Abstract
Glioblastoma Multiforme (GBM) is the most common type of brain tumor and it is uniformly fatal. The community standard of treatment for this disease is gross or subtotal resection of the tumor, followed by radiation and temozolomide. At recurrence bevacizumab can be added for increased progression free survival. Many challenges are encountered while trying to devise new drugs to treat GBM, such as the presence of the blood brain barrier which is impermeable to most drugs. Therefore in the past few years attention was turned to immunological means for the treatment of this devastating disease. EGFRvIII targeting has proven a good way to attack glioblastoma cells by using the immune system. Although in still in development, this approach holds the promise as a great first step toward immune-tailored drugs for the treatment of brain cancers.
Abbreviations
| EGFRvIII | = | The epidermal growth factor receptor variant III |
| GBM | = | Glioblastoma Multiforme |
| WHO | = | World Health Organization |
| Y | = | year |
| SEER | = | Surveillance, Epidemiology, and End Results Program |
| VEGF | = | Vascular endothelial growth factor |
| CPT-11 | = | irinotecan, Camptosar |
| OS | = | overall survival |
| PFS | = | progression-free survival |
| EORTC | = | European Organization for Research and Treatment of Cancer |
| NCIC | = | National Cancer Institute of Canada |
| CNS | = | central nervous system |
| APC | = | antigen-presenting cell |
| MHC | = | major histocompatibility complex |
| BBB | = | blood brain barrier |
| LPS | = | lipopolysaccharide |
| D | = | day |
| Ab | = | antibody |
| TGF-b | = | transforming growth factor beta |
| IL-10 | = | Interleukin-10 |
| PGE2 | = | prostaglandin E2 |
| Treg cells | = | regulatory T cells |
| TH2 cells | = | T helper type 2 cells |
| CD4 | = | cluster of differentiation 4 |
| CD25 | = | cluster of differentiation 25 |
| IL-2 | = | Interleukin-2 |
| GAGE | = | G antigen gene family |
| Ras | = | rat sarcoma genes |
| Grb2 | = | Growth factor receptor-bound protein 2 |
| KLH | = | keyhole limpet hemocyanin |
| CTL | = | Cytotoxic T lymphocytes |
| HLA | = | human leukocyte antigen |
| DTH | = | delayed-type hypersensitivity |
| ACTIVATE | = | A Complementary Trial of an Immunotherapy Vaccine against Tumor Specific EGRFvIII |
| TMZ | = | temozolomide |
| KPS | = | Karnofsky performance status |
| TTP | = | time to progression |
| GM-CSF | = | Granulocyte-macrophage colony-stimulating factor |
| MGMT | = | O-6-methylguanine-DNA methyltransferase |
| INF-g | = | Interferon gamma |
| IL-12 | = | Interleukin-12 |
Introduction
Glioblastoma multiforme (GBM) is a uniformly fatal primary brain tumor that is classified by the World Health Organization as the most malignant astrocytoma (WHO Grade IV). In the United States, the rate of GBM occurrence is approximately 3.2 in 100,000 per year, making glioblastoma the most common primary brain tumor in adults.Citation1 With maximal surgical and medical treatment, the survival at 1 y of diagnosis is approximately 43%Citation2 and median survival is currently 15 months from the time of diagnosis.Citation3 Analysis of survival data from the SEER database between 2000–2007 demonstrates that the 5 y survival for Glioblastoma is approximately 20% for patients of ages up to 39 yrs, while that for patients aged 40–65 is approximately 5%.Citation1,4 Despite strong efforts through research to find effective treatments for glioblastoma, these 5 y survival rates are only slightly improved compared to those from 1980–1989.Citation1
The current paradigm for GBM treatment was established in 2005, when a trial conducted by the European Organization for Research and Treatment of Cancer Brain Tumor and the National Cancer Institute of Canada Clinical Trials Group showed that treating patients with temozolomide, an oral alkylating agent, in addition to standard radiotherapy after surgical resection produced a 37% relative reduction in the risk of death.Citation4 Prior to this trial, the standard treatment for patients with glioblastoma consisted of surgical resection followed by targeted radiotherapy, with the median survival of approximately 12 months. Addition of temozolomide to the treatment protocol conferred a 2.5 month prolonged survival. Analysis of the SEER database reflects this result as the median survival of patients diagnosed in 2005–2006 increased to 15 months from 12 months for those diagnosed in 2000–2001.Citation3
Subsequent trials evaluated the use of bevacizumab, a monoclonal antibody targeted against VEGF in relapsed GBM patients who have failed temozolomide and radiotherapy. The first studies in recurrent patients combined bevacizumab with irinotecan.Citation5 Irinotecan, a topoisomerase inhibitor, was implemented on account of its action through a mechanism separate from that of temozolomide. Bevacizumab was chosen as a potential therapeutic agent as it had been found to augment chemotherapy for lung, breast and colorectal cancers,.Citation6-8 The use of bevacizumab alone or in combination with CPT-11 (irinotecan, Camptosar) yielded similar overall survival (OS) times of 9 months.Citation9 In 2 different studies, the use of bevacizumab has been associated with improved clinical performance and a superior 6-month progression-free survival (PFS-6) of 42–50%.Citation5,9 The significant objective response and favorable PFS seen in this phase II study resulted in US. Food and Drug Administration accelerated approval of bevacizumab for the treatment of recurrent GBM patients in 2009. As a next step, bevacizumab was tried in new-diagnosed GBM patients. Lai et al. used bevacizumab with temozolomide during and after radiation therapy for newly diagnosed GBM, and this group found the overall survival (OS) to be 19.6 months with this regimen, which is longer compared to the result of the EORTC-NCIC study (15 months).Citation10 Interestingly, this OS was shorter in comparison to that of a cohort of patients treated at the same institution with temozolomide and radiation only (21.1 months).Citation10 Vrendenburgh et al studied standard therapy followed by bevacizumab, temozolomide and irinotecan for newly diagnosed GBM.Citation11 This study found the OS survival with this regimen to be 21.2 months, however 23% of participants terminated the study prematurely due to toxicity.Citation11 Thought the role of bevacizumab as first-line therapy remains controversial, recent analysis of the SEER database has revealed an increase in the 1-year survival from 41% to 43% and a significant decrease in the odds of death with the adoption of bevacizumab.Citation2
Immunotherapy for Glioblastoma
Although the therapeutic advances mentioned above represent significant steps toward control of glioblastoma, in the past 20 years, the median survival has been lengthened by a mere few months. For this reason, many researchers have looked to other potential means besides radiation and chemotherapy for fighting this disease process. One of the major challenges in this process has been the selective targeting cancer cells without harming surrounding neurons, which are fully differentiated and incapable of regeneration. Immunotherapy has thus become vigorously pursued as a therapeutic modality that could potentially accomplish this goal.Citation12 Unfortunately, there are a number of obstacles that must be overcome to implement immunotherapy in the central nervous system (CNS), which is an immunologically privileged site in the body.Citation12 The CNS has a relative lack of lymphatic drainage and endogenous antigen-presenting cells (APCs) compared to the rest of the body.Citation12 Dendritic cells are virtually absent in the CNS, and expression of MHC class II molecules is limited to microglia, which are not as effective as antigen presenting cells.Citation12 Additionally, the CNS is isolated by blood brain barrier (BBB), which limits the movement of inflammatory cells and mediators.Citation12 The immunologic privilege that these features instill has been demonstrated in experiments that describe a delayed rejection of xenogenic tumor implants introduced into CNS parenchyma and slow clearance of virus inoculated into brain parenchyma.Citation13 Nevertheless, immune responses are able to occur in the CNS, albeit within the confines of its unique anatomy.
