Abstract
Chicken egg yolk antibodies against Vipera lebetina venom were evaluated for their antivenom potential. White leghorn hens were immunized with detoxified V. lebetina venom (γ-irradiated venom). The detoxified venom (200 μg) was mixed with an equal volume of complete Freund's adjuvant and was injected intramuscularly into the hens. The antibodies showed high activity (1.6 LD50/mL) in egg yolks after 12 d of venom injection. The eggs were collected after 12 days, and the egg yolks were removed and washed with purified water to remove any contamination with egg whites. The purification was performed using a method described by Maya Devi et al., followed by gel filtration (Sephadex G-50). The purity and molecular weight of antivenom antibodies (IgY) were determined using electrophoresis, and the molecular weight was found to be approximately 185 kDa. The potency of IgY was 6 LD50/mL (mice), i.e., 1 mL of IgY could neutralize 43.8 μg of standard V. lebetina venom). Our results showed that chicken egg yolk antibodies were effective in neutralizing the lethality and several pharmacological effects of V. lebetina venom and could be used for developing effective antivenom.
Abbreviations
| d | = | days; Fig. |
| figure | = | IgY |
| immunoglobulin Y | = | LD50 |
| lethal dose 50% | = | mL |
| milliliter | = | SDS-PAGE |
| sodium dodecyl | = | ulfate polyacrylamide gel electrophoresis |
Introduction
Envenoming and deaths due to snakebites are a major public health concern in rural tropical areas of Africa, Asia, Latin America, and New Guinea. Globally, snakebites are associated with a minimum of 421 000 envenomings and 20 000 deaths to as high as 2.5 million envenoming and >100 000 deaths each year.Citation1 Vipera lebetina obtusa (belonging to the Viperidae family) is one of the major species that is responsible for snakebites in most counties.Citation2 In Iran, snakebites are often neglected, thus resulting in high morbidity rates and healthcare costs.Citation3 Therefore, in 2009, the World Health Organization (WHO) prioritized improvement of healthcare quality delivered to snakebite victims.Citation4 Snake venom contains various proteins with biological and pharmacological importance. In addition, snake venom contains a complex composition of proteolytic enzymes that belong to 2 groups: serine proteases and metalloproteases. Enzymes belonging to both these groups affect the hemostatic system through several mechanisms.Citation5,6 Serine proteases are mainly found in venoms of snakes belonging to the Viperidae family.Citation7 The LD50 estimate of these venoms for an animal (mice) is approximately 6.4 μg; however, there is no report on humans.Citation8
Most antivenoms used to treat envenoming by snakes and scorpions are produced in horses and are purified using methods such as salting out (ammonium sulfate fractionation) and pepsin digestion.Citation9,10 These antivenoms are capable of neutralizing the toxicity and lethality of venoms and are often associated with significant clinical side effects because they contain several non-immunoglobulin protein and immunoglobulins that do not react with the venom component.Citation11,12 Commercial horse antivenom contains high concentrations of non-immunoglobulins that cause various side effects such as serum sickness and renal failureCitation13. Moreover, immunization and purification of immunoglobulin from mammalian blood are time consuming and expensive and need more facilities and larger environments.Citation14 Generally, 3 types of antivenom [IgG, F(ab')2, and Fab] are available; of these, F(ab')2 is used in many countries.Citation1 The amount of antivenom used varies with the severity of envenomation and from one case to another. The route of administration can be intravenous or intramuscular.Citation15 It is important to administer the entire initial dose of antivenom as soon as possible based on the best estimate of severity of envenomation. The following initial doses are recommended: minimal envenomation, 20–40 mL; moderate envenomation, 50–90 mL; and severe envenomation, 100–150 mL or more generally. Administration of additional doses must be considered based on the clinical response to the initial dose and continuous assessment of the severity of poisoning. If swelling continues, severity of systemic symptoms or signs of envenomation increases, or new manifestations appear (e.g., decrease in hematocrit or hypotension), an additional dose of 10–50 mL or more is administered intravenously. In case of severe envenomation, 200–400 mL of antivenom may be necessary. There is no recommended maximum dose. The total required dose is equal to the amount of antivenom needed to neutralize the venom, as determined by clinical response.Citation16 The abovementioned administration schedule is general for all snake antivenoms.
