Abstract
Cutaneous papillomaviruses are associated with specific skin diseases, such as extensive wart formation and the development of non-melanoma skin cancer (NMSC), especially in immunosuppressed patients. Hence, clinical approaches are required that prevent such lesions. Licensed human papillomavirus (HPV) vaccines confer type-restricted protection against HPV types 6, 11, 16 and 18, responsible of 90% of genital warts and 70% of cervical cancers, respectively. However, they do not protect against less prevalent high-risk types or cutaneous HPVs. Over the past few years, several studies explored the potential of developing vaccines targeting cutaneous papillomaviruses. These vaccines showed to be immunogenic and prevent skin tumor formation in certain animal models. Furthermore, under conditions mimicking the ones found in the intended target population (i.e., immunosuppression and in the presence of an already established infection before vaccination), recent preclinical data shows that immunization can still be effective. Strategies are currently focused on finding vaccine formulations that can confer protection against a broad range of papillomavirus-associated diseases. The state-of-the-art of these approaches and the future directions in the field will be presented.
Introduction
Papillomaviruses (PVs) infect keratinocytes of the skin and mucosa of different vertebrate species, including humans. Up to date there are more than 170 human papillomavirus (HPV) types known, which are associated with different clinical manifestations.Citation1 Genital HPVs cause diverse lesions ranging from benign warts (for low-risk HPV types) to different malignancies (for high-risk types) of which cervical cancer is the most prominent.Citation2 On the other hand, the wide range of cutaneous HPV types has been associated with diverse skin diseases.Citation2 Notably, recent metagenomics analysis even show that the highest prevalence of HPV is found in the skin (61%), followed by the vagina (41.5%), mouth (30%) and gut (17.3%).Citation3
Many cutaneous HPVs, mainly HPV 2, 7, 27 and 57 (α genus), HPV 4 and 65 (gamma genus), and HPV 1 (mu genus) cause benign papillomas in the skin of which the most frequent entities are common warts (verrucae vulgaris), plantar warts (verrucae plantaris) and flat warts (verrucae plana).Citation4,5 They affect almost one every 3 schoolchildrenCitation6 and represent a serious burden for immunocompromised patients, especially organ transplant recipients (OTR), who suffer from confluent wart formation all throughout the body. The prevalence in this population was reported to be 48–92% in the first 5 y after immunosuppression.Citation5 Therefore, although skin warts are benign, they cause a significant inconvenience for the patient, with a clear impact on public health.
On the other hand, HPVs from the β genus are suspected to play a role in the development of non-melanoma skin cancer (NMSC). NMSC, which comprises basal cell carcinoma (BCC, approximately 80% of cases), squamous cell carcinomas (SCC, approximately 20% of cases) and other less frequent entities, is the most frequent malignancy in the Caucasian population.Citation7 The main risk factor for NMSC is exposure to UV radiation, since more than 80% of the lesions appear in sun-exposed areas. Another important risk factor is immunosuppression, with SCC appearing 65–250 times more frequently in OTR than in the general population.Citation8 Additionally, although still controversially discussed, meta-analysis of the current literature shows cumulative evidence that infection with certain cutaneous HPVs can be a cofactor for the development of NMSC, especially SCC.Citation9-11
The oncogenic potential of β-HPV infection (e. g. HPV5 and HPV8) in NMSC has been originally identified in patients suffering from the rare inherited disease Epidermodysplasia Verruciformis (EV), who are characterized by an increased susceptibility to cutaneous HPV infection.Citation12 These patients apparently display an inherent genetic predisposition, harboring autosomal recessive mutations in the EVER1 and EVER2 genes which have been linked to the disease.Citation13 The transforming potential of the EV-HPVs has not only been shown in transgenic mouse models,Citation14,15 but also in organotypic raft cultures where EVER2-null keratinocytes can generate a hyperplastic epithelium that favors HPV8 replication.Citation16
Additionally, β-papillomaviruses have also been detected in NMSCs of non-EV patients, although usually with very low viral loads. Different studies have reported HPV DNA in 30–50% of NMSCs from immunocompetent patients,Citation17,18 whereas in lesions from immunosuppressed patients this figure goes up to 90%.Citation19 These viruses can also be found in premalignant lesions, such as actinic keratosis, where the virus is transcriptionally active,Citation20 and also in normal skin and plucked eyebrow hairs, where several studies have found an association between presence of viral DNA and increased risk of NMSC.Citation17 Remarkably, there seems to be an inverse correlation between viral load and the malignancy of the lesion,Citation21 supporting a hit-and-run mechanism of carcinogenesisCitation8 that is in contrast to the direct carcinogenic effect of genital HPVs. In other words, since some tumors either lack HPV or only one every 10–1000 cell can be found to be virus-positive, the viral oncoproteins are not necessary to maintain a proliferative and tumorigenic phenotype.Citation22 In addition to reports focused on the correlation between β-PV DNA and disease, numerous seroepidemiological studies have also contributed data suggesting an association between β-PV infection and NMSC or its precursors.Citation23-26
