Abstract
The aim of this study was to evaluate hepatitis B surface antibody (anti-HBs) levels one year after hepatitis B booster vaccination in anti-HBs-negative (<10 mIU/mL) children 11–15 y after primary vaccination. Anti-HBs titers were examined in 235 children who were negative for hepatitis B surface antigen (HBsAg), anti-HBs, and hepatitis B core antibody (anti-HBc). The children were then divided into 3 groups based on their anti-HBs levels pre-booster: Group I, <0 .1 mIU/mL; Group II, 0.1 to <1 .0 mIU/mL; and Group III, 1.0 to <10 .0 mIU/mL. They were vaccinated with 3 doses of hepatitis B vaccine (0–1–6 month, 20 ug), and anti-HBs levels were measured. One month after the first dose, the anti-HBs positive rates (≥10 mIU/mL) in Groups I–III were 56.14%, 83.61% and 100%. One month after the third dose, the anti-HBs-positive rates in Groups I–III were 96.49%, 98.36% and 100%. One year after the third dose, the anti-HBs-positive rates in Groups I–III were 73.68%, 75.41% and 98.29%, respectively. Protective levels declined more rapidly for those with lower titers. Children with pre-booster anti-HBs titers of 1–9.9 mIU/mL might not need any booster dose, and the children with pre-booster titers of 0.1–0.9 and <0 .1 mIU/mL might need more than one dose booster vaccination.
Introduction
Hepatitis B virus (HBV) infection is a global health problem. It is estimated that 2 billion people have been infected, and 350 million are chronic carriers of the virus worldwide.Citation1,2 A 1992 estimate suggested that 0.7 billion people have been infected, and 120 million are chronic carriers of the virus in China.Citation3 A number of viral antigens and their respective antibodies can be detected in serum after infection with HBVCitation2. HBV DNA followed shortly by the hepatitis B surface antigen (HBsAg) is the first viral markers detected in serum.Citation4 HBsAg persistence is a marker of hepatitis B chronicity.Citation2 Hepatitis B core antibody (anti-HBc) testing is the most reliable means of assessing previous infection with HBV, whereas hepatitis B surface antibody (anti-HBs) testing is used to assess immunity and response to the HBV vaccine.Citation5 In 1992, the universal infant vaccination program began in China to control HBV infection, and a free vaccination policy was incorporated into the national immunization plan in 2002. Before 2000, 3 doses of plasma derived vaccine or recombinant vaccine were given at zero, one, and 6 months of age. After 2000, 3 doses of recombinant vaccine were administered at the age of less than 24 hours, one month and 6 months.Citation6 The HBsAg prevalence has dropped dramatically in China after more than 10 y after the universal infant HBV vaccination program has been adjusted in China since 1992,especially among children. The national serosurvey in 2006 showed that for population aged 1–59 years, the prevalence of HBsAg was 7.2%, and for children 1–4 and 5–14 y was 0.96%, 2.24%. The Chinese population's prevalence of HBsAg was about 10% lower for all age groups, including young children, since the national serosurvey in 1992.Citation7
Long-term follow-up of neonatal hepatitis B vaccination found that the hepatitis B surface antibody (anti-HBs) titers in vaccines may attenuate over time to below the protective levels and even become undetectable.Citation8,9 Studies have shown that after 3 doses of hepatitis B vaccine, 99% of individuals had seroprotective levels of HBs after one year and 37% retained seroprotective levels after 15 yCitation10 Since 10 mIU/ml is widely considered to be a seroprotective level of anti-HBs, booster vaccination has been recommended for those whose anti-HBs decline to such a low level.Citation11-13 The European Consensus Group suggested there was no data to support the need for booster vaccination in immunocompetent individuals who responded to a primary course.Citation14 Contrary to this view, recent evidence suggested that anamnestic response also decreased over time, and the non-response rate was about 20%–50% after 10–15 y follow-up.Citation15 A Taiwanese study also found that 15–18 y after neonatal immunization, 10.1% of the total study population had lost their anamnestic response and might need a further booster. Citation16Other long-term follow-up studies also support the view that booster vaccination is needed for children whose anti-HBs titer is below the protective levelCitation17 or cannot be detected.Citation18-20 Studies in AlaskaCitation21,22 and TaiwanCitation22-25 confirmed the possibility of a higher risk of breakthrough HBV infection (anti-HBc or HBV DNA positive) in the population that had antibody titer attenuation without an immune response. The duration of protection among those vaccinated at birth may not be sufficiently long due to an immature immune system, and the risk of HBV infection increases during adolescence into early adulthood because of sexual or behavioral exposure.Citation15
Most studies in this field have only focused on the post-booster effect of hepatitis B vaccination during a short time. In this study, 235 children with anti-HBs levels that had fallen below the seroprotective levels more than 10 y after primary vaccination was received. Three doses of 20 ug vaccine were administered at zero, one, and 6 months by intramuscular injection in the deltoid region. Anti-HBs titers were examined one year after booster vaccination. We aimed to evaluate the effects (anti-HBs seroprotective rates and titers ) after one year post-booster and justify recommendations for a specific vaccination program.
