nat Rev Immunol 2007; 7:790-802; PMID:17853902; http://dx.doi.org/10.1038/nri2173
- Sallusto F, Schaerli P, Loetscher P, Schaniel C, Lenig D, Mackay CR, Qin S, Lanzavecchia A. Rapid and coordinated switch in chemokine receptor expression during dendritic cell maturation. Eur J Immunol 1998; 28:2760-9; PMID:9754563; http://dx.doi.org/10.1002/(SICI)1521-4141(199809)28:09%3c2760::AID-IMMU2760%3e3.0.CO;2-N
- Cheong C, Matos I, Choi JH, Dandamudi DB, Shrestha E, Longhi MP, Jeffrey KL, Anthony RM, Kluger C, Nchinda G, et al. Microbial stimulation fully differentiates monocytes to DC-SIGN/CD209(+) dendritic cells for immune T cell areas. Cell 2010; 143:416-29; PMID:21029863; http://dx.doi.org/10.1016/j.cell.2010.09.039
- Steinman RM, Idoyaga J. Features of the dendritic cell lineage. Immunol Rev 2010; 234:5-17; PMID:20193008; http://dx.doi.org/10.1111/j.0105-2896.2009.00888.x
- Joffre OP, Segura E, Savina A, Amigorena S. Cross-presentation by dendritic cells. Nat Rev Immunol 2012; 12:557-69; PMID:22790179; http://dx.doi.org/10.1038/nri3254
- Melief CJ. Cancer immunotherapy by dendritic cells. Immunity 2008; 29:372-83. PMID: 18799145
- Groh V, Li YQ, Cioca D, Hunder NN, Wang W, Riddell SR, Yee C, Spies T. Efficient cross-priming of tumor antigen-specific T cells by dendritic cells sensitized with diverse anti-MICA opsonized tumor cells. Proc Natl Acad Sci U S A 2005; 102:6461-6; PMID:15824323; http://dx.doi.org/10.1073/pnas.0501953102
- Wilson NS, Behrens GM, Lundie RJ, Smith CM, Waithman J, Young L, Forehan SP, Mount A, Steptoe RJ, Shortman KD, et al. Systemic activation of dendritic cells by Toll-like receptor ligands or malaria infection impairs cross-presentation and antiviral immunity. Nat Immunol 2006; 7:165-72; PMID:16415871; http://dx.doi.org/10.1038/ni1300
- Melief CJ. Mini-review: Regulation of cytotoxic T lymphocyte responses by dendritic cells: peaceful coexistence of cross-priming and direct priming? Eur J Immunol 2003; 33:2645-54; PMID:14515248; http://dx.doi.org/10.1002/eji.200324341
- Andrews DM, Maraskovsky E, Smyth MJ. Cancer vaccines for established cancer: how to make them better? Immunol Rev 2008; 222:242-55; PMID:18364006; http://dx.doi.org/10.1111/j.1600-065X.2008.00612.x
- Nestle FO, Alijagic S, Gilliet M, Sun Y, Grabbe S, Dummer R, Burg G, Schadendorf D. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat Med 1998; 4:328-32; PMID:9500607; http://dx.doi.org/10.1038/nm0398-328
- Rosenberg SA, Yang JC, Schwartzentruber DJ, Hwu P, Marincola FM, Topalian SL, Restifo NP, Dudley ME, Schwarz SL, Spiess PJ, et al. Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma. Nat Med 1998; 4:321-7; PMID:9500606; http://dx.doi.org/10.1038/nm0398-321
- O'Rourke MG, Johnson MK, Lanagan CM, See JL, O'Connor LE, Slater GJ, Thomas D, Lopez JA, Martinez NR, Ellem KA, et al. Dendritic cell immunotherapy for stage IV melanoma. Melanoma Res 2007; 17:316-22; PMID:17885587; http://dx.doi.org/10.1097/CMR.0b013e3282c3a73b
- Schadendorf D, Ugurel S, Schuler-Thurner B, Nestle FO, Enk A, Brocker EB, Grabbe S, Rittgen W, Edler L, Sucker A, et al. Dacarbazine (DTIC) versus vaccination with autologous peptide-pulsed dendritic cells (DC) in first-line treatment of patients with metastatic melanoma: a randomized phase III trial of the DC study group of the DeCOG. Ann Oncol 2006; 17:563-70; PMID:16418308; http://dx.doi.org/10.1093/annonc/mdj138