Considering a causal function of β-HPV in cancer formation, several studies have identified different transformation mechanisms that may explain a viral contribution to carcinogenesis. High-risk genital HPV types are known to degrade p53, thereby blocking downstream pathways such as apoptosis.Citation27 Although β-PV E6 protein cannot exert this direct function, it has recently been shown that the E6 proteins of some β types can inhibit HIPK2-mediated phosphorylation of p53 at serine residue 46 in response to UV damage.Citation28 This prevents the stabilization of p53 in response to genome destabilizing eventsCitation29 and blocks the transactivation of p53 target genes (i.e., MDM2, p21 and proapoptotic genes).Citation30 Additionally, β-PV E6 targets the pro-apoptotic protein Bak for degradation.Citation31,32 These effects, usually combined with mechanisms that delay DNA repair (like abrogation of ATR activityCitation33) or impairment of the telomere/telomerase systemCitation34 may explain β-PV contribution to skin carcinogenesis by favoring the accumulation of UV damaged cells. The oncogenic potential of β-PV has also been shown in in vivo transgenic models which develop SCC in response to β-PV gene expression, either spontaneously or after UV irradiation.Citation14,35
Although mortality from NMSC is rare in the immunocompetent population, this malignancy represents a considerable burden on the health-care system, particularly when considering immunosuppressed patients.Citation7 It is estimated that up to 40% of OTR will develop skin cancers, 90% of which are BCC and SCC, within the first 10 y of transplantation, and up to 80% will do so after 20 yCitation36 Additionally, skin malignancies are more aggressive in OTRs, with only 56% of 3-year disease-specific survival.Citation37 Therefore, a prophylactic strategy that could prevent or reduce the impact of these skin manifestations is much needed.
HPV Vaccines
Vaccination against genital HPV types is currently being used worldwide to prevent infection and, in turn, the development of HPV-induced lesions in the mucosa. The two licensed vaccines are composed of the L1 major capsid protein of HPV which has the ability to self-assemble into highly repetitive virus-like particles (VLPs).Citation38 One vaccine consists of VLPs of HPV16 and HPV18, the 2 more prevalent types causing cervical carcinoma, the other additionally contains VLPs of HPV6 and HPV11 to prevent the development of genital warts. Clinical trials showed a 100% efficacy in preventing HPV16-and HPV18‑associated dysplasia in women who had no evidence of infection at enrolment,Citation39 and the protection elicited was long-term, lasting for at least the first 8.4 y after vaccination.Citation40 Several lines of evidence suggest that vaccine-induced neutralizing antibodies are the primary mediators of the protection elicited by these HPV vaccines, protecting from a subsequent infection by the targeted HPV types and therefore conferring sterilizing immunity.Citation41
Although these vaccines are very effective, they also have several limitations: (i) they target only 2 mucosal high-risk types, therefore potentially preventing approximately 70% of the cervical cancer cases, which leaves 30% of the cases unattended; (ii) they do not target any cutaneous type; (iii) they are very costly, making implementation of wide-covering vaccination schedules difficult in less affluent countries. A VLP-based nonavalent vaccine, which targets 5 extra genital high-risk types, is currently in clinical trials to bridge the gap for cervical carcinoma causing types.Citation42 Although such a vaccine would increase the rate of protection to theoretically protect against more than 90% of cervical cancer cases, it is unlikely to decrease the already very high costs of HPV vaccination and, additionally, it would still not target any cutaneous type associated with skin warts or NMSC.
L1 Vaccines Against Cutaneous PV Types
The first studies which assessed the potential as prophylactic vaccines of L1-based particles from cutaneous HPV types were focused on analyzing the elicitation of neutralizing antibodies in response to vaccination. Senger et al.Citation43 explored the immunogenicity of capsomeres and VLPs from HPV α types 2, 27, and 57, the most frequent causative agents of skin warts. Antibody responses in mice resembled those observed upon vaccination with HPV 16 L1-based antigens and were type-restricted in their in vitro neutralizing capacities, as it has been observed for genital HPV types. Combination of the 3 antigens in the vaccine triggered an antibody response capable of efficiently neutralizing all 3 types. In another study, Handisurya et al.Citation44 studied the serological relationship between VLPs from HPV β types 5, 8 and 92. Again, antibody responses in rabbits were comparable with the ones elicited by genital-types VLPs and, in this case, a limited degree of cross-neutralization could be evidenced.