Results
Participant characteristics at 1 year post-booster
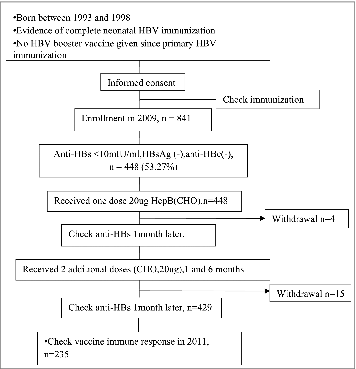
We randomly sampled 841 children (11-15 y old) who qualified for this study, and 393 children were still anti-HBs positive after primary vaccination. And the anti-HBs positive rate of 841 children was 46.73%. None of 841 children were positive for HBsAg or anti-HBc. Then 448 children enrolled in the follow-up study whose 3 items (HBsAg, anti-HBc and anti-HBs) were negative according to a serology test. One month after the first dose, 4 children rejected to collect blood samples. One month after the third dose, 15 children refused to collect blood samples. At one year after booster vaccination, there were 235 children. 194 children dropped out or were unable to follow-up when they went to a different school. The average age of the 235 children was 12.94 ± 1 .55 y. The test group was comprised of 123 boys and 112 girls. There were no significant differences in age or sex among 3 groups. Age and sex distributions of study subjects are shown in .
Table 1. Age and sex distribution of study subjects
Participant characteristics and antibody titer distribution of the children who were in or out of the follow-up study at one month post third booster dose
Children were divided up into 2 groups one month after administration of the third booster dose based on whether or not they were in the cohort study. There were no differences in age or sex between the children in the followed cohort and those who lost to follow-up in Groups I-III, respectively. Details are shown in .
At one month after the third booster dose, the anti-HBs positive rates of dropout participants in Groups I–III were 89.30%, 100%, and 100%; anti-HBs GMTs in Groups I–III were 232.90, 386.36, and 738.34 mIU/ml, respectively. showed anti-HBs titers distribution in the follow-up participants, and there were no differences in anti-HBs titers between followed cohort and dropout participants in Groups I-III, respectively (PI = 0.319, P II = 0.386, and PIII = 0.068).
Table 2. Anti-HBs titers distribution 1 month and 1 y after booster vaccination of study subjects
Antibody titers distribution at 1 year post booster vaccination
At one year after administration of the third booster dose, 203 children maintained protective antibody levels with a total anti-HBs positive rate of 86.38% and a total anti-HBs GMT of 58.61 mIU/mL. There was a significant difference between groups. The anti-HBs positive rate and GMT in Group III were the highest, but no significant difference was observed between Groups I and II. Details are shown in .
Comparison of anti-HBs titers at one month and one year post booster vaccination
One month after the first and third dose and one year after the third dose; the anti-HBs positive rates of the 235 followed children were 85.11%, 98.71%, and 86.38%, respectively. The total anti-HBs positive rate increased by 13.60% after received 2 additional dose vaccination (P < 0 .001), and decreased by 12.33% after one year (P < 0 .001). The anti-HBs titers of the 235 children increased after the third booster dose and decreased after one year, with GMTs of 169.86 mIU/mL at one month after the first dose, 614.77 mIU/mL at one month after the third dose, and 58.61 mIU/mL at one year after the third dose, respectively. The differences in GMTs post-booster were significant (all P < 0 .001).