- Ridgway D. The first 1000 dendritic cell vaccinees. Cancer Invest 2003; 21:873-86. PMID: 14735692
- Melief CJ, van der Burg SH. Immunotherapy of established (pre)malignant disease by synthetic long peptide vaccines. Nat Rev Cancer 2008; 8:351-60; PMID:18418403; http://dx.doi.org/10.1038/nrc2373
- Kenter GG, Welters MJ, Valentijn AR, Lowik MJ, Berends-van der Meer DM, Vloon AP, Drijfhout JW, Wafelman AR, Oostendorp J, Fleuren GJ, et al. Phase I immunotherapeutic trial with long peptides spanning the E6 and E7 sequences of high-risk human papillomavirus 16 in end-stage cervical cancer patients shows low toxicity and robust immunogenicity. Clin Cancer Res 2008; 14:169-77; PMID:18172268; http://dx.doi.org/10.1158/1078-0432.CCR-07-1881
- Welters MJ, Kenter GG, Piersma SJ, Vloon AP, Lowik MJ, Berends-van der Meer DM, Drijfhout JW, Valentijn AR, Wafelman AR, Oostendorp J, et al. Induction of tumor-specific CD4+ and CD8+ T-cell immunity in cervical cancer patients by a human papillomavirus type 16 E6 and E7 long peptides vaccine. Clin Cancer Res 2008; 14:178-87; PMID:18172269; http://dx.doi.org/10.1158/1078-0432.CCR-07-1880
- Bijker MS, van den Eeden SJ, Franken KL, Melief CJ, van der Burg SH, Offringa R. Superior induction of anti-tumor CTL immunity by extended peptide vaccines involves prolonged, DC-focused antigen presentation. Eur J Immunol 2008; 38:1033-42; PMID:18350546; http://dx.doi.org/10.1002/eji.200737995
- Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 2010; 363:411-22; PMID:20818862; http://dx.doi.org/10.1056/NEJMoa1001294
- Sabado RL, Bhardwaj N. Dendritic cell immunotherapy. Ann N Y Acad Sci 2013; 1284:31-45; PMID:23651191; http://dx.doi.org/10.1111/nyas.12125
- Kyte JA, Kvalheim G, Lislerud K, thor Straten P, Dueland S, Aamdal S, Gaudernack G. T cell responses in melanoma patients after vaccination with tumor-mRNA transfected dendritic cells. Cancer Immunol Immunother 2007; 56:659-75; PMID:16947019; http://dx.doi.org/10.1007/s00262-006-0222-y
- O'Rourke MG, Johnson M, Lanagan C, See J, Yang J, Bell JR, Slater GJ, Kerr BM, Crowe B, Purdie DM, et al. Durable complete clinical responses in a phase I/II trial using an autologous melanoma cell/dendritic cell vaccine. Cancer Immunol Immunother 2003; 52:387-95; PMID:12682787
- Nagayama H, Sato K, Morishita M, Uchimaru K, Oyaizu N, Inazawa T, Yamasaki T, Enomoto M, Nakaoka T, Nakamura T, et al. Results of a phase I clinical study using autologous tumour lysate-pulsed monocyte-derived mature dendritic cell vaccinations for stage IV malignant melanoma patients combined with low dose interleukin-2. Melanoma Res 2003; 13:521-30; PMID:14512794; http://dx.doi.org/10.1097/00008390-200310000-00011
- Trefzer U, Herberth G, Wohlan K, Milling A, Thiemann M, Sharav T, Sparbier K, Sterry W, Walden P. Tumour-dendritic hybrid cell vaccination for the treatment of patients with malignant melanoma: immunological effects and clinical results. Vaccine 2005; 23:2367-73; PMID:15755630; http://dx.doi.org/10.1016/j.vaccine.2005.01.081
- Hersey P, Menzies SW, Halliday GM, Nguyen T, Farrelly ML, DeSilva C, Lett M. Phase I/II study of treatment with dendritic cell vaccines in patients with disseminated melanoma. Cancer Immunol Immunother 2004; 53:125-34; PMID:14600790; http://dx.doi.org/10.1007/s00262-003-0429-0
