Abstract
The combination of multiple HIV antigens in a vaccine can broaden antiviral immune responses. In this study, we used NDV vaccine strain LaSota to generate rNDV (rLaSota/optGag) expressing human codon optimized p55 Gag protein of HIV-1. We examined the effect of co-immunization of rLaSota/optGag with rNDVs expressing different forms of Env protein gp160, gp120, gp140L [a version of gp140 that lacked cytoplasmic tail and contained complete membrane-proximal external region (MPER)] and gp140S (a version of gp140 that lacked cytoplasmic tail and distal half of MPER) on magnitude and breadth of humoral, mucosal and cellular immune responses in guinea pigs and mice. Our results showed that inclusion of rLaSota/optGag with rNDVs expressing different forms of Env HIV Gag did not affect the Env-specific humoral and mucosal immune responses in guinea pigs and that the potent immune responses generated against Env persisted for at least 13 weeks post immunization. The highest Env-specific humoral and mucosal immune responses were observed with gp140S+optGag group. The neutralizing antibody responses against HIV strains BaL.26 and MN.3 induced by gp140S+optGag and gp160+optGag were higher than those elicited by other groups. Inclusion of Gag with gp160, gp140S and gp140L enhanced the level of Env-specific IFN-γ-producing CD8+ T cells in mice. Inclusion of Gag with gp160 and gp140L also resulted in increased Env-specific CD4+ T cells. The level of Gag-specific CD8+ and CD4+ T cells was also enhanced in mice immunized with Gag along with gp140S and gp120. These results indicate lack of antigen interference in a vaccine containing rNDVs expressing Env and Gag proteins.
Introduction
HIV envelope (Env) glycoprotein is the major target antigen against which neutralizing antibodies (NAbs) are produced and their efficacy in protection against HIV-1 has been demonstrated in various animal models.Citation1-3 While the interest in NAb is clear, it should be stressed that RV144 was the only vaccine efficacy trial showing protection against HIV acquisition with little NAb activity but with new patterns of non-NAbs that may block transmission.Citation4-6 Similarly, V2 antibodies seem important for protection against SIV and SHIV challenges.Citation7 Although this study did not measure non-NAbs, in particular V1 and V2 identified as inversely correlated with risk of acquisition in RV144, this issue should be noted as deserving further studies. Apart from NAbs and non-NAbsagainst Env, the importance of cellular immunity is evident from numerous studies that have shown the correlation of reduced viremia in HIV infection with generation of cellular immune responses.Citation8-10 Several studies have indicated that CD8+T lymphocytes can control viremia in HIV-1 infected humansCitation11-14 and SIV infected monkeys.Citation11,15-18 It has also been shown that CD8+T cells targeting HIV-1 Gag epitopes are associated with control of HIV-1 infection.Citation19-21 Vaccine trials in non-human primates have also shown an association between Gag-specific cell mediated responses and virologic control in vaccinated monkeys following SIV challenge.Citation7,22,23
Newcastle disease virus (NDV), a member of the genus Avulavirus in the family Paramyxoviridae, has a number of characteristics that make it useful as a vaccine vector in humans. A wide range of NDV strains exist that can be used as vectors. NDV is an avian virus that is attenuated in humans owing to natural host range restriction.Citation24 NDV is antigenically distinct from common animal and human pathogens, and thus vaccination would not be affected by preexisting immunity. In mouse and non-human primate models, NDV can infect via the intranasal (i.n.) route and has been shown to induce humoral and cellular immune responses both at the mucosal and systemic levels. NDV was used to express protective antigens of several human pathogens and elicited high levels of serum and mucosal immune responses in non-human primate models.Citation24-30 We previously investigated the potential of recombinant (r) NDV to express HIV-1 gp160 trimers and showed that vaccination by intranasal route elicited stronger Env-specific humoral and mucosal immune responses compared to intramuscular immunization in guinea pigs.Citation31 Recently, we compared humoral as well as cellular immune responses induced in guinea pigs and mice by rNDVs expressing different forms of Env protein such as gp160, gp140 and gp120.Citation32 In this study, we have evaluated the effect of inclusion of rNDV expressing HIV-1 Gag with rNDVs expressing different forms of HIV-1 Env proteins on level of humoral, mucosal and cellular immune responses against HIV. Our results showed that immunization of rNDV expressing HIV Gag with rNDV expressing different forms of HIV Env did not affect the Env-specific systemic and mucosal immune responses and that potent immune responses generated against Env persisted for at least 13 weeks post immunization. Interestingly, inclusion of Gag with gp160, gp140S and gp140L enhanced the level of Env-specific IFN-γ-producing CD8+ T cells in mice. Inclusion of Gag with gp160 and gp140L also resulted in increase of Env-specific CD4+ T cells. In addition, Gag-specific CD8+ and CD4+ T cells were enhanced in mice immunized with rNDV expressing Gag along with gp140S and gp120.