There are many reports on the development of snake venom antibodies in chicken egg yolk against venoms of Russell's viperCitation13,14 and Bothrops and/or Crotalus.Citation17 The major antibody in chickens is immunoglobulin Y (IgY). Klemperer first described passive immunity in birds in 1893 by demonstrating the transfer of immunity against tetanus toxin from hens to chicks.Citation18 Few reports show that IgY yield of one egg is equal to that obtained from 300 mL of rabbit blood.Citation19 The quantity of antibody obtained from rabbits and chicken is 1400 and 40 000 mg per year, respectively. Studies have shown that higher concentrations of IgY are present in egg yolk than in serum.Citation19
Serum IgG antibodies from immunized chickens have been efficiently transported and accumulated in egg yolks.Citation20,21 High antibody activity can be maintained in egg yolks for several months by periodic immunization. Furthermore, vaccination of small animals such as chickens can be easily performed.Citation22
Chicken is an excellent source of antibodies, and each egg yolk contains 8–20 mg of IgY per milliliter.Citation23 Thalley and Carroll prepared specific avian antivenoms by using an efficient method.Citation24 In another report, adult white leghorn hens hyperimmunized with Brazilian Bothrops and/or Crotalus viper venom produced antibodies that recognized and neutralized toxic and lethal components of venoms.Citation25 In 2002, Maya Devi et al. reported the immunization of chicken by γ-irradiated Vipera venom and isolation and purification of chicken egg yolks. They found that immunized hens produced antivenom in considerable amounts and that γ-irradiated venom was safe for immunizing hens.
The abovementioned study described a simple method for purifying antivenom with 90% consistency, which showed good neutralization of venom in vitro. Antibodies were found in eggs for up to 100 d after the immunization, and the antivenom purified from immunized chicken egg yolks was biologically active.Citation13,14 Chickens, as a source of antivenom antibodies, can be used as an alternative animal system that offers some advantages with respect to animal care, high productivity, reduction in the number of animals required, and refinement. The present study evaluated the effectiveness of chicken egg yolk antibodies obtained by immunizing chicken with irradiated V. lebetina venom.
Results
Purpose
This study aimed to produce antibodies against V. lebetina venom in chicken eggs and develop a method to purify and evaluate these antibodies both in vivo and in vitro.
Toxicity of crude venom and properties of crude egg yolk antibodies
The toxicity (LD50) of crude V. lebetina venom was 7.3 μg (as a dry weight) per mice. Crude egg yolk antibodies were detected using Ouchterlony double diffusion method. As shown in , 30 μg of venom was added to the middle well; 30 μg of crude egg yolk from test and control groups was added to wells 1 and 2, respectively; and 30 μg of fraction 1 of purified egg yolk was added to well 3. The results showed multiple precipitin lines in well 1 (). No reaction was observed in well 2, and a specific reaction between antigen and antibody was observed in well 3. These results indicated that egg yolks contained specific antibodies after hyperimmunization, as indicated by the precipitation of Ouchterlony test venom by IgY antibodies.
Figure 1. The Ouchterlony's double diffusion profile of egg yolk antibodies. middle well: 30 μg venom, wells no 1: 30 μg of crud egg yolk of test group, wells no 2: 30 μg of crud egg yolk of control group, wells no 3: 30 μg of purified fraction 1 of egg yolk from test group.
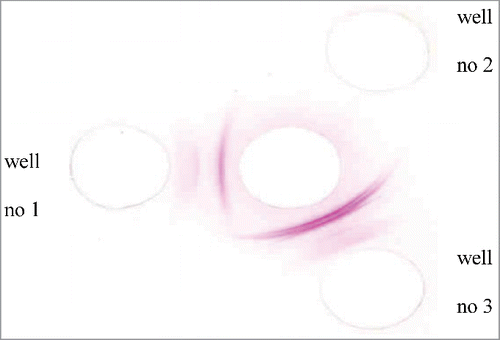
The antivenom activity of crude egg yolk antibodies was analyzed by performing serum neutralization assay. The antivenom activity was found to be 1.6 LD50/mL after 12 d of immunization.