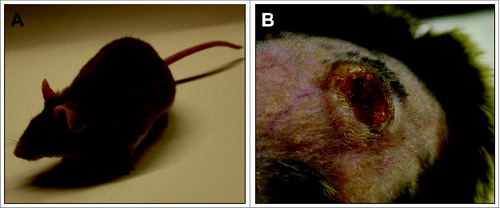
However, to test a vaccine in a preclinical setting, the mere measurement of appearing antibodies is not sufficient. In other words, to show the efficacy of a vaccine, appropriate in vivo read-out systems are required where skin tumors are prevented in a natural host. Evidence that a VLP-based vaccine against cutaneous papillomaviruses can indeed avoid spontaneous skin tumor formation even under immunosuppressed conditions came recently from the animal model Mastomys coucha.Citation45 These rodents are naturally and persistently infected with a cutaneous PVCitation46 and spontaneously develop not only benign skin tumors, such as papillomas and keratoacanthomas, but also squamous cell carcinomas for which the Mastomys PV is the etiological agent.Citation47 Infection occurs early in lifetime, similarly to cutaneous HPVs, and high viral loads can be detected in the skin of older animals, which trigger the onset of tumor development (). Although other reliable animal systems exist (e.g. the cervicovaginal murine challenge model or the cottontail rabbit model),Citation48,49 Mastomys coucha allows to study the impact of a skin papillomavirus in an immunocompetent animal, starting from primary infection in newborns until the final manifestation of a tumor in a natural host. Moreover, in contrast to mice or rabbit inbred strains, the outbred character of these animals does not only mimic the genetic heterogeneity in humans, but also allows determining whether a VLP vaccine is readily protective and finally of translational importance for patients.
Figure 1. Mastomys coucha as animal model for papillomavirus-induced tumors. (A) Mastomys coucha. M. coucha is an African multimammate mouse of the Muridae family. (B) Skin keratoacanthoma. Mastomys coucha is latently infected with MnPV, which induces skin tumors in older animals.
In a recent study, we evaluated the efficacy of a VLP-based vaccine on either previously or newly established infections in Mastomys coucha.Citation45 Upon inoculation, the VLPs induce a long-lasting response of neutralizing antibodies that confers protection against benign as well as malignant skin tumors even under systemic immune suppression. Notably, protection includes the maintenance of a low viral load in the skin by an antibody-dependent prevention of virus spread. These results prove the principle that VLPs elicit an effective immune response in the skin under immunocompetent and immunosuppressed conditions in an outbred animal model, irrespective of the infection status at the time of vaccination. This may provide the basis for follow-up studies that should finally lead to the clinical implementation of a vaccine against cutaneous HPV-induced tumors to avoid SCCs especially in OTR.
Second Generation Vaccines and Future Directions
Since there are several cutaneous HPV types potentially involved in the development of non-melanoma skin cancer in humans,Citation50 a broadly protective vaccine would be ideal. The papillomavirus minor capsid protein L2, contains a major cross-neutralizing epitope that could be used to develop a second generation vaccine.Citation51 However, L2-derived linear peptides are in general weakly immunogenic, which is why different strategies have been explored to overcome this poor immunogenicity, such as conjugation of L2 peptides to a T-helper epitope and a Toll-like receptor ligand,Citation52 concatemer formation of multiple L2 peptides,Citation53 L2 peptide display on structures like bacteriophagesCitation54 or on adeno-associated viruses,Citation55 chimeric HPV L2 peptide/L1-VLPsCitation56 or integration into the thioredoxin active site.Citation57 All these approaches have shown that production of neutralizing antibodies with a broad range of cross-neutralization is achievable, but the titers are usually lower than the ones obtained against L1 when using VLPs.