After stratifying by groups, the anti-HBs positive rates increased from 56.14% to 96.49% in Group I (P <0 .001); and from 83.61% to 98.36% in group II (P = 0.004) at one month after the third dose. Anti-HBs positive rates decreased from 96.49% to 73.68% in Group I (P <0 .001); and from 98.36% to 75.41% in group II (P < 0 .001) at one year after the third dose. The anti-HBs positive rates in Group III were the same from one month after the first dose to one year after the third dose (100%, 100%, and 98.29%; P = 1).
showed the anti-HBs GMTs of group I-II at one month after the third dose were higher than at one month after the first dose, while the anti-HBs GMTs of group III were the same. At one year post booster, the GMTs of group I-III were all lower than at one month after the third booster dose.
Discussion
The duration of protection after hepatitis B vaccination in early infancy remains incompletely understood. A previous study conducted in China showed that long term protection of nearly 20 y appeared to occur for those infants who received 3 doses of hepatitis B vaccine, starting at birth, in high HBV-endemic areas.Citation27
After stratification by pre-booster anti-HBs titers, this study clearly pointed out that the children who had pre-booster anti-HBs titers of 1–9.9 mIU/mL (Group III) might not support any booster dose. All of them developed a rapid and robust antibody anamnestic response after a booster dose. This phenomenon highlighted that anamnestic response is related to the persistence of immune memory to the vaccine antigens. The study results demonstrated that protection against hepatitis B virus can persist at least 11–15 y in the majority of these children within the Group III category, who were inoculated with hepatitis B vaccine from birth.
In general, it is considered that protection against HBV depends on immunological memory after the primary vaccination schedule.Citation28 Anamnestic response was evaluated in those who were administered a booster of hepatitis B vaccine in some previous studies.Citation29,30 Different from those children above, the seroprotective rates after the first booster dose of hepatitis B vaccine in the children who had pre-booster anti-HBs titers of 0.1–0.9 and <0 .1 mIU/ml (Groups II and I ) were only 82.61% and 59.34%, respectively. A part of these children who complete primary hepatitis B immunization in their infants might lose immunological memory.
This study also showed that the great majority of children who had pre-booster anti-HBs titers of 0.1–0.9 and <0 .1 mIU/mL (Groups II and I) have been anti-HBs seroprotective levels after 3-dose booster. Studies in Great BritainCitation31 and TaiwanCitation32-35 demonstrated that a 3-dose revaccination ensured that all non- and low-responders eventually reached sufficient anti-HBs levels.
Therefore, it is suggested to give a booster only for those who showed lower titers (<1 .0 mIU/mL), rather than for all individuals with anti-HBs titers under 10 mIU/mL, which is different from the conclusion published previously. The distinctive difference in the current study compared to the article published before is that the anti-HBs titers were investigated for one year after third booster dose.
A fast decrease in seroprotection levels and anti-HBs titers were also observed in group I and II during the first year after receiving the third booster dose of hepatitis B vaccine. It may mean that a 3 dose booster vaccination has short a short-term effectiveness in these individuals.
Kazuhiko NakaoCitation36 analyzed the persistence of anti-HBs after a 3 dose booster vaccination, and observed higher anti-HBs seroprotective levels one year after the third booster dose. Wang et al Citation37 also demonstrated the seroprotective levels persistence maintained at 92. Five% after 3 y post 3 dose revaccination. The differences might suggest that immunity against HBV in some of these individuals may have impaired. The duration of the anti-HBs seroprotective levels persistence for these individuals merits further investigation. The necessity of booster vaccination largely depends on the duration of immune memory rather than the duration of protective antibody after booster immunization. Thus, detection of anti-HBs titer one year after booster vaccination might have little benefit in determining whether booster vaccination is needed or effective for these children. A long-term follow-up study would be required to confirm these conclusions.
In this study, we clustered participants based on school enrolment, and nearly half of the children selected for the follow-up study were lost when they went to another school or graduated. The loss rate was too high and impacted the reliability and sample size of this study. The children's characteristics were analyzed as well as antibody titer distributions of the children who were in or out of the follow-up study at one month after the third dose of booster vaccination. The study found that there were no differences in age, sex, or antibody titer distribution between them. However, the reliability and statistical power of the study might still have been impacted by dropout participants.