Purification of egg yolk antibodies
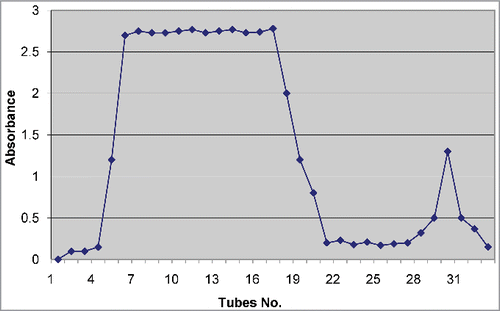
IgY antibodies in the egg yolk were purified using gel chromatography. The pattern of protein elution from the column is shown in . The pooled first peak (F1) contained 3.76% w/v of proteins, and the second peak (F2) contained 1.56% w/v of proteins. Quantitative affinity chromatography showed that approximately 10.1% immunoglobulins in F1 and 3.5% immunoglobulins in F2 were venom specific IgY.
Results of SDS-PAGE and purity test
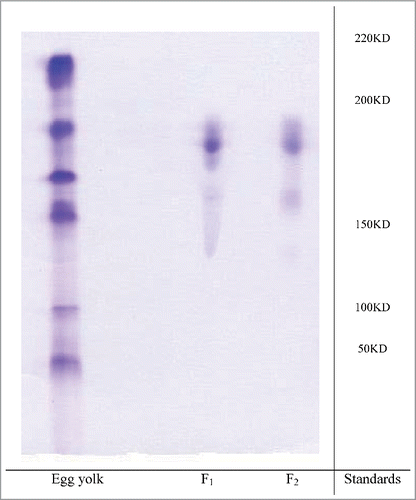
The purity and molecular weight of IgY were identified by electrophoresis. A main protein band in the electrophoresis pattern showed the purity of antibody, which was obtained by gel chromatography. The immunoglobulin band intensities for F1 and F2 constituted ∼93% and ∼90% of the cumulative band intensities on SDS-PAGE (). The molecular weight of IgY (F1 and F2) was found to be approximately 185 kDa.
Potency
The antibody activity of both the fractions was evaluated using serum neutralization assay. F1, with a total protein concentration of 0.75 g/mL, showed efficacy in neutralization assays and was able to protect animals from death. The potency of F1 was approximately 6 LD50/mL (mice) while that of F2, which had the same protein concentration of 0.75 g/mL, was approximately 2 LD50/mL (mice) ().
Table 1. Vipera lebetina snake venom neutralization potency of chicken egg yolk antibody Fractions 1 (sample from the pooled first peak) and 2 (sample from the pooled second peak)
Discussion
As shown in , multiple precipitin lines in well 1 indicated the presence of antibodies in the egg yolk before purification. No reaction was observed in well 2, indicating that specific antibodies against the venom were not present in the control group. However, a specific reaction between antigen and antibody was observed in well 3, indicating that all the animals showed good response to venom and suggesting that chicken eggs are a good source of antibodies because they have high levels of antibodies against V. lebetina venom. The purity of egg yolks after the purification was determined using SDS-PAGE. As observed in the electrophoresis pattern, IgY purified in the present study had formed a main band and was highly pure.