A few studies have focused in the use of an L2-derived vaccine to also extend the range of protection to cutaneous papillomaviruses. Vaccination with an HPV16-derived L2 peptide can generate antibodies which cross-neutralize in vitro the non-cognate cutaneous types HPV 2, HPV 3, HPV 5, HPV 8, HPV 23, HPV 27, HPV 38, HPV 57 and HPV 76.Citation52,58,59 Cross-neutralization could also be achieved in vivo by cutaneous challenge with pseudovirions, highlighting the potential of the L2-derived vaccines to confer protection to a broad range of cutaneous HPVs. However, the effectivity of such vaccines in preventing skin tumors has not been assessed so far, and fundamental questions remain to be addressed to confirm the prophylactic potential of these vaccines. One important issue to be answered is whether L2 vaccination can elicit enough neutralizing antibodies to confer protection against tumor formation. Using the cervicovaginal murine challenge model, passive immunization with different dilutions of anti-L1 antibodies apparently interferes with infection at 2 stages (i.e. blocking of the binding to the acellular basement membrane at high titers, inhibition of the association to the epithelial cell surface at low antibody titers).Citation60 However, there is no direct correlate known between antibody levels elicited by the HPV L1 vaccines and the ultimate protection from lesion development. Therefore, although L2-vaccines generate lower neutralization titers as compared with their L1 counterparts, there is a high chance that these titers are enough for tumor prevention. However, there is still the need to prove such a vaccine in a preclinical model in which spontaneous development of PV-induced tumors can be used as a read-out. For this, Mastomys coucha stands out as an excellent animal system to test this new generation of vaccines. Being infected with both a cutaneous and a mucosal PV type,Citation46 Mastomys provides the possibility of not only assessing whether the antibody level raised by an L2-vaccine is sufficient, but also of testing for the first time whether a vaccine targeted against the L2-peptide of one PV-type can prevent tumors caused by an unrelated type under normal and immunosuppressed conditions.
In conclusion, there is currently a large body of results in preclinical models pointing to the fact that a vaccine against cutaneous papillomaviruses is possible and might prevent HPV-associated skin disease, such as warts and NMSC. However, the high number of both cutaneous and genital HPV types which are linked to human diseases, demands for a vaccine that can protect from all these types at once. In this regard, L2 vaccines are promising candidates, and validation of such vaccines in suitable animal models will provide the basis for the clinical implementation of these prophylactic tools.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by a grant from the Wilhelm Sander Stiftung, Munich, Germany (Förderprojekt 2010.019.1).
References
- de Villiers EM. Cross-roads in the classification of papillomaviruses. Virology 2013; 445:2-10; PMID:23683837; http://dx.doi.org/10.1016/j.virol.2013.04.023
- Grce M, Mravak-Stipetic M. Human papillomavirus-associated diseases. Clin Dermatol 2014; 32:253-8; PMID:24559561; http://dx.doi.org/10.1016/j.clindermatol.2013.10.006
- Ma Y, Madupu R, Karaoz U, Nossa CW, Yang L, Yooseph S, Yachimski PS, Brodie EL, Nelson KE, Pei Z. Human papillomavirus community in healthy persons, defined by metagenomics analysis of human microbiome project shotgun sequencing data sets. J Virol 2014; 88:4786-97; PMID:24522917; http://dx.doi.org/10.1128/JVI.00093-14