Immune surveillance of the CNS takes place under normal physiologic conditions and is predominantly carried out by memory T lymphocytes.Citation14 Immunohistochemical and immunofluorescent studies in mice models have demonstrated that fluorescent-labeled lymphocytes injected into the blood stream can be found in the choroid plexus stroma and meninges a couple hours following the injection.Citation15 Leukocytes may enter the CNS through the choroid plexus, through postcapillary venules and Virchow-Robin spaces into the subarachnoid space, and in the case of activated T cells, directly cross the blood brain barrier.Citation14 Inflammatory responses are able to occur in the CNS, but they occur more slowly than in other tissues. Injection of 20 ng of lipopolysaccharide (LPS) into a mouse's ear has been shown to produce a robust immune response within hours, while injection of 2 μg of LPS into the mouse hippocampus will draw a few polymorphonuclear cells into the brain parenchyma, followed by dramatic increase in the number of monocytes and microglia at 3 d.Citation16 Although the blood BBB significantly limits the passage of Abs and cells into the CNS, humoral immunity can also play a role in some CNS immune responses.Citation17 Inflammatory states, such as viral and autoimmune encephalitis, in which the BBB becomes “leaky,” may allow for the passage of Ab and Ab-secreting cells into the CNS. Viral infection of the CNS has been shown to elicit the migration of Ab-secreting cells from lymphoid tissues into the CNS,Citation18 and intrathecal Ab secretion plays an important role in controlling viral replication following the initial infection.Citation19
Adding to the immunologically privileged status of the CNS is the relatively immunosuppressed state that is induced in the presence of glioblastoma. For decades, a reduction of T cell activity has been observed and recognized in patients with primary brain tumors, and this feature poses an additional challenge for enacting immunotherapy for glioblastoma. Decreased cell-mediated immunity,Citation20 impaired delayed-type hypersensitivity reactions,Citation21 decreased proliferative potential of T lymphocytes,Citation22 and release of substances that inhibit inflammatory mediatorsCitation23 have all been described in patients with primary brain tumors, including glioblastoma (see ).
Figure 1. Immunosuppressive Effects of Glioblastoma. The presence of malignant glioma cells causes the activation of multiple immunosuppressive pathways, such as decreased cell-mediated immunity, impaired delayed-type hypersensitivity reactions, decreased proliferative potential of T lymphocytes, and release of substances that inhibit inflammatory mediators.
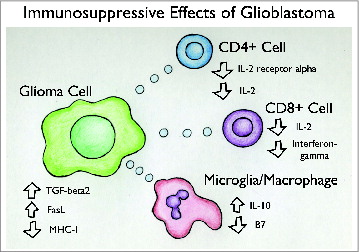
Mechanisms of immune suppression in glioblastoma may include secretion of immunosuppressive chemokines, such as transforming growth factor β (TGF-B), IL-10, and PGE2Citation23,24 as well as recruitment of regulatory T cells (Treg cells) into tumor tissue. An increased proportion of Treg cells has been found in the tumors of glioblastoma patients, and they have been shown to impair T cell proliferation and reduce TH2 cytokines.Citation25,26 This increased Treg cell activity is believed to contribute greatly to the immune suppression observed in glioblastoma. Treg cells suppress CD4 function by inhibiting the production of IL-2, which is a co-stimulator required for CD4 T cell activation.Citation27 Fecci et al. demonstrated that in a mouse model of glioblastoma, inoculation with an anti CD25 monoclonal Ab directed at Treg cells is capable of allowing for tumor rejection in 50% of mice, and in 100% of mice when administered along with an anti-tumor dendritic cell vaccine.Citation25 This suggests that the inhibition of Treg cells could effectively restore cell-mediated immunity in patients with glioblastoma.Citation25 Tumor cells may induce immune suppression by promoting the recruitment and proliferation of Treg cells.Citation25,28 This is suggested by in vitro studies in which glioblastoma stem cells isolated from human tumors were found to induce Treg cells, inhibit T cell proliferation and activation, and induce T cell apoptosis.Citation28
EGFR as a Potential Target
Considering the effects of glioblastoma on the immune system, one would anticipate that immunotherapy would be most successful in young patients with healthy immune systems and in those who received gross total resection of their tumors. Additionally, a target for a vaccine therapy would need to be an antigen that could elicit a sufficiently strong immune response. To best accomplish this, the target should be an antigen that is expressed by the tumor and not by normal cells so that it would be perceived by the immune system as “foreign.” Tumor antigens may arise from normal gene products that are over-expressed by tumor cells or mutated gene products that are not expressed in normal cells, and such antigens may serve as targets for immune-based therapies. For example, melanoma tumor cells express unique gene products, such as the GAGE gene family, which are not found in normal cells.Citation29 In a murine model of melanoma, these tumor-specific antigens were able to be recognized as foreign by cytotoxic T lymphocytes and elicit an immune response.Citation30 Similarly, a mutant, constitutively active epidermal growth factor receptor (EGFR) has been identified in some glioblastoma tumors, and has been extensively investigated as a tumor-specific antigen. Amplified EGFR occurs in approximately 50% of cases of GBM and mutated EGFR is found in about 30% of cases.Citation31 Mutated EGFR is expressed in multiple human tumors, including breast, ovarian and glial tumors, and plays an important role in tumorigenicity.Citation32
EGFR is a 170-kDa transmembrane glycoprotein receptor, which when stimulated by epidermal growth factor (EGF) acts as a tyrosine kinase, initiating a cascade of events resulting in gene transcription.Citation33 Multiple tumor types, including GBM, express a mutated form of the EGFR, which is constitutively active and enhances tumorigenicity by activating the Ras-Shc-Grb2 pathway.Citation34 The most common mutant form of EGFR in GBM is the EGFR class III variant (EGFRvIII), which has a truncated extracellular domain due to an 801 base pair in-frame deletion of the wild-type receptor.Citation35 This deletion results in the fusion of the 2 ends of the peptide and creation of an antigenic site which contains a novel glycine residue not included in the wild-type peptide.Citation36 Therefore the EGFRvIII serves as an ideal tumor-specific antigen for GBM. EGFRvIII is expressed in approximately 30% of GBMs,Citation31 and is found to be expressed on 37–86% of cells in a given tissue sample.Citation37 There is evidence that EGFRvIII may be “shared” among neighboring tumor cells by means of transfer via membrane-derived microvesicles, which may lead to the activation of oncogenic signaling pathways in recipient cells.Citation38 GBMs that express EGFRvIII are usually primary GBMs in which amplification or mutation of EGRF plays a central role in the pathway of tumor development.Citation39 Expression of EGRFvIII has been found to augment proliferation and inhibit apoptosis,Citation40-42 promote tumor cell motility,Citation43 and confer resistance to radiation and chemotherapy.Citation44-46 Interestingly, clinical and biochemical characteristics associated with poor prognosis in EGFRvIII-negative GBMs do not predict outcome in EGFRvIII-positive GBM.Citation47 Among GBM patients who have undergone gross total resection of their tumors and survived beyond 1 y of diagnosis, expression of EGFRvIII has been found to be an independent negative prognostic indicator.Citation31
Preclinical Results of EGFRvIII Immunologic Targeting
Rindopepimut, or CDX-110, is a 14 amino acid peptide that has been implemented in raising an immune response against EGFRvIII. This synthetic peptide, also known as PEPvIII, corresponds to the fusion junction of EGFRvIII, which includes residues 1–5, the novel glycine residue, and residues 274–280 with a terminal cysteine.Citation48 To enhance its immunogenicity, the peptide is conjugated to the carrier protein keyhole limpet hemocyanin (KLH). PEPvIII has been shown to be capable of eliciting immune responses in in vivo models, and antibodies produced from vaccination with PEPvIII, have been shown to bind tumor cells expressing EGFRvIII.Citation48 In early in vivo studies, PEPvIII was shown to induce antibody (Ab) production in mice, rabbits, goats and Macaques.Citation48 In these experiments, the antisera produced from vaccination of the rabbits and one goat with PEPvIII alone was shown to exhibit specific binding to EGFRvIII on the surface of human glioma cells. In mice, immunization with PEPvIII needed to be combined with immunization with EGFRvIII to produce an efficient response.Citation48 Subsequently, high affinity mouse monoclonal Abs (mAbs) were able to be created using long term immunization protocols involving PEPvIII, and these mAbs were shown to be selectively reactive with EGFRvIII on the surface of breast cancer, non-small cell lung cancer, and glioma cells.Citation37 One of these antibodies, Y10, which is of the IgG2a class, was shown to inhibit DNA synthesis, impair cellular proliferation and induce antibody-dependent cell-mediated toxicity in vitro.Citation49 Mice that were pretreated with injections of Y10 and then inoculated with melanoma tumor cells never developed palpable tumors unlike their control counterparts. In mice with established intracranial melanoma tumors, systemic injections with Y10 failed to increase survival, however, a single intra-tumoral injection of Y10 was found to increase median survival by 286% on average.Citation49 These data provided compelling support that immunotherapies such as vaccination and tumor-directed Abs could effectively treat malignancies of the central nervous system.