Results
Generation of rNDVs expressing HIV-1 Gag and Env proteins
NDV vaccine strain LaSota was used to construct 2 viruses, one expressing wild type Gag and other expressing human codon optimized Gag protein of HIV-1 strain BaL.1 and were designated rLaSota/wtGag and rLaSota/optGag, respectively (). The rLaSota/wtGag and rLaSota/optGag viruses were found to be stable after 10 passages in chicken eggs. The construction and generation of rNDVs expressing different forms of the Env glycoprotein of HIV-1 strain BaL.1 are described in materials and methods and contribution of each of the 4 Env proteins to immunogenicity in guinea pigs and mice was evaluated separately in our previous study.Citation32
Figure 1. Genome maps of parental recombinant NDV LaSota (rLaSota) and derivative bearing an insert encoding either HIV wild type (wt) Gag (rLaSota/wtGag) or human codon optimized (opt) Gag (rLaSota/optGag). (A) A transcription cassette encoding Gag was cloned into the PmeI (italicized) site at the junction of the P and M genes of the NDV LaSota antigenomic cDNA. The Gag ORF (ATG initiation and TGA termination signals in bold) was flanked by an NDV gene end (GE) transcription signal [boxed], an intergenic T nucleotide, and a gene start (GS) transcription signal [boxed]. (B) Maps of rLaSota/wtGag, rLaSota/optGag and rLaSota. NDV genes (NP, nucleoprotein; P, phosphoprotein; M, matrix protein; F, fusion glycoprotein; HN, hemagglutinin-neuraminidase protein; L, large polymerase protein) are shown as open boxes.
![Figure 1. Genome maps of parental recombinant NDV LaSota (rLaSota) and derivative bearing an insert encoding either HIV wild type (wt) Gag (rLaSota/wtGag) or human codon optimized (opt) Gag (rLaSota/optGag). (A) A transcription cassette encoding Gag was cloned into the PmeI (italicized) site at the junction of the P and M genes of the NDV LaSota antigenomic cDNA. The Gag ORF (ATG initiation and TGA termination signals in bold) was flanked by an NDV gene end (GE) transcription signal [boxed], an intergenic T nucleotide, and a gene start (GS) transcription signal [boxed]. (B) Maps of rLaSota/wtGag, rLaSota/optGag and rLaSota. NDV genes (NP, nucleoprotein; P, phosphoprotein; M, matrix protein; F, fusion glycoprotein; HN, hemagglutinin-neuraminidase protein; L, large polymerase protein) are shown as open boxes.](/cms/asset/03b87815-8bef-45b8-b5c6-9e53c686b546/khvi_a_987006_f0001_b.gif)
Expression of HIV-1 wt and opt Gag proteins
Analysis of cell lystates of DF1 cells infected with rLaSota/wtGag and rLaSota/optGag viruses revealed the presence of 4 bands representing (i) p55 Gag precursor protein, with an apparent molecular mass of ∼55 kDa; (ii) cleavage product with an apparent molecular mass of ∼47 kDa probably representing p17 matrix + p24 capsid + spacer peptides p1 and p2 proteins; (iii) cleavage product with an apparent molecular mass of ∼41 kDa probably representing p17+p24+p1 Gag proteins (iv) cleavage product with an apparent molecular mass of ∼24 kDa representing p24 Gag protein (). The densitometric analysis showed a very low level of p24 protein in lysate of rLaSota/wtGag infected cells compared to p24 in lysate of rLaSota/optGag infected cells. These results indicated that the secondary structure of rNDV expressed mRNA of codon optimized Gag may be more stable and conformationally more optimal than mRNA of wild type Gag for cleavage of precursor polyprotein by cellular proteases. As p55 Gag precursor protein expressed by rLaSota/optGag cleaved efficiently, rLasota/optGag was chosen for further biological characterization and immunogenicity studies in guinea pig and mice.