Currently available antibodies are mostly mammalian monoclonal or polyclonal antibodies. Conventionally, larger animals such as horse and sheep are used for producing polyclonal antibodies. However, production and purification of antibodies from the blood of these animals result in low yield, and the obtained antibodies contain a mixture of foreign proteins that may cause severe allergic reactions in patients. Steps involved in both the technologies, such as (i) immunization, (ii) collection of blood samples, and (iii) bleeding that are a prerequisite for antibody production, cause distress to the involved animals. An average volume of egg yolk (15 mL) contains 50–100 mg of IgY, with 2%–10% of specific antibodies. The yield of IgY is significantly greater than the yield of polyclonal antibodies from mammals such as horse.Citation26
Chicken polyclonal antibodies were produced against a number of various antigens (such as Helicobacter pylori, cholera toxin B, and different animal venoms) and can be used for various purposes (such as for research, diagnosis, and therapy; as tools for purifying or detecting antigens; and as protective agents in passive immunization). They are an excellent alternative to their mammalian counterparts. IgY can be used in place of mammalian IgG for all immunological tests. In the last 5 years, IgY has been successfully used for passive and protective immunization against gastrointestinal pathogens in humans and animals; as an immunotherapeutic agent against pathogens that are difficult to treat using traditional antibiotics; and as an useful tool in cancer research, diagnosis, and therapy.Citation26
Nowadays, chickens are being increasingly used instead of mammals for antibody production. A major advantage of using chickens is that antibodies can be harvested from egg yolks instead of serum, thus making blood sampling obsolete. In addition, antibody productivity of an egg-laying hen is much higher than that of a similar-sized mammal.Citation27
Michael et al. reported that chicken egg yolk antibodies can be considered as an alternative to mammalian antibodies. IgY antibodies in chicken blood are transferred to eggs and accumulate in the egg yolk in large quantities. Yolks of eggs laid by immunized chickens have been recognized as an excellent source of polyclonal antibodies for over a decade.Citation28
However, Landon et al. have reported that avian proteins can induce allergic reactions, and their use may be associated with high incidence of side effects. This suggests that more purification steps may be required to produce a more specific product.Citation29
Purification of immunoglobulins from mammalian plasma is complicated, time consuming, and expensive. Today, hens are recognized as a convenient and inexpensive source of antibodies. The amount of immunoglobulins obtained from an egg laid by an immunized hen is almost the same as that obtained from 300 mL of rabbit blood.
Mandal et al. reported the immunization of rabbits with γ-irradiated venom (Russell's viper venom) mixed with aluminum phosphate adjuvant on days 0, 15, and 30 to produce antivenom that could effectively neutralize the lethal activity of the crude venom.Citation30 In addition, they reported the neutralization potency of antivenom (produced in eggs laid by chickens immunized using irradiated venom) against V. lebetina venom.
Larsson et al. found that the concentration of IgY was higher in egg yolks than in serum.Citation31 The concentration of antibodies in egg yolks peaked from days 12 to 14 after immunization. Stuart et al. reported that a secondary response was observed at least 3 weeks after primary immunization.Citation32 The results of the present study are in agreement with those of previous studies. Moreover, the biological activity of the antivenom, determined using the serum neutralization assay, was demonstrated in this study (). To the best of our knowledge, this is the first study in the world to report and snakebites by V. lebetina have high frequency. Because we are the main manufacturer of antivenom in the Middle East, this study will form an important basis for research and development at our institute.
In conclusion, γ-irradiated venom is safe for immunizing hens. This study reports a simple method for purifying and isolating antivenom against V. lebetina venom from chicken egg yolks. The purified antibodies have a good purity profile and desirable potency. This study also indicates that IgY is a promising candidate as an alternative to mammalian antibodies.
Materials and Methods
Animals
Healthy normal leghorn hens were purchased from Damavand Poultry Farm and were housed in individual cages in an isolated condition. The hens were vaccinated against poultry diseases. Balb/c mice (weight, 18–20 g) were obtained from Razi Institute and were used for lethality and potency tests.
Venom
Freeze-dried snake venom (from V. lebetina) was obtained from Razi Institute. One milliliters of the venom was exposed to 60 Co γ irradiation with 100-KR strength for 3 hours.Citation13,30 In order to confirm inactivation, before and after γ irradiation toxicity of venom was determined by Finney LD50 (50% lethal doses) method.Citation33
Immunization of hens
Hens were divided into 2 groups with 10 hens: control group and test group. Hens in the test group were immunized with the irradiated venomCitation13,30 while those in the control group were injected with normal saline. For immunization, 0.5-mL solution of irradiated venom containing protein concentration of 400 μg/mL (200 μg/dose) was mixed with an equal volume of Freund's complete adjuvant and was injected intramuscularly in the hens. Eggs laid by the hens were collected immediately before and from 12 day onwards after first injection during the course of immunization. The eggs were stored at 4°C.Citation13 Equal volumes of normal saline (0.5 mL) were injected in hens in the control group.