Another mAb directed against EGFRvIII, mAb 806, was produced by immunizing mice with mouse fibroblasts expressing EGFRvIII. MAb 806 was shown to inhibit the growth of developing and established EGFRvIII-positive human glioma xenografts in mice in a dose-dependent manner.Citation50 Interestingly, mAb 806 was also shown to inhibit the growth of xenografts which overexpressed wild-type EGFRCitation50–52 and to reduce phosphorylation of EGFR and increase apoptosis of these tumor cells.Citation51 mAb 806 did not bind cells that expressed normal levels of EGFR, and was found to recognize about 10% of the total number of receptors present on cells overexpressing EGFR.Citation53 These findings suggested that certain EGFR- directed antibodies may be used to target EGFRvIII-expressing and EGFR-overexpressing tumor cells without binding to other tissues that naturally express EGFR, such as liver and skin.
Vaccination against EGFR-vIII was also proven to be efficacious in murine models of intracerebral tumors. PEPvIII conjugated to keyhole limpet hemocyanin (PEPvIII-KLH) was effectively used to vaccinate mice against EGFR-vIII, rendering them capable of developing immune responses against developed EGFR-vIII-positive melanoma tumors.Citation54 Heimberger et al showed that 70% mice vaccinated with PEPvIII-KLH failed to develop subcutaneous EGFR-vIII- positive melanoma tumors when challenged with subcutaneous injection of tumor cells, and those that did develop tumors had significantly smaller tumors compared to controls.Citation54 The response that the mice developed as a result of vaccination was found to be humoral in nature and to be dependent on CD8+ Cytotoxic T lymphocytes (CTLs) and natural killer cells.Citation54 In a mouse strain in which peptides spanning the splicing site of EGFRvIII were predicted to bind strongly to MHC class I, vaccination with PEPvIII-KLH prior to an intracerebral tumor challenge was found to increase median survival > 173% compared to controls.Citation54 Furthermore, a one-time vaccination of mice having established intracerebral tumors was shown to increase the median survival by 26% with 40% of the mice having long-term survival.Citation54 Subcutaneous tumors that recurred were tested with immunohistochemistry, and interestingly, 80% of relapsing tumors were found to have lost EGFR expression.Citation54
To boost the cell-mediated immune response against EGFRvIII-expressing tumors, dendritic cells were later implemented in the vaccination paradigm.Citation55 Dendritic cells are potent antigen-presenting cells that express high levels of MHC class II molecules for effectively presenting antigens to and activating naive T cells.Citation55 Vaccination with dendritic cells pulsed with glioma peptides had previously shown to prolong survival of rats having intracranial gliomas.Citation56 In experiments conducted by Heimberger et al., mice that received a vaccine of dendritic cells mixed with PEPvIII-KLH and then administered a lethal intracerebral injection of EGFRvIII -expressing melanoma cells exhibited a 600% increase in median survival with 5 of 8 mice surviving long-term.Citation57 Those mice that survived the lethal tumor challenge were then re-challenged with an intracerebral injection of tumor cells into the contralateral hemisphere, and all immunized mice survived.Citation57 This experiment demonstrated that vaccination with dendritic cells mixed with PEPvIII-KLH could induce lasting immunity.Citation57
As vaccination with PEPvIII-KLH had previously been shown to be weakly effective in a mice of the C57BL/6J background,Citation54 which was believed to be due to poor binding to MHC class I molecules, experiments with the dendritic cell vaccine were designed to identify an epitope in the EGFRvIII peptide that would most strongly bind HLA-A0201 on U87 glioma cells.Citation58 Peptides were created based on analyses of software that could predict the binding affinities of certain peptide sequences spanning the splice site of the EGFRvIII protein to a specific HLA class I molecule on U87 glioma cells.Citation58 Three peptides were created and 3 dendritic cell vaccines were produced with these peptides and used to stimulate CTLs, with one peptide eliciting a significantly higher response than the other 2. CTLs that were stimulated with this peptide were shown to effectively lyse tumor cells, while the CTLs stimulated by the other 2 peptides did not.Citation58 These findings demonstrate that that the HLA haplotype expressed by glioma cells may affect CTL responses to dendritic cell tumor vaccines.Citation58
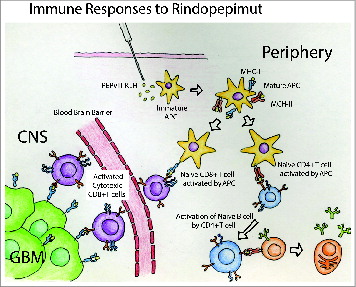
The findings of these experiments support that both humoral responses, such as Ab production, and cytotoxic immune responses, such as the generation of tumor-specific cytotoxic T cells, may be useful for glioblastoma immunotherapy (). However, the presence of the BBB may create an additional barrier for Ab-based immunotherapies, and methods of enabling CNS penetration may be needed for such therapies to be effective. Immunotherapies which lead to the generation of tumor-directed cytotoxic T cells may be particularly useful for glioblastoma because activated T cells can cross the BBB and lyse tumor cells. Furthermore, these experiments demonstrate the importance of HLA haplotype to the efficacy of EGRFvIII vaccination. The affinity with which MHC class I proteins bind to PEPvIII determines the strength of the immune response that is produced. Heimberger et al. demonstrated that vaccination of mice with a background having an MHC class I haplotype with poor binding affinity to PEPvIII yielded a median survival only 2 d longer compared to that of the control group, while vaccination of mice with a background having an MCH class I haplotype with strong binding affinity yielded a median survival of almost 3 times that of the control group.Citation54 Based on the frequencies of human haplotypes that are predicted to bind strongly to PEPvIII, this group calculated that the chance that a glioma patient would be able to elicit a strong response to PEPvIII vaccination is about 64%.Citation54
Clinical Results of EGFRvIII Vaccination
The first clinical trial investigating the use of PEPvIII-KLH for vaccination of human subjects with glioblastoma implemented the dendritic cell mediated approach, in which mature dendritic cells were pulsed with Pep3-KLH. The VICTORI trial was a phase I trial conducted at Duke University Medical Center, which enrolled 15 newly diagnosed GBM patients who had gross total resection of their tumors and underwent standard external beam radiation therapy.Citation59 Given that this study was designed primarily to assess toxicity, patients were not pre-screened for EGFRvIII-expression.Citation59 The 12 patients out of 15 who did not have progression during radiation therapy received 3 vaccinations of an autologous dendritic cell vaccine with each vaccination spaced 2 weeks apart.Citation59 Toxicity from the vaccine was minimal, and patients did not develop any symptoms of autoimmunity. To assess the cellular immune response to vaccination, patients underwent delayed-type hypersensitivity (DTH) testing with Pep3 and KLH before and after vaccination.Citation59 None of the patients had a response to KLH or PEPvIII before vaccination, but 9 of 9 who were tested showed a positive response to KLH after vaccination and 5 of 9 showed a positive response to Pep3. The median time to progression after vaccination was found to be 6.8 months, and the proportion of patients without disease progression at 6 months was 0.67 and at 12 months was 0.33.Citation59 The median survival after diagnosis was 22.8 months and 18.7 months after vaccination.Citation59 Curran's recursive partition analysis was used to determine if the patients had better outcomes than would be expected by chance, and while 9 out of 12 patients had better than expected outcomes, this result did not reach statistical significance.Citation59