Another limitation of this study was that it lacked primary immunization results. Therefore, we could not find out if the children with a negative response post one or 3 booster doses had failed respond to the primary immunization (up to about 5% of subjects do not respond after 3 doses). The result indicated that, for those who had completed primary immunizations, there was a significant relationship between the anti-HBs titers before and after immune booster. Primary series non-responders were more likely in Groups I-II; hence leading to the lower effects after booster vaccination in Groups I-II. This is the main limitation of the studyt. In addition, there were noresults for the second booster dose because the parents or guardians of the subjects rejected the collection of blood samples twice within a time frame of less than 3 months, which was also one of the limitations of this study.
In conclusion, the children with pre-booster anti-HBs titers of 1–9.9 mIU/mL might not need any booster dose. The children with pre-booster titer lower than 1 mIU/mL might need more than one dose booster vaccination, but a further long-term study is needed to confirm the conclusions.
Materials and Methods
Study participants and methods
This research was performed in China from September 1, 2009 to April 30, 2011, and 2 towns were randomly selected in each county of Qingyuan, Kaihua and Xianju Counties in the Zhejiang Province as research sites. We clustered sample subjects based on school enrolment. Selected children were born between May 1, 1993 and September 30, 1998 and had received a course of 3 doses of hepatitis B vaccine during infancy at zero, one, and 6 months after birth, but had not received further booster vaccination. For all the children, immunization status was recorded from the child's immunization certificate kept by the parents or by review of the child's immunization card kept at the township hospital immunization clinic. All children's parents or guardians completed a questionnaire that requested demographic data about the child, including sex, date of birth, telephone number, home address, and HBV vaccination history. Detailed characteristics of the participants are presented elsewhere.Citation26
We collected 3-mL blood samples from each participant and preserved them for further testing of anti-HBs hepatitis B core antibody (anti-HBc) and HBsAg. 841 children ages 11 to 15 y who qualified for this study were randomly sampled, and 448 children were selected whose anti-HBs, anti-HBc, and HBsAg were all negative according to a serology test as final study subjects. Anyone positive for anti-HBs, anti-HBc, or HBsAg were excluded. Then they were administered booster doses of hepatitis B vaccine made by recombinant DNA (DNA) techniques in Chinese hamster ovary (CHO) cells, (hepatitis B vaccine (CHO) lot number: 200802A21 (01–05), dose: 20 μg; NCPC GeneTech Biotechnology Pharmaceutical Co. Ltd., Hebei, China) by intramuscular injection in the upper arm deltoid, according to the immunization procedure for months zero, one, and 6. One month after the first and the third dose, 3 mL blood samples were collected from each participant and checked for anti-HBs. One year after the third dose, 2-mL blood samples were collected from the follow-up participants based on school enrolment and tested for anti-HBs. Study flow chart was shown in .
This study was approved by the Institutional Review Board of the Zhejiang Center for Disease Control and Prevention (Hangzhou, China). We obtained informed consent from every participants’ parents or guardians.
Laboratory procedures
Frozen separated serum samples were sent to ADICON Clinical Laboratories (Hangzhou, China) for quantification of HBsAg, anti-HBs, and anti-HBc by chemiluminescence immunoassay (Architect-i2000; Abbott, Abbott Park, IL, USA). The reagent lot number for HBsAg tests was 70318HN00, with the criterion that an S/N≥0 .05 was considered to be positive. The reagent lot number for the anti-HBc test was 72448M100, with the criterion that an anti-HBc ≥1 mIU/ml was defined as positive. The reagent lot number for the anti-HBs test after one month post the first and third dose was 75684M100 , and 10259F00 for the anti-HBs test after one year post the third dose. Anti-HBs titer ≥10 mIU/mL is considered to be positive and was defined as having protective effects against HBV infection. Samples with anti-HBs ≥ 1,000 mIU/mL were diluted for further testing. While samples with antibody titer >15 ,000 mIU/mL were excluded from further testing and recorded as >15 ,000 mIU/mL.