- Morton DL, Foshag LJ, Hoon DS, Nizze JA, Famatiga E, Wanek LA, Chang C, Davtyan DG, Gupta RK, Elashoff R, et al. Prolongation of survival in metastatic melanoma after active specific immunotherapy with a new polyvalent melanoma vaccine. Ann Surg 1992; 216:463-82; PMID:1417196; http://dx.doi.org/10.1097/00000658-199210000-00010
- Ridolfi R, Petrini M, Fiammenghi L, Stefanelli M, Ridolfi L, Ballardini M, Migliori G, Riccobon A. Improved overall survival in dendritic cell vaccination-induced immunoreactive subgroup of advanced melanoma patients. J Transl Med 2006; 4:36; PMID:16914047; http://dx.doi.org/10.1186/1479-5876-4-36
- Salcedo M, Bercovici N, Taylor R, Vereecken P, Massicard S, Duriau D, Vernel-Pauillac F, Boyer A, Baron-Bodo V, Mallard E, et al. Vaccination of melanoma patients using dendritic cells loaded with an allogeneic tumor cell lysate. Cancer Immunol Immunother 2006; 55:819-29; PMID:16187085; http://dx.doi.org/10.1007/s00262-005-0078-6
- Lesterhuis WJ, Aarntzen EH, De Vries IJ, Schuurhuis DH, Figdor CG, Adema GJ, Punt CJ. Dendritic cell vaccines in melanoma: from promise to proof? Crit Rev Oncol Hematol 2008; 66:118-34; PMID:18262431; http://dx.doi.org/10.1016/j.critrevonc.2007.12.007
- Bercovici N, Haicheur N, Massicard S, Vernel-Pauillac F, Adotevi O, Landais D, Gorin I, Robert C, Prince HM, Grob JJ, et al. Analysis and characterization of antitumor T-cell response after administration of dendritic cells loaded with allogeneic tumor lysate to metastatic melanoma patients. J Immunother 2008; 31:101-12; PMID:18157017; http://dx.doi.org/10.1097/CJI.0b013e318159f5ba
- Palucka AK, Ueno H, Connolly J, Kerneis-Norvell F, Blanck JP, Johnston DA, Fay J, Banchereau J. Dendritic cells loaded with killed allogeneic melanoma cells can induce objective clinical responses and MART-1 specific CD8+ T-cell immunity. J Immunother 2006; 29:545-57; PMID:16971810; http://dx.doi.org/10.1097/01.cji.0000211309.90621.8b
- Vilella R, Benitez D, Mila J, Lozano M, Vilana R, Pomes J, Tomas X, Costa J, Vilalta A, Malvehy J, et al. Pilot study of treatment of biochemotherapy-refractory stage IV melanoma patients with autologous dendritic cells pulsed with a heterologous melanoma cell line lysate. Cancer Immunol Immunother 2004; 53:651-8; PMID:14999431; http://dx.doi.org/10.1007/s00262-003-0495-3
- Hatfield P, Merrick AE, West E, O'Donnell D, Selby P, Vile R, Melcher AA. Optimization of dendritic cell loading with tumor cell lysates for cancer immunotherapy. J Immunother 2008; 31:620-32; PMID:18600182; http://dx.doi.org/10.1097/CJI.0b013e31818213df
- Moyer JS, Maine G, Mule JJ. Early vaccination with tumor-lysate-pulsed dendritic cells after allogeneic bone marrow transplantation has antitumor effects. Biol Blood Marrow Transplant 2006; 12:1010-9; PMID:17084367; http://dx.doi.org/10.1016/j.bbmt.2006.06.009
- Yasuda T, Kamigaki T, Nakamura T, Imanishi T, Hayashi S, Kawasaki K, Takase S, Ajiki T, Kuroda Y. Dendritic cell-tumor cell hybrids enhance the induction of cytotoxic T lymphocytes against murine colon cancer: a comparative analysis of antigen loading methods for the vaccination of immunotherapeutic dendritic cells. Oncol Rep 2006; 16:1317-24; PMID:17089056
- Yoshida S, Morii K, Watanabe M, Saito T, Yamamoto K, Tanaka R. The generation of anti-tumoral cells using dentritic cells from the peripheral bloood of patients with malignant brain tumors. Cancer Immunol Immunother 2001; 50:321-7; PMID:11570586; http://dx.doi.org/10.1007/s002620100201