The ACTIVATE (A Complementary Trial of an Immunotherapy Vaccine against Tumor Specific EGRFvIII) trial was the first phase II study to evaluate the efficacy and safety of PEPvIII-KLH as a vaccine therapy for GBM.Citation60 Due to the variability and high cost associated with the autologous dendritic cell vaccine, patients enrolled in the ACTIVATE and subsequent trials were vaccinated with PEPvIII-KLH and GM-CSF alone.Citation60 Eighteen patients with newly diagnosed GBM who were screened for EGFRvIII expression were enrolled at MD Anderson Cancer Center and Duke University Medical Center.Citation60 Each patient received gross total resection of their tumor, underwent external beam radiation therapy and concurrent temozolomide (TMZ) with no radiographic progression, and had a Karnofsky performance status (KPS) of > or = to 80. Patients were vaccinated 3 times at 2 week intervals and then every month until progression occurred.Citation60 The outcome of the treatment group compared favorably with a control group that received the standard surgical resection with radiation therapy and TMZ with a median time to progression (TTP) of 14.2 months and a median overall survival (OS) of 26.0 months (hazard ratio: 5.1; 95% CI: 1.9–13.9, P = 0.001).Citation60 Six of the 14 patients who had their serum tested were found to have antibodies specific for PEPvIII, and the median OS of these patients was found to be significantly higher compared to the OS of those who did not develop a humoral response (47.7 months vs. 22.8 months, P = 0.025). Patients who developed a cellular immune response to PEPvIII (3 out of 17 patients) also demonstrated significantly longer PFS and OS (P = 0.03) with median OS from time of histologic diagnosis being unreached after 50 months of follow up compared to 23.1 months for those that did not develop cellular immune responses.Citation60 In cases of recurrent GBM, most tumors were found to have lost EGFRvIII expression.Citation60
Another phase II trial, entitled ACT II, was constructed to determine if TMZ-induced lymphopenia would enhance immune responses to the PEPvIII-KLH vaccine.Citation61 This hypothesis was based on results from experiments in animals and humans that suggested that lymphopenia may support anti-tumor immunity by eliminating the effect of Treg cells and homeostatic mechanisms inducing T cell tolerance.Citation62, 63 This study enrolled 22 patients with newly diagnosed EGFRvIII-positive GBM.Citation61 All patients underwent gross total resection of their tumors followed by standard radiation therapy with TMZ and were subsequently screened for progression.Citation60 Twelve patients were then assigned to receive TMZ at a targeted dose of 200 mg/m2 for the first 5 d of a 28-day cycle (standard dose) and the remaining 10 patients were assigned to receive a targeted dose of 100 mg/m2 for the first 21 d of a 28 day cycle (intensified dose).Citation61 Vaccinations with PEPvIII-KLH with GM-CSF were initiated within 6 weeks of completing radiation therapy.Citation57 The first 3 vaccinations were given at 2 week intervals and subsequent vaccinations were administered monthly until progression.Citation61
Four patients in the group receiving the dose-intensified regimen of TMZ experienced possible allergic drug reactions, and one was removed for toxicity-related reasons while 2 others were removed for tumor progression.Citation61 The grade of lymphopenia was greater and sustained in the dose-intensified group compared to the standard dose TMZ group, however, the proportion of Treg cells was actually found to be higher in the dose-intensified group. All patients in the study developed antibody titers to PEPvIII, however those patients in the dose intensified group demonstrated increasing antibody titers over time such that their titers became higher than those of the standard dose group.Citation61 In addition, more patients in the dose intensified group developed DTH reactions to PEPvIII compared to the standard dose group. In the standard dose group, 9 out of 12 patients were progression free 6 months after vaccination, and 10 of 12 patients were alive at 12 months.Citation61 In the dose-intensified group, 9 of 10 patients were progression free 6 months after vaccination, and 9 of 10 patients were alive at 12 months. The median PFS from the time of histologic diagnosis for all patients was 15.2 months (95% CI: 11.0–18.5), and the OS for all patients was 23.6 months (95% CI: 18.5–33.1). This compared favorably with the PFS and OS of the historic control group (6.3 months [95% CI: 4.1–9.0] and 15.0 months [95% CI: 11.4–19.7], respectively.Citation61 After adjusting for KPS and age, there was no difference in OS between the 2 TMZ cohorts, although the study had not been powered to detect such a difference.Citation61
The ACT III trial was the third phase II trial to investigate the efficacy and safety of the Rindopepimut vaccine.Citation64 This study included 65 patients at 31 different centers with newly diagnosed primary, EGFRvIII-positive GBM who underwent gross total resection and did not show evidence of progression after radiation and TMZ therapy.Citation64 The treatment regimen consisted of 3 vaccinations with PAPvIII-KLH/GM-CSF at 2 week intervals and then vaccinations monthly until progression.Citation64 This vaccination regimen was accompanied by 150–200 mg/m2 doses of TMZ administered for the first 5 d of a 28 day cycle, which was repeated until progression.Citation64 Almost all patients developed injection site reactions and 2 patients had to discontinue treatment because of toxicity.Citation64 85% of patients developed anti EGFRvIII antibody titers, which were observed to increase over the duration of the study.Citation64 The PFS and OS after histological diagnosis was found to be 12.3 months and 24.6 months, respectively, and the PSF at 8.5 months from diagnosis was 66%.Citation64 These survival lengths compare favorably to that of an EGFRvIII-positive control group who received the standard treatment of gross total resection and radiation therapy with concurrent TMZ (P = 0.00088 for PFS and P= < 0.0001 for OS)Citation64. Additionally, patient outcomes were analyzed based on methylation status of MGMT, and those with methylated MGMT were found to have a significantly longer PFS (17.5 months vs 11.2 months, P = 0.0057) and OS (32.3 months vs 20.9 months, P = 0.0067).Citation64 These results support that the rindopepimut vaccine may be advantageous for augmenting standard therapy for GBM.Citation64 The rindopepimut vaccine has been shown to be generally well-tolerated and to lengthen PSF and OS in patients with both methylated MGMT, and unmethylated MGMT.Citation64
Due to the promising results of the previous phase II trials, multiple further studies of rindopepimut for GBM have been initiated. Current ongoing studies include a phase III trial investigating the efficacy of rindopepimut for newly diagnosed GBM (ACTIV). This study is planned to include 374 patients with newly diagnosed EGFR-positive GBM, and will be conducted internationally at over 150 different centers. ACT IV is a randomized, double-blind trial of rindopepimut vaccination compared to vaccination with KLH alone. The primary outcomes will be PFS, OS and quality of life. Patients with incomplete resection will be included so that the efficacy of rindopepimut on residual tumor burden may be assessed. The reACT trial is another phase II trial that evaluates the efficacy of the rindopepimut vaccine with concurrent bevacizumab in patients with EGFR-positive recurrent GBM. In this study, one subset of patients is randomized to receive vaccination with either rindopepimut or KLH, both in conjunction with bevacizumab therapy. The other subset, which will consist of patients who are refractory to bevacizumab, will receive vaccination with rindopepimut in conjunction with bevacizumab. Ninety-five patients are planned to be enrolled in this study, and the effect of residual tumor burden will be evaluated. As the previous ACT trials adhered to strict selection criteria favoring healthier patients with less disease burden, the results of the ReACT trial will more clearly demonstrate the efficacy of the vaccine in a study group which is more representative of the majority of patients living with glioblastoma. The ACT III and ReAct trials were expected to be completed in November 2016 and March 2014, respectively.