- Bruggink SC, de Koning MN, Gussekloo J, Egberts PF, Ter Schegget J, Feltkamp MC, Bavinck JN, Quint WG, Assendelft WJ, Eekhof JA. Cutaneous wart-associated HPV types: prevalence and relation with patient characteristics. J Clin Virol 2012; 55:250-5; PMID:22884670; http://dx.doi.org/10.1016/j.jcv.2012.07.014
- Jablonska S, Majewski S, Obalek S, Orth G. Cutaneous warts. Clin Dermatol 1997; 15:309-19; PMID:9255438; http://dx.doi.org/10.1016/S0738-081X(96)00170-8
- van Haalen FM, Bruggink SC, Gussekloo J, Assendelft WJ, Eekhof JA. Warts in primary schoolchildren: prevalence and relation with environmental factors. Br J Dermatol 2009; 161:148-52; PMID:19438464; http://dx.doi.org/10.1111/j.1365-2133.2009.09160.x
- Eisemann N, Waldmann A, Geller AC, Weinstock MA, Volkmer B, Greinert R, Breitbart EW, Katalinic A. Non-melanoma skin cancer incidence and impact of skin cancer screening on incidence. J Invest Dermatol 2014; 134:43-50; PMID:23877569; http://dx.doi.org/10.1038/jid.2013.304
- Nindl I, Rösl F. Molecular concepts of virus infections causing skin cancer in organ transplant recipients. Am J Transplant 2008; 8:2199-204; PMID:18785959; http://dx.doi.org/10.1111/j.1600-6143.2008.02392.x
- Wang J, Aldabagh B, Yu J, Arron ST. Role of human papillomavirus in cutaneous squamous cell carcinoma: a meta-analysis. J Am Acad Dermatol 2014; 70:621-9; PMID:24629358; http://dx.doi.org/10.1016/j.jaad.2014.01.857
- Iannacone MR, Gheit T, Pfister H, Giuliano AR, Messina JL, Fenske NA, Cherpelis BS, Sondak VK, Roetzheim RG, Silling S, et al. Case-control study of genus-beta human papillomaviruses in plucked eyebrow hairs and cutaneous squamous cell carcinoma. Int J Cancer 2014; 134:2231-44; PMID:24136717; http://dx.doi.org/10.1002/ijc.28552
- Farzan SF, Waterboer T, Gui J, Nelson HH, Li Z, Michael KM, Perry AE, Spencer SK, Demidenko E, Green AC, et al. Cutaneous alpha, beta and gamma human papillomaviruses in relation to squamous cell carcinoma of the skin: a population-based study. Int J Cancer 2013; 133:1713-20; PMID:23536363; http://dx.doi.org/10.1002/ijc.28176
- Orth G. Human papillomaviruses associated with epidermodysplasia verruciformis in non-melanoma skin cancers: guilty or innocent? J Invest Dermatol 2005; 125:xii-xiii; PMID:15982294; http://dx.doi.org/10.1111/j.0022-202X.2005.23811.x
- Lazarczyk M, Cassonnet P, Pons C, Jacob Y, Favre M. The EVER proteins as a natural barrier against papillomaviruses: a new insight into the pathogenesis of human papillomavirus infections. Microbiol Mol Biol Rev 2009; 73:348-70; PMID:19487731; http://dx.doi.org/10.1128/MMBR.00033-08
- Schaper ID, Marcuzzi GP, Weissenborn SJ, Kasper HU, Dries V, Smyth N, Fuchs P, Pfister H. Development of skin tumors in mice transgenic for early genes of human papillomavirus type 8. Cancer Res 2005; 65:1394-400; PMID:15735026; http://dx.doi.org/10.1158/0008-5472.CAN-04-3263
- Pfefferle R, Marcuzzi GP, Akgul B, Kasper HU, Schulze F, Haase I, Wickenhauser C, Pfister H. The human papillomavirus type 8 E2 protein induces skin tumors in transgenic mice. J Invest Dermatol 2008; 128:2310-5; PMID:18401427; http://dx.doi.org/10.1038/jid.2008.73
- Borgogna C, Zavattaro E, De Andrea M, Griffin HM, Dell'Oste V, Azzimonti B, Landini MM, Peh WL, Pfister H, Doorbar J, et al. Characterization of beta papillomavirus E4 expression in tumours from Epidermodysplasia Verruciformis patients and in experimental models. Virology 2012; 423:195-204; PMID:22217391; http://dx.doi.org/10.1016/j.virol.2011.11.029
- Harwood CA, Surentheran T, Sasieni P, Proby CM, Bordea C, Leigh IM, Wojnarowska F, Breuer J, McGregor JM. Increased risk of skin cancer associated with the presence of epidermodysplasia verruciformis human papillomavirus types in normal skin. Br J Dermatol 2004; 150:949-57; PMID:15149508; http://dx.doi.org/10.1111/j.1365-2133.2004.05847.x
- Pfister H. Chapter 8: human papillomavirus and skin cancer. J Natl Cancer Inst Monogr 2003; 31:52-6; PMID:12807946; http://dx.doi.org/10.1093/oxfordjournals.jncimonographs.a003483