The protein concentration in the yolks was determined using the method described by Lowry et al.Citation34 with bovine serum albumin as the standard.
Immunodiffusion
Antibodies (IgY) present in the egg yolks were identified using Ouchterlony double diffusion methodCitation35 by using 1.5% agar. Stock solutions containing 300 μg of crude and purified egg yolks were prepared in phosphate buffered saline (PBS). Next 100 μl of each sample was added to specified wells. After 48 h at 4°C, the gels were washed for 24 h with several changes of saline, dried and visualized with staining by 1% Coomasie brilliant blue stain.
Purification of immunoglobulin from egg yolks
Purification was performed at room temperature. Yolks of eggs laid by hens in both the groups were purified in parallel. In brief, egg yolks were separated from egg whites, pooled, and washed 2 to 3 times by using 2-times volume of purified water (with slow shaking for 5 minutes) to remove contamination by egg whites. The washed egg yolks were measured, diluted with equal volumes of purified water, and frozen overnight at −70°C. On the next day, the frozen egg yolks were thawed slowly (at 4°C) and centrifuged at 20000 × g for one hour at 4°C to remove small particles. The supernatant was separated, filtered using Whatman No. 1 filter paper (USA), and concentrated to approximately 5 mL by dialyzing in a dialysis tube (Sigma-Aldrich, USA), with a molecular cut-off 2000, for 24–48 hours against solid sucrose.Citation6 The concentrated solution was fractionated by gel filtration chromatography with Sephadex G-50 (column: 2 × 60 cm; mobile phase: 10 mM PBS, pH 7.5). All the fractions (5 mL) were collected, and absorbance was measured at 280 nm by using spectrophotometer (Shimadzu, Japan). Specific antibodies to whole venom were purified from F1 and F2 by passing the fractions through Vipera lebetina venom antigen matrices by coupling of venom to affinity resin.Citation24 Protein concentration of F1 and F2 fractions before affinity chromatography and affinity purified antibody concentrations were determined using by Lowry protein assay.Citation34 Total protein of F1 and F2 fractions and affinity purified antibody of both fractions were measured and percent of venom specific antibodies calculated.
Electrophoresis
The purity and molecular weight of immunoglobulins were determined by performing non-reduced electrophoresis on 12.5% polyacrylamide gel according to a method described by Shapiro.Citation36 Band intensities were analyzed using the image analysis software Multi-Analyst (Bio-Rad, USA).
Neutralization of lethality
The lethality of crude V. lebetina venom was determined using the method described by Theakston and Reid.Citation37 Neutralization of lethality was determined by injecting incubated venom-antivenom mixture into mice. Mixtures containing a constant concentration of the venom and varying dilutions of antivenom were prepared in normal saline and were incubated at 37°C for one hour. Aliquots (0.5 mL) of the mixtures containing venom concentration corresponding to 5 LD50 were injected intravenously into groups of 4 mice (weight, 17–21 g). Deaths were recorded over 96 hours, and potency of the antivenom was estimated and expressed in terms of LD50. Commercially available horse antivenom (Razi Institute, Iran) was used as positive control, and saline premixed with venom was used as negative control; these were injected intravenously in control mice. The weight in milligrams equivalent to LD50 of the venom should be neutralized by a specific quantity of antivenom based on the protection of a stated proportion of animals (e.g., 100%). Serum potency was expressed as the largest amount of venom neutralized by 1 mL of serum.Citation8
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by grant from the Razi Vaccine and Serum Research Institute, Karaj, Iran.