- Pandha HS, John RJ, Hutchinson J, James N, Whelan M, Corbishley C, Dalgleish AG. Dendritic cell immunotherapy for urological cancers using cryopreserved allogeneic tumour lysate-pulsed cells: a phase I/II study. BJU Int 2004; 94:412-8; PMID:15291878; http://dx.doi.org/10.1111/j.1464-410X.2004.04922.x
- Palmer DH, Midgley RS, Mirza N, Torr EE, Ahmed F, Steele JC, Steven NM, Kerr DJ, Young LS, Adams DH. A phase II study of adoptive immunotherapy using dendritic cells pulsed with tumor lysate in patients with hepatocellular carcinoma. Hepatology 2009; 49:124-32; PMID:18980227; http://dx.doi.org/10.1002/hep.22626
- Holtl L, Ramoner R, Zelle-Rieser C, Gander H, Putz T, Papesh C, Nussbaumer W, Falkensammer C, Bartsch G, Thurnher M. Allogeneic dendritic cell vaccination against metastatic renal cell carcinoma with or without cyclophosphamide. Cancer Immunol Immunother 2005; 54:663-70; PMID:15918076; http://dx.doi.org/10.1007/s00262-004-0629-2
- Mantia-Smaldone GM, Corr B, Chu CS. Immunotherapy in ovarian cancer. Hum Vaccin Immunother 2012; 8:1179-91; PMID:22906947; http://dx.doi.org/10.4161/hv.20738
- Mantia-Smaldone GM, Chu CS. A review of dendritic cell therapy for cancer: progress and challenges. BioDrugs 2013; 27:453-68; PMID:23592406; http://dx.doi.org/10.1007/s40259-013-0030-9
- Watchmaker PB, Berk E, Muthuswamy R, Mailliard RB, Urban JA, Kirkwood JM, Kalinski P. Independent regulation of chemokine responsiveness and cytolytic function versus CD8+ T cell expansion by dendritic cells. J Immunol 2010; 184:591-7; PMID:20018619; http://dx.doi.org/10.4049/jimmunol.0902062
- Krysko DV, Garg AD, Kaczmarek A, Krysko O, Agostinis P, Vandenabeele P. Immunogenic cell death and DAMPs in cancer therapy. Nat Rev Cancer 2012; 12:860-75; PMID:23151605; http://dx.doi.org/10.1038/nrc3380
- Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol 1994; 12:991-1045.
- Tsan MF, Gao B. Endogenous ligands of Toll-like receptors. J Leukoc Biol 2004; 76:514-9; PMID:15178705; http://dx.doi.org/10.1189/jlb.0304127
- Vacchelli E, Eggermont A, Sautes-Fridman C, Galon J, Zitvogel L, Kroemer G, Galluzzi L. Trial Watch: Toll-like receptor agonists for cancer therapy. Oncoimmunology 2013; 2:e25238. PMID: 24083080
- Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol 2004; 5:987-95; PMID:15454922; http://dx.doi.org/10.1038/ni1112
- Medzhitov R, Preston-Hurlburt P, Janeway CA, Jr. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 1997; 388:394-7; PMID:9237759; http://dx.doi.org/10.1038/41131
- Kaisho T, Akira S. Toll-like receptor function and signaling. J Allergy Clin Immunol 2006; 117:979-87; quiz 88; PMID:16675322; http://dx.doi.org/10.1016/j.jaci.2006.02.023
- Mandron M, Aries MF, Brehm RD, Tranter HS, Acharya KR, Charveron M, Davrinche C. Human dendritic cells conditioned with Staphylococcus aureus enterotoxin B promote TH2 cell polarization. J Allergy Clin Immunol 2006; 117:1141-7; PMID:16675344; http://dx.doi.org/10.1016/j.jaci.2005.12.1360
- Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E, Saulnier P, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med 2007; 13:1050-9; PMID:17704786; http://dx.doi.org/10.1038/nm1622