- Harwood CA, Surentheran T, McGregor JM, Spink PJ, Leigh IM, Breuer J, Proby CM. Human papillomavirus infection and non-melanoma skin cancer in immunosuppressed and immunocompetent individuals. J Med Virol 2000; 61:289-97; PMID:10861635; http://dx.doi.org/10.1002/1096-9071(200007)61:3%3c289::AID-JMV2%3e3.0.CO;2-Z
- Borgogna C, Lanfredini S, Peretti A, De Andrea M, Zavattaro E, Colombo E, Quaglia M, Boldorini R, Miglio U, Doorbar J, et al. Improved detection reveals active beta-papillomavirus infection in skin lesions from kidney transplant recipients. Mod Pathol 2014; 27(8):1101-15; PMID:24390217
- Weissenborn SJ, Nindl I, Purdie K, Harwood C, Proby C, Breuer J, Majewski S, Pfister H, Wieland U. Human papillomavirus-DNA loads in actinic keratoses exceed those in non-melanoma skin cancers. J Invest Dermatol 2005; 125:93-7; PMID:15982308; http://dx.doi.org/10.1111/j.0022-202X.2005.23733.x
- Akgül B, Cooke JC, Storey A. HPV-associated skin disease. J Pathol 2006; 208:165-75; PMID:16362995; http://dx.doi.org/10.1002/path.1893
- Feltkamp MC, Broer R, di Summa FM, Struijk L, van der Meijden E, Verlaan BP, Westendorp RG, ter Schegget J, Spaan WJ, Bouwes Bavinck JN. Seroreactivity to epidermodysplasia verruciformis-related human papillomavirus types is associated with nonmelanoma skin cancer. Cancer Res 2003; 63:2695-700; PMID:12750299
- Struijk L, Hall L, van der Meijden E, Wanningen P, Bavinck JN, Neale R, Green AC, Ter Schegget J, Feltkamp MC. Markers of cutaneous human papillomavirus infection in individuals with tumor-free skin, actinic keratoses, and squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev 2006; 15:529-35; PMID:16537712; http://dx.doi.org/10.1158/1055-9965.EPI-05-0747
- Plasmeijer EI, Pandeya N, O'Rourke P, Pawlita M, Waterboer T, Feltkamp MC, Green AC, Neale RE. The Association between Cutaneous Squamous Cell Carcinoma and Betapapillomavirus Seropositivity: a Cohort Study. Cancer Epidemiol Biomarkers Prev 2011; 20:1171-7; PMID:21527580; http://dx.doi.org/10.1158/1055-9965.EPI-11-0110
- Andersson K, Michael KM, Luostarinen T, Waterboer T, Gislefoss R, Hakulinen T, Forslund O, Pawlita M, Dillner J. Prospective study of human papillomavirus seropositivity and risk of nonmelanoma skin cancer. Am J Epidemiol 2012; 175:685-95; PMID:22419740; http://dx.doi.org/10.1093/aje/kwr373
- Moody CA, Laimins LA. Human papillomavirus oncoproteins: pathways to transformation. Nat Rev Cancer 2010; 10:550-60; PMID:20592731; http://dx.doi.org/10.1038/nrc2886
- Muschik D, Braspenning-Wesch I, Stockfleth E, Rösl F, Hofmann TG, Nindl I. Cutaneous HPV23 E6 prevents p53 phosphorylation through interaction with HIPK2. PLoS One 2011; 6:e27655; PMID:22110707; http://dx.doi.org/10.1371/journal.pone.0027655
- Wallace NA, Robinson K, Galloway DA. Beta human papillomavirus E6 expression inhibits stabilization of p53 and increases tolerance of genomic instability. J Virol 2014; 88:6112-27; PMID:24648447; http://dx.doi.org/10.1128/JVI.03808-13
- White EA, Walther J, Javanbakht H, Howley PM. Genus Beta HPV E6 Proteins Vary in their Effects on the Transactivation of p53 Target Genes. J Virol 2014; 88(15):8201-12.; PMID:24850740
- Jackson S, Storey A. E6 proteins from diverse cutaneous HPV types inhibit apoptosis in response to UV damage. Oncogene 2000; 19:592-8; PMID:10698529; http://dx.doi.org/10.1038/sj.onc.1203339
- Underbrink MP, Howie HL, Bedard KM, Koop JI, Galloway DA. E6 proteins from multiple human betapapillomavirus types degrade Bak and protect keratinocytes from apoptosis after UVB irradiation. J Virol 2008; 82:10408-17; PMID:18715924; http://dx.doi.org/10.1128/JVI.00902-08