Road Blocks to EGFRvIII Targeting
EGFRvIII targeting is a very attractive strategy for developing immunotherapies for GBM as EGFRvIII is one of the few antigens that can be found exclusively on tumor cells and not on normal tissues. The rindopepimut vaccine has been successful in stimulating immune responses to EGFRvIII in GBM patients as it incorporates a unique epitope including a novel glycine not present in the wild-type peptide, which contributes to its immunogenicity.Citation49,59–61,64 Although rindopepimut has been shown to prolong survival of patients with glioblastoma, limitations of this modality have been encountered in clinical trials.Citation59–61,64 First, EGFRvIII targeting is useful in only a subset of GBM patients, which comprises about 20–40% of the total number of cases. Therefore, patients must be screened for EGFRvIII before they can be considered for vaccination. Additionally, in all 3 completed clinical trials (ACTIVATE, ACT II, and ACT III), recurrent GBMs were found to have lost EGFR expression, indicating that the tumor is able to escape through the selection and proliferation of cells that are EGFR negative.Citation59–61,64 Therefore, the rindopepimut vaccine may lose its value in treatment once the GBM recurs. Other alternative therapies would then need to be implemented for continued control of tumor growth.Citation59–61,64
One strategy that attempts to overcome this obstacle is vaccination against multiple tumor antigens simultaneously. The aim of this approach is to decrease the ability of the tumor to escape as each cell should be positive for at least one of the antigens included in the vaccine.Citation59–61 As these antigens may not be very antigenic themselves, dendritic cells could be also potentially used to present the antigens to T cells to stimulate an immune response. Several groups have used whole tumor lysates to vaccinate patients with GBM.Citation65 Prim et al. vaccinated a mixed group of newly diagnosed and recurrent GBM patients (n = 23) with a vaccine consisting of autologous dendritic cells pulsed with autologous tumor lysate obtained from tumor samples from surgical resection.Citation65 Although this Phase I study was not powered to evaluate clinical efficacy, the median PFS was found to be 15.9 months and the median OS was 31.4 months. Interestingly, 3 patients in this group survived over 6 y In this study, no serious adverse events or autoimmune reactions were observed.Citation65 Similarly, Wheeler et al. conducted a phase II clinical trial using an autologous dendritic cell vaccine to vaccinate 23 recurrent and 11 newly diagnosed GBM patients.Citation66 Seventeen patients exhibited a positive immune response after vaccination, as measured by levels of INF-γ, while 14 showed no response, and the remaining 3 were not able to be tested.Citation66 Mean PSF was 308 +/− 55 d in responders and 167 +/− 22 d in non-responders while mean OS was 642 +/− 61 d in responders and 430 +/− 50 in non-responders. As expected, responders to the vaccine tended to be newly diagnosed patients.Citation66 These patients, similar to those in the rindopepimut trials, eventually experienced progression, and so it is obvious that escape from immunity must have occurred with this vaccine as well.Citation66 Attempting to vaccinate against multiple tumor antigens may not always provide complete protection as many tumor antigens may also be expressed on normal tissues and may not be immunogenic.Citation66
One of the benefits of using tumor lysates in vaccination is that patients do not require screening for a particular antigen and any tumor, regardless of molecular markers, may receive vaccine therapy. The disadvantage with this approach is that autologous dendritic cell vaccines are very costly to manufacture and they can only be manufactured on an individual basis. The inherent lack of efficiency with autologous dendritic cell vaccine production inevitably causes this therapy to appear less attractive to corporate sponsors, and lack of sponsorship impedes the production of expensive phase III trials required for FDA approval.Citation67 Efforts to maximize the efficacy of dendritic cell vaccines through promoting a robust cytotoxic T cell response have been initiated with the development of α-type 1 polarized dendritic cells.Citation68 The maturation process of dendritic cells in culture has been found to lead to decreased ability to produce IL-12, which is critical to inducing a cytotoxic-supporting Th 1 response.Citation69 When dendritic cells are matured in the presence of INF-gamma and tumor necrosis factor they have been demonstrated to produce significantly higher levels of IL-12,Citation68 and this maturation process has been associated with prolonged progression free survival in some patients with recurrent glioblastoma.Citation70,71 Further studies would be needed to determine if the efficacy of Th 1 polarized dendritic cell vaccines could offset the inefficiency of dendritic cell vaccine production. Peptide vaccines may be mass-produced and are relatively inexpensive, and large, multi-center phase III clinical trials are more manageable.Citation67 Therefore, while PEPvIII-KLH vaccination may not be indicated for all GBM patients, this vaccine may be easily implemented as an efficacious adjuvant to standard therapy in a relatively large subset of patients.Citation59,67
Peptide vaccines which aim to immunize GBM patients against multiple tumor peptides have been developed as well. Similar to rindopepimut, such vaccines consist simply of tumor-derived peptides mixed with an immune adjuvant. For example, Ishikawa et al. treated 24 newly diagnosed GBM patients with a formalin-fixed vaccine made from autologous tumor lysates.Citation72 The median OS and PFS were 22.2 months and 8.2 months, respectively.Citation72 Of note, this group found that positive DTH responses of equal or greater than 10 mm of induration correlated with longer PFS durations, however this correlation did not reach statistical significance.Citation72 Terasaki et al. developed a vaccine for GBM patients comprised of a personalized combination of several synthetic tumor peptides.Citation73 The peptides were identified from a group of over 100 genes encoding proteins that are expressed at high levels in cancer cells and that play a role in cell proliferation.Citation73 Fourteen peptides were selected based on their ability to elicit peptide-specific immune responses in HLA-A24-positive patients with GBM or other cancers.Citation73 For the individual vaccines designed for each of the 12 patients with recurrent GBM included in a phase I trial, up to 4 peptides out of the 14 were selected based on those which elicited the strongest immune responses in each patient.Citation73 Although the trial was designed specifically to determine the safety and highest tolerated dose of vaccine, the PFS at 6 months was noted to be 16.7% and the OS survival was 10.6 months.Citation73 Compared to PEPvIII, the peptides included in these vaccines may not be as immunogenic because many of them may be expressed at low levels in normal cells. Nevertheless, with the aid of immune adjuvants, tumor-derived peptides may be capable of eliciting anti-tumor immune responses in certain patients.
As some peptide vaccines include a variety of tumor-specific antigens, PEPvIII might be combined with other tumor peptides to induce a cytotoxic response targeting multiple tumor antigens. This might help prevent the antigenic escape of tumor cells as observed with immunization against EGFRvIII alone. Another option may be to combine EGFRvIII vaccination with other adjuvant immunotherapies. One example is anti-programmed cell death-1 antibodies, which have shown some promising results in patients with widespread malignancies, including melanoma, colon cancer, and prostate cancer.Citation74 Programmed cell death-1 is an inhibitory co-stimulator located on the surface of activated T and B cells, and its expression may contribute to the immune resistance of tumors.Citation75 Inhibition of this co-stimulator has been shown to have anti-tumor effects in murine models of carcinomas,Citation75,76 and if able to be effectively implemented for glioblastoma, this therapy could theoretically boost the cytotoxic response initiated by vaccination against EGFRvIII. Of course, use of antibody-based therapies for GBM may require direct inoculation into the CNS, as transfer across the blood-brain-barrier is highly inefficient.
In summary, rindopepimut is a peptide vaccine that has been shown to potentially prolong survival in EGFRvIII-positive GBM patients – pending the final confirmation in a large, ongoing phase III clinical trial (ACTIV). This vaccine targets the EGFRvIII surface antigen, a mutated form of the EGFR receptor that is present only on tumor cells and not in normal healthy tissues. Due to the foreign nature of this peptide, vaccination is capable of eliciting significant anti-tumor immune responses. This immunotherapy has the potential to contribute to extended survival when used in conjunction with standard therapies, including surgical resection, radiation therapy and TMZ chemotherapy. In the future, tumor-directed vaccination may be incorporated into the standard therapeutic regimen for GBM, however, the timing of vaccination to best induce a clinically relevant anti-tumor immune response may require further investigation. Although GBMs may eventually escape EGFRvIII targeting through loss of expression of EGFRvIII, the rindopepimut vaccine may be efficacious as an initial therapy to augment radiation and chemotherapy in newly diagnosed EFGRvIII-positive GBM patients. As more discoveries are made regarding the pathophysiology of GBM and more targets for immunotherapies are discovered, future treatment of GBM will likely consist of combined modalities that are customized for unique genetic makeup of an individual's tumor.
Disclosure of Potential Conflicts of Interest
FH and DB have served as investigators in the ACT III, ACT IV, and ReACT studies. MP and DA-A have no potential conflicts of interest to disclose.