Data analysis
The participants were divided into 3 groups according to their anti-HBs levels prior to administration of the booster: Group I, <0 .1 mIU/mL; Group II, 0.1 to <1 .0 mIU/mL; and Group III, 1.0 to <10.0 mIU/mL. The T-Test was used to compare normal continuous variables between 2 groups. The One-way Anova Test was used to compare normal continuous variables between all 3 groups. The Chi-square Test was used to compare categorical variables. The Kruskal–Wallis Test was used to compare abnormal continuous variables between groups. A two-tailed probability in statistical tests was used, with an α of 0.05 considered to be significant. Because we were unable to detect anti-HBs less than 0.01 mIU/ml, we assigned a value of 0.005 mIU/ml to these subjects when calculating Geometric Mean Titer (GMT) of anti-HBs. Anti-HBs >15 ,000 mIU/mL were assigned a value of 15,000 mIU/mL when a log transformation was used for calculating GMT. To account for any effect of age, the Cochran-Mantel- Haenszel test was used to compare the anti-HBs positive rates between group I-III.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by the National Scientific and Technological Major Project of China (No. 2011ZX10004-901 and 2013ZX10004-904), and the Scientific Research Fund of Medical Technology and Education in Zhejiang Province (No. 2013C25114).
References
- El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 2012; 142:1264-73; PMID:22537432; http://dx.doi.org/10.1053/j.gastro.2011.12.061
- Liang TJ. Hepatitis B: the virus and disease. Hepatology 2009; 49(5Suppl):S13-S21; PMID:19399811; http://dx.doi.org/10.1002/hep.22881
- ZC Dai, QG Ming. Chinese hepatitis B viral serum epidemiology investigation report. Beijing: Taylor & Francis. 1997; 39-58.
- Krugman S, Overby LR, Mushahwar IK, Ling CM, Frosner GG, Deinhardt F. Viral hepatitis, typeB. Studies on natural history and prevention re-examined. N Engl J Med 1979; 300: 101-6; PMID:758598; http://dx.doi.org/10.1056/NEJM197901183000301
- Tabor E, Hoofnagle JH, Barker LF, Pineda-Tamondong G, Nath N, Smallwood LA, et al. Antibody to hepatitis B core antigen in blood donors with a history of hepatitis. Transfusion 1981;21:366-371; PMID:7233523; http://dx.doi.org/10.1046/j.1537-2995.1981.21381201816.x
- H Zhuang. Hepatitis B vaccination in China. Basic Medical Sciences and Clinics 2004; 24(2):136-40.
- Liang X, Bi S, Yang W, Wang L, Cui G, Cui F, Zhang Y, Liu J, Gong X, Chen Y, et al. Reprint of: Epidemiological serosurvey of Hepatitis B in China—Declining HBV prevalence due to Hepatitis B vaccination. Vaccine 2013;31 Suppl 9:J21-8; PMID:23948229; http://dx.doi.org/10.1016/j.vaccine.2013.08.012
- Lu L , Chen YH, Liu X, He JX . Research of hepatitis B vaccine immune effect. Chinese Journal of Public Health Management 2006; 22(6):485-7.