- Wallace NA, Robinson K, Howie HL, Galloway DA. HPV 5 and 8 E6 abrogate ATR activity resulting in increased persistence of UVB induced DNA damage. PLoS Pathog 2012; 8:e1002807; PMID:22807682; http://dx.doi.org/10.1371/journal.ppat.1002807
- Gabet AS, Accardi R, Bellopede A, Popp S, Boukamp P, Sylla BS, Londono-Vallejo JA, Tommasino M. Impairment of the telomere/telomerase system and genomic instability are associated with keratinocyte immortalization induced by the skin human papillomavirus type 38. FASEB J 2008; 22:622-32; PMID:17898088; http://dx.doi.org/10.1096/fj.07-8389com
- Viarisio D, Mueller-Decker K, Kloz U, Aengeneyndt B, Kopp-Schneider A, Gröne H-J, Gheit T, Flechtenmacher C, Gissmann L, Tommasino M. E6 and E7 from Beta Hpv38 Cooperate with Ultraviolet Light in the Development of Actinic Keratosis-Like Lesions and Squamous Cell Carcinoma in Mice. PLoS Pathog 2011; 7:e1002125; PMID:21779166; http://dx.doi.org/10.1371/journal.ppat.1002125
- Rangwala S, Tsai KY. Roles of the immune system in skin cancer. Br J Dermatol 2011; 165:953-65; PMID:21729024; http://dx.doi.org/10.1111/j.1365-2133.2011.10507.x
- Lott DG, Manz R, Koch C, Lorenz RR. Aggressive behavior of nonmelanotic skin cancers in solid organ transplant recipients. Transplantation 2010; 90:683-7; PMID:20808266; http://dx.doi.org/10.1097/TP.0b013e3181ec7228
- Kirnbauer R, Booy F, Cheng N, Lowy DR, Schiller JT. Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic. Proc Natl Acad Sci U S A 1992; 89:12180-4; PMID:1334560; http://dx.doi.org/10.1073/pnas.89.24.12180
- Lehtinen M, Paavonen J, Wheeler CM, Jaisamrarn U, Garland SM, Castellsague X, Skinner SR, Apter D, Naud P, Salmeron J, et al. Overall efficacy of HPV-16/18 AS04-adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol 2012; 13:89-99; PMID:22075171; http://dx.doi.org/10.1016/S1470-2045(11)70286-8
- Roteli-Martins CM, Naud P, De Borba P, Teixeira JC, De Carvalho NS, Zahaf T, Sanchez N, Geeraerts B, Descamps D. Sustained immunogenicity and efficacy of the HPV-16/18 AS04-adjuvanted vaccine: up to 8.4 years of follow-up. Hum Vaccin Immunother 2012; 8:390-7; PMID:22327492; http://dx.doi.org/10.4161/hv.18865
- Schiller JT, Lowy DR. Understanding and learning from the success of prophylactic human papillomavirus vaccines. Nat Rev Microbiol 2012; 10:681-92; PMID:22961341; http://dx.doi.org/10.1038/nrmicro2872
- Drolet M, Laprise JF, Boily MC, Franco EL, Brisson M. Potential cost-effectiveness of the nonavalent human papillomavirus (HPV) vaccine. Int J Cancer 2014; 134:2264-8; PMID:24174175; http://dx.doi.org/10.1002/ijc.28541
- Senger T, Schadlich L, Textor S, Klein C, Michael KM, Buck CB, Gissmann L. Virus-like particles and capsomeres are potent vaccines against cutaneous alpha HPVs. Vaccine 2010; 28:1583-93; PMID:20003923; http://dx.doi.org/10.1016/j.vaccine.2009.11.048
- Handisurya A, Gambhira R, Schellenbacher C, Shafti-Keramat S, Forslund O, Favre M, Kirnbauer R. Serological relationship between cutaneous human papillomavirus types 5, 8 and 92. J Gen Virol 2009; 90:136-43; PMID:19088282; http://dx.doi.org/10.1099/vir.0.006189-0
- Vinzon SE, Braspenning-Wesch I, Müller M, Geissler EK, Nindl I, Gröne HJ, Schäfer K, Rösl F. Protective vaccination against papillomavirus-induced skin tumors under immunocompetent and immunosuppressive conditions: a preclinical study using a natural outbred animal model. PLoS Pathog 2014; 10:e1003924; PMID:24586150; http://dx.doi.org/10.1371/journal.ppat.1003924
- Nafz J, Schäfer K, Chen SF, Bravo IG, Ibberson M, Nindl I, Stockfleth E, Rösl F. A novel rodent papillomavirus isolated from anogenital lesions in its natural host. Virology 2008; 374:186-97; PMID:18234262; http://dx.doi.org/10.1016/j.virol.2007.12.012
- Nafz J, Köhler A, Ohnesorge M, Nindl I, Stockfleth E, Rösl F. Persistence of Mastomys natalensis papillomavirus in multiple organs identifies novel targets for infection. J Gen Virol 2007; 88:2670-8; PMID:17872518; http://dx.doi.org/10.1099/vir.0.82955-0