References
- World Health Organization (WHO). Guidelines for the production, control and regulation of snake antivenom immunoglobulins. 2010; 8-9. Available at http://www.who.int/bloodproducts/Snakeantivenoms
- Firouz E. The complete fauna of Iran. London: I. B. Tauris; 2005.
- Warrell DA. Snake bite. Lancet 2010; 375:77-88;PMID:20109866; http://dx.doi.org/10.1016/S0140-6736(09)61754-2
- World Health Organization (WHO). Snakebite 2009. Available from: http://www.who.int/neglected_diseases/diseases/snakebites/en/index.html [Last accessed on 2013 May 10]
- White J. Snake venoms and coagulopathy. Toxicon 2005; 45(8):951-67. View at Publisher View at Google Scholar View at Scopus; PMID:15922768; http://dx.doi.org/10.1016/j.toxicon.2005.02.030
- Jia YH, Jin Y, Lu QM, Li DS, Wang WY, Xiong YL. Jerdonase, a novel serine protease with kinin-releasing and fibrinogenolytic activity from Trimeresurus jerdonii venom. Acta Biochimica et Biophysica Sinica 2003; 35(8):689-94. View at Scopus; PMID:12897962
- Serrano SMT, Maroun RC. Snake venom serine proteinases: sequence homology versus substrate specificity, a paradox to be solved. Toxicon 2005; 45(8):1115-32. View at Publisher View at Google Scholar View at Scopus; PMID:15922778; http://dx.doi.org/10.1016/j.toxicon.2005.02.020
- Latifi M, Manhouri H. [Antivenin production]. Mem Inst Butantan 1966; 3: 893-7; PMID:6002969
- Christensen PA. Snakebite and the use of antivenom in Southern Africa. S Afr Med J 1981; 59:934-8.
- Russell FE. Snake venom immunology: historical and practical considerations. J Toxicol Toxin Rev 1989; 7:1-82; PMID:NOT_FOUND; http://dx.doi.org/10.3109/15569548809059725
- Sutherland SK. Serum reactions. An analysis of commercial antivenoms and a possible role of anticomplementary activity in de novo reactions of antivenoms and toxins. Med J Aust 1977; 1:613-5.
- Morais JF, De Freitas MCW, Yamaguchi IK, Dos Santos MC, Dias Da Silva W. Snake antivenoms from hyperimmunized horses: comparison of the antivenom activity and biological properties of their whole IgG and F(ab)2 fragments. Toxicon 1994; 32:725-34; PMID:7940578; http://dx.doi.org/10.1016/0041-0101(94)90341-7
- Maya Devi C, Vasantha Bai M, Krishnan LK. development of vipera venom antibodies in chicken egg yolk and assay of their antigen binding capacity. Toxicon 2002b; 40:857-61; http://dx.doi.org/10.1016/S0041-0101(01)00258-6
- Maya Devi C, Mary VB, Arthur VL, Umashankar PR, Lissy KK. An improved method for isolation of anti-viper venom antibodies from chicken egg yolk. J Biochem Biophys Methods 2002a; 51:129-38; http://dx.doi.org/10.1016/S0165-022X(02)00002-7
- Warrell DA. Guidelines for the management of snakebites. World Health Organization 2010. ISBN 978-92-9022-377-4, 85-89. Available at http://www.searo.who.int
- Wingert W, Wainschel J. Diagnosis and management of envenomation by poisonous snakes. South. Med. J 1975; 68:1015; http://dx.doi.org/10.1097/00007611-197508000-00019
- Almeida CMC, Kanashiro MM, Rangel FFB, Mata MFR, Kipnis TL, Dias da Silva W. Development of snake antivenom antibodies in chickens and their purification from yolk. Veterinary Record 1998; 143:579-84; http://dx.doi.org/10.1136/vr.143.21.579
- Klemperer F. Uber naturliche immunitat und ihre verwertungfur die immuniserungstherapie. Archiv fur die experimentelle pathologie und pharmakologie 1893; 31:356-82.
- Schade R, Pfister C, HaLatsch R, Henklein P. polyclonal IgY antibody from chicken egg yolk—an alternative to the production of mammalian IgG type antibody in rabbits. ATLA Alter Lab Anim 1991; 19:403-19.