References
- Kohler BA, >Ward E, McCarthy BJ, Schymura MJ, Ries LA, Eheman C, Jemal A, Anderson RN, Ajani UA, Edwards BK. Annual report to the nation on the status of cancer, 1975-2007, featuring tumors of the brain and other nervous system. J Natl Cancer I May 4 2011; 103(9):714-736; PMID:21454908; http://dx.doi.org/10.1093/jnci/djr077
- Wachtel MS, Yang S. Odds of death after glioblastoma diagnosis in the United States by chemotherapeutic era. Cancer medicine Mar 10 2014; 3(3):660-6; PMID:24610705; http://dx.doi.org/10.1002/cam4.213
- Koshy M, Villano JL, Dolecek TA, Howard A, Mahmood U, Chmura SJ, Weichselbaum RR, McCarthy BJ. Improved survival time trends for glioblastoma using the SEER 17 population-based registries. J Neuro-Oncol Mar 2012; 107(1):207-212; PMID:21984115; http://dx.doi.org/10.1007/s11060-011-0738-7
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med Mar 10 2005; 352(10):987-996; PMID:15758009; http://dx.doi.org/10.1056/NEJMoa043330
- Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, Marcello J, Reardon DA, Quinn JA, Rich JN, Sathornsumetee S, Gururangan S, Sampson J, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol Oct 20 2007; 25(30):4722-4729; PMID:17947719; http://dx.doi.org/10.1200/JCO.2007.12.2440
- Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med Jun 3 2004; 350(23):2335-2342; PMID:15175435; http://dx.doi.org/10.1056/NEJMoa032691
- Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, Lilenbaum R, Johnson DH. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. New Engl J Med Dec 14 2006; 355(24):2542-2550; PMID:17167137; http://dx.doi.org/10.1056/NEJMoa061884
- Wedam SB, Low JA, Yang SX, Chow CK, Choyke P, Danforth D, Hewitt SM, Berman A, Steinberg SM, Liewehr DJ, et al. Antiangiogenic and antitumor effects of bevacizumab in patients with inflammatory and locally advanced breast cancer. J Clin Oncol Feb 10 2006; 24(5):769-777; PMID:16391297; http://dx.doi.org/10.1200/JCO.2005.03.4645
- Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE, Yung WK, Paleologos N, Nicholas MK, Jensen R, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol Oct 1 2009; 27(28):4733-4740; PMID:19720927; http://dx.doi.org/10.1200/JCO.2008.19.8721
- Lai A, Tran A, Nghiemphu PL, Pope WB, Solis OE, Selch M, Filka E, Yong WH, Mischel PS, Liau LM, et al. Phase II study of bevacizumab plus temozolomide during and after radiation therapy for patients with newly diagnosed glioblastoma multiforme. J Clin Oncol Jan 10 2011; 29(2):142-148; PMID:21135282; http://dx.doi.org/10.1200/JCO.2010.30.2729
- Vredenburgh JJ, Desjardins A, Reardon DA, Peters KB, Herndon JE 2nd, Marcello J, Kirkpatrick JP, Sampson JH, Bailey L, Threatt S, et al. The addition of bevacizumab to standard radiation therapy and temozolomide followed by bevacizumab, temozolomide, and irinotecan for newly diagnosed glioblastoma. Clin Cancer Res Jun 15 2011; 17(12):4119-4124; PMID:21531816; http://dx.doi.org/10.1158/1078-0432.CCR-11-0120
- Bo L, Mork S, Kong PA, Nyland H, Pardo CA, Trapp BD. Detection of MHC class II-antigens on macrophages and microglia, but not on astrocytes and endothelia in active multiple sclerosis lesions. J neuroimmunol May 1994; 51(2):135-146; PMID:8182113; http://dx.doi.org/10.1016/0165-5728(94)90075-2
- Stevenson PG, Austyn JM, Hawke S. Uncoupling of virus-induced inflammation and anti-viral immunity in the brain parenchyma. J Gen virol Jul 2002; 83(Pt 7):1735-1743; PMID:12075093
- Ransohoff RM, Kivisakk P, Kidd G. Three or more routes for leukocyte migration into the central nervous system. Nat Rev Immunol Jul 2003; 3(7):569-581; PMID:12876559; http://dx.doi.org/10.1038/nri1130
- Carrithers MD, Visintin >I, Viret C, Janeway CS, Jr. Role of genetic background in P selectin-dependent immune surveillance of the central nervous system. J Neuroimmunol Aug 2002; 129(1-2):51-57; PMID:12161020; http://dx.doi.org/10.1016/S0165-5728(02)00172-8
- Perry VH, Andersson PB. The inflammatory response in the CNS. Neuropath appl neuro Oct 1992; 18(5):454-459; PMID:1454134; http://dx.doi.org/10.1111/j.1365-2990.1992.tb00811.x
- Knopf, PM, Harling-Berg CJ, Cserr HF, Basu D, Sirulnick EJ, Nolan SC, Park JT Keir G, Thompson EJ, Hickey WF, Antigen-dependent intrathecal antibody synthesis in the normal rat brain: tissue entry and local retention of antigen- specific B cells. J Immunol 1998; 161:692-701; PMID:9670944
- Tschen S, Stohlman SA, Ramakrishna C Hinton DR, Atkinson RD, Bergmann CC. CNS viral infection diverts homing of antibody-secreting cells from lymphoid organs to the CNS. Eur. J. Immunol. 2006; 36:603-612; PMID:16437540; http://dx.doi.org/10.1002/eji.200535123
- Phares TW, Stholman SA, Bergmann CC. Intrathecal humoral immunity to encephalitic RNA viruses. Viruses 2013, 5:732-752; PMID:23435240; http://dx.doi.org/10.3390/v5020732
- Brooks WH, Netsky MG, Normansell DE, Horwitz DA. Depressed cell-mediated immunity in patients with primary intracranial tumors. Characterization of a humoral immunosuppressive factor. J Exp Med Dec 1 1972; 136(6):1631-1647; PMID:4345108; http://dx.doi.org/10.1084/jem.136.6.1631
- Brooks WH, Roszman TL, Rogers AS. Impairment of rosette-forming T lymphocytes in patients with primary intracranial tumors. Cancer Apr 1976; 37(4):1869-1873; PMID:769940; http://dx.doi.org/10.1002/1097-0142(197604)37:4%3c1869::AID-CNCR2820370435%3e3.0.CO;2-Q
- Miescher S, Whiteside TL, de Tribolet N, von Fliedner V. In situ characterization, clonogenic potential, and antitumor cytolytic activity of T lymphocytes infiltrating human brain cancers. J Neurosurg Mar 1988; 68(3):438-448; PMID:3257792; http://dx.doi.org/10.3171/jns.1988.68.3.0438
- Fontana A, Hengartner H, de Tribolet N, Weber E. Glioblastoma cells release interleukin 1 and factors inhibiting interleukin 2-mediated effects. J Immunol Apr 1984; 132(4):1837-1844; PMID:6607949
- Bodmer S, Strommer K, Frei K, Siepl C, de Tribolet N, Heid I, Fontana A. Immunosuppression and transforming growth factor-β in glioblastoma. Preferential production of transforming growth factor-β 2. J Immunol Nov 15 1989; 143(10):3222-3229; PMID:2809198
- Fecci PE, Mitchell DA, Whitesides JF, Xie W, Friedman AH, Archer GE, Herndon JE 2nd, Bigner DD, Dranoff G, Sampson JH. Increased regulatory T-cell fraction amidst a diminished CD4 compartment explains cellular immune defects in patients with malignant glioma. Cancer Res Mar 15 2006; 66(6):3294-3302; PMID:16540683; http://dx.doi.org/10.1158/0008-5472.CAN-05-3773
- El Andaloussi A, Lesniak MS. An increase in CD4+CD25+FOXP3+ regulatory T cells in tumor-infiltrating lymphocytes of human glioblastoma multiforme. Neuro-oncology Jul 2006; 8(3):234-243; PMID:16723631; http://dx.doi.org/10.1215/15228517-2006-006
- Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med Jul 20 1998; 188(2):287-296; PMID:9670041; http://dx.doi.org/10.1084/jem.188.2.287
- Wei J, Barr J, Kong LY, Wang Y, Wu A, Sharma AK, Gumin J, Henry V, Colman H, Sawaya R, et al. Glioma-associated cancer-initiating cells induce immunosuppression. Clinical Cancer Res Jan 15 2010; 16(2):461-473; PMID:20068105; http://dx.doi.org/10.1158/1078-0432.CCR-09-1983