- Roberts JN, Buck CB, Thompson CD, Kines R, Bernardo M, Choyke PL, Lowy DR, Schiller JT. Genital transmission of HPV in a mouse model is potentiated by nonoxynol-9 and inhibited by carrageenan. Nat Med 2007; 13:857-61; PMID:17603495; http://dx.doi.org/10.1038/nm1598
- Breitburd F, Kirnbauer R, Hubbert NL, Nonnenmacher B, Trin-Dinh-Desmarquet C, Orth G, Schiller JT, Lowy DR. Immunization with viruslike particles from cottontail rabbit papillomavirus (CRPV) can protect against experimental CRPV infection. J Virol 1995; 69:3959-63; PMID:7745754
- de Koning MN, Weissenborn SJ, Abeni D, Bouwes Bavinck JN, Euvrard S, Green AC, Harwood CA, Naldi L, Neale R, Nindl I, et al. Prevalence and associated factors of betapapillomavirus infections in individuals without cutaneous squamous cell carcinoma. J Gen Virol 2009; 90:1611-21; PMID:19321753; http://dx.doi.org/10.1099/vir.0.010017-0
- Gambhira R, Karanam B, Jagu S, Roberts JN, Buck CB, Bossis I, Alphs H, Culp T, Christensen ND, Roden RB. A protective and broadly cross-neutralizing epitope of human papillomavirus L2. J Virol 2007; 81:13927-31; PMID:17928339; http://dx.doi.org/10.1128/JVI.00936-07
- Alphs HH, Gambhira R, Karanam B, Roberts JN, Jagu S, Schiller JT, Zeng W, Jackson DC, Roden RB. Protection against heterologous human papillomavirus challenge by a synthetic lipopeptide vaccine containing a broadly cross-neutralizing epitope of L2. Proc Natl Acad Sci U S A 2008; 105:5850-5; PMID:18413606; http://dx.doi.org/10.1073/pnas.0800868105
- Jagu S, Karanam B, Gambhira R, Chivukula SV, Chaganti RJ, Lowy DR, Schiller JT, Roden RB. Concatenated multitype L2 fusion proteins as candidate prophylactic pan-human papillomavirus vaccines. J Natl Cancer Inst 2009; 101:782-92; PMID:19470949; http://dx.doi.org/10.1093/jnci/djp106
- Tumban E, Peabody J, Peabody DS, Chackerian B. A pan-HPV vaccine based on bacteriophage PP7 VLPs displaying broadly cross-neutralizing epitopes from the HPV minor capsid protein, L2. PLoS One 2011; 6:e23310; PMID:21858066; http://dx.doi.org/10.1371/journal.pone.0023310
- Nieto K, Weghofer M, Sehr P, Ritter M, Sedlmeier S, Karanam B, Seitz H, Müller M, Kellner M, Hörer M. Development of AAVLP (HPV16/31L2) Particles as Broadly Protective HPV Vaccine Candidate. PLoS One 2012; 7:e39741; PMID:22761884; http://dx.doi.org/10.1371/journal.pone.0039741
- Schellenbacher C, Roden R, Kirnbauer R. Chimeric L1-L2 virus-like particles as potential broad-spectrum human papillomavirus vaccines. J Virol 2009; 83:10085-95; PMID:19640991; http://dx.doi.org/10.1128/JVI.01088-09
- Rubio I, Bolchi A, Moretto N, Canali E, Gissmann L, Tommasino M, Muller M, Ottonello S. Potent anti-HPV immune responses induced by tandem repeats of the HPV16 L2 (20–38) peptide displayed on bacterial thioredoxin. Vaccine 2009; 27:1949-56; PMID:19368776; http://dx.doi.org/10.1016/j.vaccine.2009.01.102
- Schellenbacher C, Kwak K, Fink D, Shafti-Keramat S, Huber B, Jindra C, Faust H, Dillner J, Roden RB, Kirnbauer R. Efficacy of RG1-VLP vaccination against infections with genital and cutaneous human papillomaviruses. J Invest Dermatol 2013; 133:2706-13; PMID:23752042; http://dx.doi.org/10.1038/jid.2013.253
- Kwak K, Jiang R, Wang JW, Jagu S, Kirnbauer R, Roden RB. Impact of Inhibitors and L2 Antibodies upon the Infectivity of Diverse Alpha and Beta Human Papillomavirus Types. PLoS One 2014; 9:e97232; PMID:24816794; http://dx.doi.org/10.1371/journal.pone.0097232
- Day PM, Kines RC, Thompson CD, Jagu S, Roden RB, Lowy DR, Schiller JT. In vivo mechanisms of vaccine-induced protection against HPV infection. Cell Host Microbe 2010; 8:260-70; PMID:20833377; http://dx.doi.org/10.1016/j.chom.2010.08.003