- Patterson R, Youngner JS, Weigle WO, Dixon FJ. Antibody production and transfer to egg yolk in chickens. J Immunol 1962; 89:272-8.
- BarJoseph M, Malkinson M. Hen egg yolk as a source of antiviral antibodies in the enzyme linked immunosorbent assay (ELISA): a comparison of two plant viruses. J Virol Meth 1980; 1:179-84; http://dx.doi.org/10.1016/0166-0934(80)90014-2
- Altschul D, Hennache G, Van Regenmortel NH. Determination of IgG and IgM level in serum by rocket immunoelectrophorosis using yolk antibodies from immunized chickens. J Immunol Meth 1984; 69:1-7; http://dx.doi.org/10.1016/0022-1759(84)90270-9
- Akita EM, Nakai S. Comparison of four purification methods for the production of immunoglobulins from eggs laid by hens immunized with an enterotoxigenic E. coli strain. J Immunol Methods 1993; 160(2):207-14; http://dx.doi.org/10.1016/0022-1759(93)90179-B
- Thalley BT, Carroll SB. Rattlesnake and scorpion antivenin from the egg yolks of immunized hens. Biotechnology 1990; 8:934-8; http://dx.doi.org/10.1038/nbt1090-934
- Almeida CMC, Kanashiro MM, Rangel FFB, Mata MFR, Kipnis TL, Diasda Silva W. Development of snake antivenom antibodies in chickens and their purification from yolk. Veterinary Record 1998; 143:579-84; http://dx.doi.org/10.1136/vr.143.21.579
- Mojca N. Production of antibodies in chickens. Food Technol Biotechnol 2003; 41(3):259-67.
- Hau J, Hendriksen C. Refinement of polyclonal antibody production by combining oral immunization of chickens with harvest of antibodies from the egg yolk. ILAR 2005; 46:294-9; http://dx.doi.org/10.1093/ilar.46.3.294
- Michael A, Meenatchisundaram S, Parameswari G, Subbraj T, Selvakumaran R, Ramalingam S. Chicken egg yolk antibodies (IgY) as an alternative to mammalian antibodies. Indian J Sci Technol [Online] 2010; 3.4:468-74. Web. 2 Jan. 2014.
- Landon J, Woolley JA, Mclean C. Antibody production in the han. In: Landon J, Chard T. Ed. Therapeutic antibodies. Berlin: Springer Verlag 1995; 47-68.
- Mandal M, Hati RN, Hati AK. Neutralization potency of russells vipera venom as compared with standard antivenin. Indian J Med Res 1992; 96:219-22.
- Larsson A, Balow RM, Lindahl TL, Forsberg PO. Chicken antibodies: taking advantage of evolution. A Rev Poultry Sci 1993; 72:1807-12; http://dx.doi.org/10.3382/ps.0721807
- Stuart CA, Pietrzyk RA, Furlanetto RW, Green A. High affinity antibody from hen eggs directed against the human insulin receptor and the human IGFI receptor. Anal Biochem 1998; 173(1):142-50; http://dx.doi.org/10.1016/0003-2697(88)90171-6
- Finney D. The median lethal dose and its estimation. Arch Toxicol 1985; 56:208-15; http://dx.doi.org/10.1007/BF00295156
- Lowry OH, Rosenbrough NJ, Farr, AL, Randall, RJ. Protein measurement with the phenol reagent. J Biol Chem 1951; 193:265-75.
- Ouchteriony O. Antigen-antibody reaction in gels. Ark Kemi Mineral Geol 1949; 26:1.
- Shapiro AL, Viñuela E, Maizel JV. Molecular weight estimation of polypeptide chains by electrophoresis in SDS–polyacrylamide gels. Biochem Biophys Res Commun 1967; 28(5):815-20; http://dx.doi.org/10.1016/0006-291X(67)90391-9
- Theakston RDG, Reid HA. Development of simple standard assay procedures for the characterization of snake venoms. Bull World Health Organ 1983; 61(6):949-56.