- Backer O, Arden KC, Borretti M, Vantomme V, De Smet C, Czekay S, Viars CS, De Plaen E, Brasseur F, Chomez P, et al. Characterization of the GAGE genes that are expressed in various human cancers and in normal testis. Cancer Res 1999; 59:3157-65; PMID:10397259
- Van den Eynde B, Peeters O, De Backer O, Gaugler B, Lucas S, Boon T. A new family of genes coding for an antigen recognized by autologous cytolytic T lymphocytes on a human melanoma. J Exp Med Sep 1 1995; 182(3):689-698; PMID:7544395; http://dx.doi.org/10.1084/jem.182.3.689
- Heimberger AB, Hlatky R, Suki D, Yang D, Weinberg J, Gilbert M, Sawaya R, Aldape K. Prognostic effect of epidermal growth factor receptor and EGFRvIII in glioblastoma multiforme patients. Clin Cancer Res Feb 15 2005; 11(4):1462-1466; PMID:15746047; http://dx.doi.org/10.1158/1078-0432.CCR-04-1737
- Moscatello DK, Holgado-Madruga M, Godwin AK, Ramirez G, Gunn G, Zoltick PW, Biegel JA, Hayes RL, Wong AJ. Frequent expression of a mutant epidermal growth factor receptor in multiple human tumors. Cancer Re Dec 1 1995; 55(23):5536-5539; PMID:7585629
- Carpenter G, Cohen S. Epidermal growth factor. J Biol Chem May 15 1990; 265(14):7709-7712; PMID:2186024
- Prigent SA, Nagane M, Lin H, Huvar I, Boss GR, Feramisco JR, Cavenee WK, Huang HS. Enhanced tumorigenic behavior of glioblastoma cells expressing a truncated epidermal growth factor receptor is mediated through the Ras-Shc-Grb2 pathway. J Biol Chem Oct 11 1996; 271(41):25639-25645; PMID:8810340; http://dx.doi.org/10.1074/jbc.271.41.25639
- Bigner SH, Humphrey PA, Wong AJ, Vogelstein B, Mark J, Friedman HS, Bigner DD. Characterization of the epidermal growth factor receptor in human glioma cell lines and xenografts. Cancer Res Dec 15 1990; 50(24):8017-8022; PMID:2253244
- Humphrey PA, Wong AJ, Vogelstein B, Zalutsky MR, Fuller GN, Archer GE, Friedman HS, Kwatra MM, Bigner SH, Bigner DD. Anti-synthetic peptide antibody reacting at the fusion junction of deletion-mutant epidermal growth factor receptors in human glioblastoma. Proc Natl Acad Sci U S A Jun 1990; 87(11):4207-4211; PMID:1693434; http://dx.doi.org/10.1073/pnas.87.11.4207
- Wikstrand CJ, McLendon RE, Friedman AH, Bigner DD. Cell surface localization and density of the tumor-associated variant of the epidermal growth factor receptor, EGFRvIII. Cancer Res Sep 15 1997; 57(18):4130-4140; PMID:9307304
- Al-Nedawi K, Meehan B, Micallef J, Lhotak V, May L, Guha A, Rak J. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat Cell Biol May 2008; 10(5):619-624; PMID:18425114; http://dx.doi.org/10.1038/ncb1725
- Ohgaki H, Kleihues P. Genetic pathways to primary and secondary glioblastoma. Am J Pathol May 2007; 170(5):1445-1453; PMID:17456751; http://dx.doi.org/10.2353/ajpath.2007.070011
- Nishikawa R, Ji XD, Harmon RC, Lazar CS, Gill GN, Cavenee WK, Huang HJ. A mutant epidermal growth factor receptor common in human glioma confers enhanced tumorigenicity. Proc Natl Acad Sci U S A Aug 2 1994; 91(16):7727-7731; PMID:8052651; http://dx.doi.org/10.1073/pnas.91.16.7727
- Batra SK, Castelino-Prabhu S, Wikstrand CJ, Zhu X, Humphrey PA, Friedman HS, Bigner DD. Epidermal growth factor ligand-independent, unregulated, cell-transforming potential of a naturally occurring human mutant EGFRvIII gene. Cell Growth Differ Oct 1995; 6(10):1251-1259; PMID:8845302.
- Nagane M, Coufal F, Lin H, Bogler O, Cavenee WK, Huang HJ. A common mutant epidermal growth factor receptor confers enhanced tumorigenicity on human glioblastoma cells by increasing proliferation and reducing apoptosis. Cancer Res Nov 1 1996; 56(21):5079-5086; PMID:8895767
- Pedersen MW, Tkach V, Pedersen N, Berezin V, Poulsen HS. Expression of a naturally occurring constitutively active variant of the epidermal growth factor receptor in mouse fibroblasts increases motility. Int J Cancer JInt du Cancer Feb 20 2004; 108(5):643-653; PMID:14696090; http://dx.doi.org/10.1002/ijc.11566
- Lammering G, Valerie K, Lin PS, Hewit TH, Schmidt-Ullrich RK. Radiation-induced activation of a common variant of EGFR confers enhanced radioresistance. Radiother Oncol Sep 2004; 72(3):267-273; PMID:15450724; http://dx.doi.org/10.1016/j.radonc.2004.07.004
- Lammering G, Hewit TH, Holmes M, Valerie K, Hawkins W, Lin PS, Mikkelsen RB, Schmidt-Ullrich RK. Inhibition of the type III epidermal growth factor receptor variant mutant receptor by dominant-negative EGFR-CD533 enhances malignant glioma cell radiosensitivity. Clin Cancer Res Oct 1 2004; 10(19):6732-6743; PMID:15475464; http://dx.doi.org/10.1158/1078-0432.CCR-04-0393
- Montgomery RB, Guzman J, O'Rourke DM, Stahl WL. Expression of oncogenic epidermal growth factor receptor family kinases induces paclitaxel resistance and alters β-tubulin isotype expression. J Biol Chem Jun 9 2000; 275(23):17358-17363; PMID:10749863; http://dx.doi.org/10.1074/jbc.M000966200
- Pelloski CE, Ballman KV, Furth AF, Zhang L, Lin E, Sulman EP, Bhat K, McDonald JM, Yung WK, Colman H, et al. Epidermal growth factor receptor variant III status defines clinically distinct subtypes of glioblastoma. J Clin Oncol Jun 1 2007; 25(16):2288-2294; PMID:17538175; http://dx.doi.org/10.1200/JCO.2006.08.0705
- Wikstrand CJ, Stanley SD, Humphrey PA, Pegram CN, Archer GE, Kurpad S, Shibuya M, Bigner DD. Investigation of a synthetic peptide as immunogen for a variant epidermal growth factor receptor associated with gliomas. J Neuroimmunol Jul 1993; 46(1-2):165-173; PMID:8360327; http://dx.doi.org/10.1016/0165-5728(93)90246-U
- Sampson JH, Crotty LE, Lee S, Archer GE, Ashley DM, Wikstrand CJ, Hale LP, Small C, Dranoff G, Friedman AH, et al. Unarmed, tumor-specific monoclonal antibody effectively treats brain tumors. Proc Natl Acad Sci U S A Jun 20 2000; 97(13):7503-7508; PMID:10852962; http://dx.doi.org/10.1073/pnas.130166597
- Luwor RB, Johns TG, Murone C, Huang HJ, Cavenee WK, Ritter G, Old LJ, Burgess AW, Scott AM. Monoclonal antibody 806 inhibits the growth of tumor xenografts expressing either the de2-7 or amplified epidermal growth factor receptor (EGFR) but not wild-type EGFR. Cancer Res Jul 15 2001;61(14):5355-5361; PMID:11454674
- Mishima K, Johns TG, Luwor RB, Scott AM, Stockert E, Jungbluth AA, Ji XD, Suvarna P, Voland JR, Old LJ, et al. Growth suppression of intracranial xenografted glioblastomas overexpressing mutant epidermal growth factor receptors by systemic administration of monoclonal antibody (mAb) 806, a novel monoclonal antibody directed to the receptor. Cancer Res Jul 15 2001; 61(14):5349-5354; PMID:11454673
- Jungbluth AA, Stockert E, Huang HJ, Collins VP, Coplan K, Iversen K, Kolb D, Johns TJ, Scott AM, Gullick WJ. A monoclonal antibody recognizing human cancers with amplification/overexpression of the human epidermal growth factor receptor. Proc Natl Acad Sci U S A. Jan 21 2003; 100(2):639-644; PMID:12515857; http://dx.doi.org/10.1073/pnas.232686499