- Osorio F, Reis e Sousa C. Myeloid C-type lectin receptors in pathogen recognition and host defense. Immunity 2011; 34:651-64; PMID:21616435; http://dx.doi.org/10.1016/j.immuni.2011.05.001
- Zitvogel L, Kepp O, Kroemer G. Decoding cell death signals in inflammation and immunity. Cell 2010; 140:798-804; PMID:20303871; http://dx.doi.org/10.1016/j.cell.2010.02.015
- Pasare C, Medzhitov R. Toll-like receptors: linking innate and adaptive immunity. Adv Exp Med Biol 2005; 560:11-8; PMID:15932016; http://dx.doi.org/10.1007/0-387-24180-9_2
- Nembrini C, Abel B, Kopf M, Marsland BJ. Strong TCR signaling, TLR ligands, and cytokine redundancies ensure robust development of type 1 effector T cells. J Immunol 2006; 176:7180-8; PMID:16751361; http://dx.doi.org/10.4049/jimmunol.176.12.7180
- Yrlid U, Milling SW, Miller JL, Cartland S, Jenkins CD, MacPherson GG. Regulation of intestinal dendritic cell migration and activation by plasmacytoid dendritic cells, TNF-α and type 1 IFNs after feeding a TLR7/8 ligand. J Immunol 2006; 176:5205-12; PMID:16621985; http://dx.doi.org/10.4049/jimmunol.176.9.5205
- Ahrens S, Zelenay S, Sancho D, Hanc P, Kjaer S, Feest C, Fletcher G, Durkin C, Postigo A, Skehel M, et al. F-actin is an evolutionarily conserved damage-associated molecular pattern recognized by DNGR-1, a receptor for dead cells. Immunity 2012; 36:635-45; PMID:22483800; http://dx.doi.org/10.1016/j.immuni.2012.03.008
- Iyer SS, Pulskens WP, Sadler JJ, Butter LM, Teske GJ, Ulland TK, Eisenbarth SC, Florquin S, Flavell RA, Leemans JC, et al. Necrotic cells trigger a sterile inflammatory response through the Nlrp3 inflammasome. Proc Natl Acad Sci U S A 2009; 106:20388-93; PMID:19918053; http://dx.doi.org/10.1073/pnas.0908698106
- Tesniere A, Apetoh L, Ghiringhelli F, Joza N, Panaretakis T, Kepp O, Schlemmer F, Zitvogel L, Kroemer G. Immunogenic cancer cell death: a key-lock paradigm. Curr Opin Immunol 2008; 20:504-11; PMID:18573340; http://dx.doi.org/10.1016/j.coi.2008.05.007
- Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, Castedo M, Mignot G, Panaretakis T, Casares N, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med 2007; 13:54-61; PMID:17187072; http://dx.doi.org/10.1038/nm1523
- Srivastava P. Roles of heat-shock proteins in innate and adaptive immunity. Nat Rev Immunol 2002; 2:185-94. PMID: 11913069
- Li Z, Menoret A, Srivastava P. Roles of heat-shock proteins in antigen presentation and cross-presentation. Curr Opin Immunol 2002; 14:45-51; PMID:11790532; http://dx.doi.org/10.1016/S0952-7915(01)00297-7
- Fucikova J, Kralikova P, Fialova A, Brtnicky T, Rob L, Bartunkova J, Spisek R. Human tumor cells killed by anthracyclines induce a tumor-specific immune response. Cancer Res 2011; 71:4821-33; PMID:21602432; http://dx.doi.org/10.1158/0008-5472.CAN-11-0950
- Garg AD, Nowis D, Golab J, Vandenabeele P, Krysko DV, Agostinis P. Immunogenic cell death, DAMPs and anticancer therapeutics: an emerging amalgamation. Biochim Biophys Acta 2010; 1805:53-71; PMID:19720113
- Garg AD, Krysko DV, Verfaillie T, Kaczmarek A, Ferreira GB, Marysael T, Rubio N, Firczuk M, Mathieu C, Roebroek AJ, et al. A novel pathway combining calreticulin exposure and ATP secretion in immunogenic cancer cell death. Embo J 2012; 31:1062-79; PMID:22252128; http://dx.doi.org/10.1038/emboj.2011.497