- Chen DS. Hepatitis B vaccination: The key towards elimination and eradication of hepatitis B. J Hepatol 2009; 50(4):805-16; PMID:19231008; http://dx.doi.org/10.1016/j.jhep.2009.01.002
- Ma JC , Liu HB, Zhang YL , Meng ZD , Han CQ, Zhang YW et al. Rural neonatal hepatitis b vaccine at 14 years after the immune effect evaluation. Chinese Immunizat. 2002, 8(4):181-4
- Immunisation against hepatitis B. Lancet 1988; i:875-6. PMID: 2895375
- Hadler SC, Francis DP, Maynard JE, Thompson SE, Judson FN, Echenberg DF, Ostrow DG, O'Malley PM, Penley KA, Altman NL, et al. Long-term immunogenicity and efficacy of hepatitis B vaccine in homosexual men. N Engl J Med 1986;315:209-14; PMID:2941687; http://dx.doi.org/10.1056/NEJM198607243150401
- Yoshida T, Saito I. Hepatitis B booster vaccination for healthcare workers. Lancet 2000; 355:1464; PMID:10791552; http://dx.doi.org/10.1016/S0140-6736(05)74663-8
- Are booster immunisations needed for lifelong hepatitis B immunity? European consensus group on hepatitis B immunity. Lancet 2000; 355(9203):561-5; PMID:10683019; http://dx.doi.org/10.1016/S0140-6736(99)07239-6
- Chaves SS, Groeger J, Helgenberger L, Auerbach SB, Bialek SR, Hu DJ, Drobeniuc J. Improved anamnestic response among adolescents boosted with a higher dose of the hepatitis B vaccine. Vaccine 2010; 28(16):2860-4; PMID:20153793; http://dx.doi.org/10.1016/j.vaccine.2010.01.059
- Lu CY, Ni YH, Chiang BL, Chen PJ, Chang MH, Chang LY, et al. Humoral and cellular immune responses to a hepatitis B vaccine booster 15-18 years after neonatal immunization. J Infect Dis 2008;197(10):1419-26; PMID:18444799; http://dx.doi.org/10.1086/587695
- Lankarani KB. The necessity of booster vaccination after neonatal hepatitis B vaccination. Hepat Mon 2011; 11(6):419-21; PMID:22087171
- Young BW, Lee SS, Lim WL, Yeoh EK. The long-term efficacy of plasma-derived hepatitis B vaccine in babies born to carrier mothers. J Viral Hepat 2003;10(1):23-30; PMID:12558908; http://dx.doi.org/10.1046/j.1365-2893.2003.00386.x
- van der Sande MA, Waight P, Mendy M, Rayco-Solon P, Hutt P, Fulford T, Doherty C, McConkey SJ, Jeffries D, Hall AJ, et al. Long-term protection against carriage of hepatitis B virus after infant vaccination. J Infect Dis 2006; 193(11):1528-35; PMID:16652281; http://dx.doi.org/10.1086/503433
- Dentinger CM, McMahon BJ, Butler JC, Dunaway CE, Zanis CL, Bulkow LR, Bruden DL, Nainan OV, Khristova ML, Hennessy TW, et al. Persistence of antibody to hepatitis B and protection from disease among Alaska natives immunized at birth. Pediatr Infect Dis J 2005; 24(9):786-92; PMID:16148845; http://dx.doi.org/10.1097/01.inf.0000176617.63457.9f
- McMahon BJ, Bruden DL, Petersen KM, Bulkow LR, Parkinson AJ, Nainan O, Khristova M, Zanis C, Peters H, Margolis HS. Antibody levels and protection after hepatitis B vaccination: results of a 15-year follow-up. Ann Intern Med 2005; 142:333-41; PMID:15738452; http://dx.doi.org/10.7326/0003-4819-142-5-200503010-00008
- van der Sande MA, Waight PA, Mendy M, Zaman S, Kaye S, Sam O et al. Long-term protection against HBV chronic carriage of Gambian adolescents vaccinated in infancy and immune response in HBV booster trial in adolescence. J PLoS One 2007; 8:e753; PMID:17710152; http://dx.doi.org/10.1371/journal.pone.0000753
- Su FH, Cheng SH, Li CY, Chen JD, Hsiao CY, Chien CC, Yang YC, Hung HH, Chu FY. Hepatitis B seroprevalence and anamnestic response amongst Taiwanese young adults with full vaccination in infancy, 20 years subsequent to national hepatitis B vaccination. Vaccine 2007;25:8085-90; PMID:17920732; http://dx.doi.org/10.1016/j.vaccine.2007.09.013
- Kao JT, Wang JH, Hung CH, Yen YH, Hung SF, Hu TH, Lee CM, Lu SN. Long-term efficacy of plasma-derived and recombinant hepatitis B vaccines in a rural township of Central Taiwan. Vaccine 2009; 27:1858-62; PMID:19186203; http://dx.doi.org/10.1016/j.vaccine.2009.01.027