- Johns TG, Stockert E, Ritter G, Jungbluth AA, Huang HJ, Cavenee WK, Smyth FE, Hall CM, Watson N, Nice EC, et al. Novel monoclonal antibody specific for the de2-7 epidermal growth factor receptor (EGFR) that also recognizes the EGFR expressed in cells containing amplification of the EGFR gene. Int J Cancer J Int du Cancer Mar 20 2002; 98(3):398-408; PMID:11920591; http://dx.doi.org/10.1002/ijc.10189
- Heimberger AB, Crotty LE, Archer GE, Hess KR, Wikstrand CJ, Friedman AH, Friedman HS, Bigner DD, Sampson JH. Epidermal growth factor receptor VIII peptide vaccination is efficacious against established intracerebral tumors. Clin Cancer Res Sep 15 2003; 9(11):4247-4254; PMID:14519652
- Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol 1991; 9:271-296; PMID:1910679; http://dx.doi.org/10.1146/annurev.iy.09.040191.001415
- Liau LM, Black KL, Prins RM, Sykes SN DiPatre PL, Cloughesy TF, Becker DP, Bronstein JM. Treatment of intracranial gliomas with bone marrow-derived dendritic cells pulsed with tumor antigens. J Neurosurg Jun 1999; 90(6):1115-1124; PMID:10350260; http://dx.doi.org/10.3171/jns.1999.90.6.1115
- Heimberger AB, Learn CA, Archer GE, McLendon RE, Chewning TA, Tuck FL, Pracyk JB, Friedman AH, Friedman HS, Bigner DD, et al. Brain tumors in mice are susceptible to blockade of epidermal growth factor receptor (EGFR) with the oral, specific, EGFR-tyrosine kinase inhibitor ZD1839 (iressa). Clin Cancer Res Nov 2002; 8(11):3496-3502; PMID:12429640
- Wu AH, Xiao J, Anker L, Hall WA, Gregerson DS, Cavenee WK, Chen W, Low WC. Identification of EGFRvIII-derived CTL epitopes restricted by HLA A0201 for dendritic cell based immunotherapy of gliomas. J Neuro-oncol Jan 2006; 76(1):23-30; PMID:16155724; http://dx.doi.org/10.1007/s11060-005-3280-7
- Sampson JH, Archer GE, Mitchell DA, Heimberger AB, Herndon JE 2nd, Lally-Goss D, McGehee-Norman S, Paolino A, Reardon DA, Friedman AH, et al. An epidermal growth factor receptor variant III-targeted vaccine is safe and immunogenic in patients with glioblastoma multiforme. Mol Cancer Ther Oct 2009; 8(10):2773-2779; PMID:19825799; http://dx.doi.org/10.1158/1535-7163.MCT-09-0124
- Sampson JH, Heimberger AB, Archer GE, Aldape KD, Friedman AH, Friedman HS, Gilbert MR, Herndon JE 2nd, McLendon RE, Mitchell DA, et al. Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. J Clin Oncol Nov 1 2010; 28(31):4722-4729; PMID:20921459; http://dx.doi.org/10.1200/JCO.2010.28.6963
- Sampson JH, Aldape KD, Archer GE, Coan A, Desjardins A, Friedman AH, Friedman HS, Gilbert MR, Herndon JE, McLendon RE, et al. Greater chemotherapy-induced lymphopenia enhances tumor-specific immune responses that eliminate EGFRvIII-expressing tumor cells in patients with glioblastoma. Neuro-oncol Mar 2011; 13(3):324-333; PMID:21149254; http://dx.doi.org/10.1093/neuonc/noq157
- Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry R, Restifo NP, Hubicki AM, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science Oct 25 2002; 298(5594):850-854; PMID:12242449; http://dx.doi.org/10.1126/science.1076514
- Dummer W, Niethammer AG, Baccala R, Lawson BR, Wagner N, Reisfeld RA, Theofilopoulos AN. T cell homeostatic proliferation elicits effective antitumor autoimmunity. J Clin Invest Jul 2002; 110(2):185-192; PMID:12122110; http://dx.doi.org/10.1172/JCI0215175
- Lai RK RL, Reardon DA. Long-term follow-up of ACT III: A Phase II trial of rindopepimut (CDX-110) in newly diagnosed glioblastoma. Neuro-oncol 2011 2011; 13(suppl 3):iii34-iii40.
- Prins RM, Soto H, Konkankit V, Odesa SK, Eskin A, Yong WH, Nelson SF, Liau LM. Gene expression profile correlates with T-cell infiltration and relative survival in glioblastoma patients vaccinated with dendritic cell immunotherapy. Clin Cancer Res Mar 15 2011; 17(6):1603-1615; PMID:21135147; http://dx.doi.org/10.1158/1078-0432.CCR-10-2563
- Wheeler CJ, Black KL, Liu G, Mazer M, Zhang XX, Pepkowitz S, Goldfinger D, Ng H, Irvin D, Yu JS. Vaccination elicits correlated immune and clinical responses in glioblastoma multiforme patients. Cancer Res Jul 15 2008; 68(14):5955-5964; PMID:18632651; http://dx.doi.org/10.1158/0008-5472.CAN-07-5973
- Wheeler CJ. Dendritic cell vaccines to combat glioblastoma. Expert Review Neurother Apr 2010; 10(4):483-486; PMID:20367199; http://dx.doi.org/10.1586/ern.10.26
- Mailliard RB, Wankowics-Kalinska A, Cai Q, Wesa A, Hilkens CM, Kapsenberg ML, Kirkwood JM, Storkus WJ, Kalinski P. Alpha-type 1 polarized dendritic cells: A novel immunization tool with optimized CTL-inducing activity. Cancer Res 2004; 64:5934-5937; PMID:15342370; http://dx.doi.org/10.1158/0008-5472.CAN-04-1261
- Kalinski P, Schuitemaker JHN, Hilkens CMU Wierenga EA, Kapsenberg ML. Final maturation of dendritic cells is associated with impaired responsiveness to IFN-gamma and to bacterial IL-12 Inducers: decreased ability of mature dendritic cells to produce IL-12 during interaction with Th cells. J Immunol 1999; 162:3231-3231; PMID:10092774
- Okada H, Kalinski P, Ueda A, Hoji A, Kohanbash G, Donegan TE, Mintz AH, Engh JA, Bartlett DL, Brown CK et al. Induction of CD8+ T cell responses against novel glioma associated antigen peptides and clinical activity by vaccinations with α- type 1 polarized dendritic cells and polyincosinci-polycytidylic acid stabilized by Lycine and carboxymethylcellulose in patients with recurrent Malignant Glioma. J Clin Onc 2011; 29.3:330-336; PMID:21149657; http://dx.doi.org/10.1200/JCO.2010.30.7744
- Akiyama Y, Oshita C, Kume A, Iizuka A, Miyata H, Komiyama M, Ashizawa T, Yagoto M, Abe Y, Mitsuya K et al. Alpha Type 1 polarized dendritic cell-based vaccination in recurrent high-grade glioma: a phase I clinical trial. Cancer 2012; 12:623; PMID:23270484
- Ishikawa I., Yoshihiro, M, Tetsuya, Y., Maruyama T, Tsuboi K, Ikuta S, Hashimoto K, Uemae Y, Ishihara T, Matsuda M et al. Phase I/IIa trial of fractionated radiotherapy, temozolomide, and autologous formalin-fixed tumor vaccine for newly-diagnosed glioblastoma. J Neurosurg 2014; 121:543-553; PMID:24995786; http://dx.doi.org/10.3171/2014.5.JNS132392
- Terasaki M, Shibui S, Narita Y, Fujimaki T, Aoki T, Kajiwara K, Sawamura Y, Kurisu K, Mineta T, Yamada A et al. Phase I trial of a personalized peptide vaccine for patients positive for humanleukocyte antigen a24 with recurrent or progressive glioblastoma multiforme. J Clin Oncol 2010; 29:337-344; PMID:21149665; http://dx.doi.org/10.1200/JCO.2010.29.7499
- Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, Stankevich E, Pons A, Salay TM, McMiller TL et al. Phase I study of single agent snti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol 2010; 28.19:3167-3175; PMID:20516446; http://dx.doi.org/10.1200/JCO.2009.26.7609
- Iwai Y, Ishida M, Tanaka Y Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from the host immune system and tumor immunotherapy by PD-L1 blockade. Prc Natl Acad Sci USA 2002; 99:12293-12297; PMID:12218188; http://dx.doi.org/10.1073/pnas.192461099
- Strome SE, Dong H, Tamura H, Voss SG, Flies DB, Tamada K, Salomao D, Cheville J, Hirano F, Lin W et al. B7-H1 blockade augments T cell immunotherapy for squamous cell carcinoma. Cancer Res 2003; 63:6501-6505; PMID:14559843