- Casares N, Pequignot MO, Tesniere A, Ghiringhelli F, Roux S, Chaput N, Schmitt E, Hamai A, Hervas-Stubbs S, Obeid M, et al. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J Exp Med 2005; 202:1691-701; PMID:16365148; http://dx.doi.org/10.1084/jem.20050915
- Chen Z, Xia D, Bi X, Saxena A, Sidhu N, El-Gayed A, Xiang J. Combined radiation therapy and dendritic cell vaccine for treating solid tumors with liver micro-metastasis. J Gene Med 2005; 7:506-17; PMID:15580588; http://dx.doi.org/10.1002/jgm.692
- den Brok MH, Sutmuller RP, Nierkens S, Bennink EJ, Frielink C, Toonen LW, Boerman OC, Figdor CG, Ruers TJ, Adema GJ. Efficient loading of dendritic cells following cryo and radiofrequency ablation in combination with immune modulation induces anti-tumour immunity. Br J Cancer 2006; 95:896-905; PMID:16953240; http://dx.doi.org/10.1038/sj.bjc.6603341
- Rock KL, Kono H. The inflammatory response to cell death. Annu Rev Pathol 2008; 3:99-126; PMID:18039143; http://dx.doi.org/10.1146/annurev.pathmechdis.3.121806.151456
- Palm NW, Medzhitov R. Pattern recognition receptors and control of adaptive immunity. Immunol Rev 2009; 227:221-33; PMID:19120487; http://dx.doi.org/10.1111/j.1600-065X.2008.00731.x
- Krysko DV, Agostinis P, Krysko O, Garg AD, Bachert C, Lambrecht BN, Vandenabeele P. Emerging role of damage-associated molecular patterns derived from mitochondria in inflammation. Trends Immunol 2011; 32:157-64; PMID:21334975; http://dx.doi.org/10.1016/j.it.2011.01.005
- Ladoire S, Hannani D, Vetizou M, Locher C, Aymeric L, Apetoh L, Kepp O, Kroemer G, Ghiringhelli F, Zitvogel L. Cell-Death-Associated Molecular Patterns As Determinants of Cancer Immunogenicity. Antioxid Redox Signal 2013; PMID:23394620
- Martins I, Tesniere A, Kepp O, Michaud M, Schlemmer F, Senovilla L, Seror C, Metivier D, Perfettini JL, Zitvogel L, et al. Chemotherapy induces ATP release from tumor cells. Cell Cycle 2009; 8:3723-8; PMID:19855167; http://dx.doi.org/10.4161/cc.8.22.10026
- Kepp O, Tesniere A, Schlemmer F, Michaud M, Senovilla L, Zitvogel L, Kroemer G. Immunogenic cell death modalities and their impact on cancer treatment. Apoptosis 2009; 14:364-75; PMID:19145485; http://dx.doi.org/10.1007/s10495-008-0303-9
- Obeid M, Panaretakis T, Joza N, Tufi R, Tesniere A, van Endert P, Zitvogel L, Kroemer G. Calreticulin exposure is required for the immunogenicity of gamma-irradiation and UVC light-induced apoptosis. Cell Death Differ 2007; 14:1848-50; PMID:17657249; http://dx.doi.org/10.1038/sj.cdd.4402201
- Chiang CL, Kandalaft LE, Tanyi J, Hagemann AR, Motz GT, Svoronos N, Montone K, Mantia-Smaldone GM, Smith L, Nisenbaum HL, et al. A dendritic cell vaccine pulsed with autologous hypochlorous acid-oxidized ovarian cancer lysate primes effective broad antitumor immunity: from bench to bedside. Clin Cancer Res 2013; 19:4801-15; PMID:23838316; http://dx.doi.org/10.1158/1078-0432.CCR-13-1185
- Shi H, Cao T, Connolly JE, Monnet L, Bennett L, Chapel S, Bagnis C, Mannoni P, Davoust J, Palucka AK, et al. Hyperthermia enhances CTL cross-priming. J Immunol 2006; 176:2134-41; PMID:16455969; http://dx.doi.org/10.4049/jimmunol.176.4.2134
- Gonzalez FE, Ortiz C, Reyes M, Dutzan N, Patel V, Pereda C, Gleisner MA, Lopez MN, Gutkind JS, Salazar-Onfray F. Melanoma cell lysate induces CCR7 expression and in vivo migration to draining lymph nodes of therapeutic human dendritic cells. Immunology 2014; 142:396-405; PMID:24673602; http://dx.doi.org/10.1111/imm.12264