- Chang HC, Yen CJ, Lee YC, Chiu TY, Jan CF. Seroprevalence of hepatitis B viral markers among freshmen – 20 years after mass hepatitis B vaccination program in Taiwan. J Formos Med Assoc 2007; 106:513-9; PMID:17660140; http://dx.doi.org/10.1016/S0929-6646(07)60001-1
- Yao J, Ren J, Shen L, Chen Y, Liang X, Cui F, Li Q, Jiang Z, Wang F. The effects of booster vaccination of hepatitis B vaccine on anti-HBV surface antigen negative children 11-15 years after primary vaccination. Hum Vaccin 2011; 7(10):1055-9; PMID:21989290; http://dx.doi.org/10.4161/hv.7.10.15990
- Wu Q, Zhuang G, Wang X, Wang L, Li N, Zhang M. Antibody levels and immune memory 23 years after primary plasma-derived hepatitis B vaccination: Results of a randomized placebo-controlled trial cohort from China where endemicity is high. Vaccine 2011; 29(12):2302-7; PMID:21277403; http://dx.doi.org/10.1016/j.vaccine.2011.01.025
- Banatvala JE, Van Damme P. Hepatitis B vaccine – do we need boosters? J Viral Hepat 2003; 10(1):1-6; PMID:12558904; http://dx.doi.org/10.1046/j.1365-2893.2003.00400.x
- Poovorawan Y, Chongsrisawat V, Theamboonlers A, Bock HL, Leyssen M, Jacquet JM. Persistence of antibodies and immune memory to hepatitis B vaccine 20 years after infant vaccination in Thailand. Vaccine 2010; 28(3):730-6; PMID:19892043; http://dx.doi.org/10.1016/j.vaccine.2009.10.074
- Gilca V, De Serres G, Boulianne N, Murphy D, De Wals P, Ouakki M, Trudeau G, Massé R, Dionne M. Antibody persistence and the effect of a booster dose given 5, 10 or 15 years after vaccinating preadolescents with a recombinant hepatitis B vaccine. Vaccine 2013; 31(3):448- 51; PMID:23206974; http://dx.doi.org/10.1016/j.vaccine.2012.11.037
- Clemens R, Sanger R, Kruppenbacher J, Hobel W, Stanbury W, Bock HL, Jilg W. Booster immunization of low- and non-responders after a standard three dose hepatitis B vaccine schedule–results of a post-marketing surveillance. Vaccine 1997; 15(4):349-52; PMID:9141203; http://dx.doi.org/10.1016/S0264-410X(96)00205-8
- Su FH, Chu FY, Bai CH, Lin YS, Hsueh YM, Sung FC, Yeh CC. Efficacy of hepatitis B vaccine boosters among neonatally vaccinated university freshmen in Taiwan. J Hepatol 2013; 58(4):684-9; PMID:23207141; http://dx.doi.org/10.1016/j.jhep.2012.11.036
- Lu JJ, Cheng CC, Chou SM, Hor CB, Yang YC, Wang HL. Hepatitis B immunity in adolescents and necessity for boost vaccination: 23 years after nationwide hepatitis B virus vaccination program in Taiwan. Vaccine 2009; 27:6613-8; PMID:19698812; http://dx.doi.org/10.1016/j.vaccine.2009.08.007
- Jan CF, Huang KC, Chien YC, Greydanus DE, Davies HD, Chiu TY, Huang LM, Chen CJ, Chen DS. Determination of immune memory to hepatitis B vaccination through early booster response in college students. Hepatology 2010; 51:1547-54; PMID:20209603; http://dx.doi.org/10.1002/hep.23543
- Kao JT, Wang JH, Hung CH, Yen YH, Hung SF, Hu TH, et al. Long-term efficacy of plasma-derived and recombinant hepatitis B vaccines in a rural township of Central Taiwan. Vaccine 2009; 27:1858-62; PMID:19186203; http://dx.doi.org/10.1016/j.vaccine.2009.01.027
- Nakao K, Hamasaki K, Wakihama N, Maeda M, Ohtsubo N, Sagiike T, Ichikawa T, Ishikawa H, Eguchi K, Ishii N. Analysis of anti-HBs levels in healthcare workers over 10 years following booster vaccination for hepatitis B virus. Vaccine 2003; 21(25-26):3789-94; PMID:12922112; http://dx.doi.org/10.1016/S0264-410X(03)00313-X
- JJ Wang, YT Qiang, QS Li, ZL Wen, GL Xia, CB Liu. Long-term protection against HBV vaccinated in infancy and immune response in HBV booster trial in adolescence. Chinese Journal of Vaccines and Immunization .2002(4):184-8.