Figure 2. Analysis of rNDV-expressed Gag proteins and viral growth kinetics. (A) DF1 cells were infected with indicated viruses at an MOI of 0.01 PFU. After 48 h, the cell culture medium supernatants and cells were collected and processed. (A) The cell lysates were prepared from cells and subjected to SDS-PAGE under reducing conditions. (B) The cell culture medium supernatants were concentrated 10x by passing through Amicon filters and subjected to SDS-PAGE under reducing conditions. The gels were analyzed by Western blotting using a pool Gag p24 specific mAbs. The positions of HIV-1 Gag precursor protein (p55) and cleavage products are indicated by arrows in the right margin. Molecular masses of marker proteins (in kilodaltons) are shown in the left margin. (C) Vero cells were infected with rLaSota/optGag (panel a) and rLaSota (panel b) at an MOI of 0.1 PFU. Twenty-four h post-infection, the infected cells were fixed with paraformadehyde and permeabilized with Triton X-100 for detection of total antigen inside the cell. The cells were probed with Gag p24 specific mAbs followed by staining with Alexa Fluor 488-conjugated goat anti-mouse IgG antibodies (green) and DAPI (blue), and analyzed by immunofluorescence. The cells were visualized under Nikon Eclipse TE fluorescent microscope. (D) Comparison of multicycle growth kinetics of rLaSota and rLaSota/optGag viruses in DF1 cells. Cells were infected with each virus at an MOI of 0.01 and cell culture media supernatant aliquots were harvested at 8 h intervals until 64 h post-infection. The virus titers in the aliquots were determined by plaque assay in DF1 cells. Values are averages from 3 independent experiments.
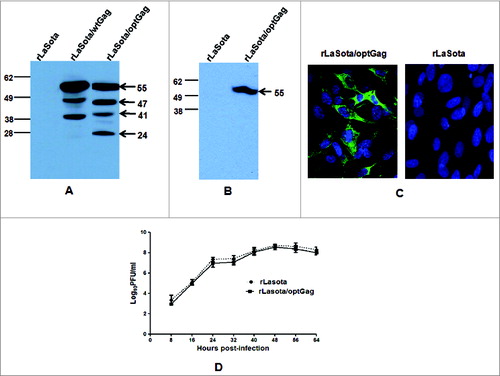
Analysis of cell culture supernatant showed that p55 Gag precursor protein was secreted in medium of DF1 cells infected with rLaSota/optGag (). Expression of Gag in Vero cells infected with rLaSota/optGag was also subjected to indirect immunoflouresecence analysis using p24-specific mAbs. The results showed that Gag was expressed efficiently in cytoplasm of Vero cells by rLaSota/optGag at 24 h post infection ().
Biological characterization of rNDV expressing opt Gag protein
The multicycle growth kinetics of rLaSota/optGag was evaluated in DF1 cells. The replication of this recombinant virus was similar to that of parental rLaSota virus (). The MDT (mean death time) test was used to determine the pathogenicity of this recombinant and parental rLaSota virus in 9-day-old embryonated chicken eggs The values of MDT for rLaSota and rLaSota/optGag were 105 h and 107 h, respectively. These results indicated that the insertion of the foreign gene slightly attenuated the pathogenicity of vaccine strain LaSota.
Humoral immune responses
Six groups of female Hartley guinea pigs (n = 3 ) were immunized on days 0 and 14 with rLaSota vector control and rLaSota/optGag individually or combination of rLaSota/optGag with either rLaSota/gp160 or rLaSota/gp140L or rLaSota/gp140S or rLaSota/gp120 as described in materials and methods and shown in . The animals in all the groups did not show any overt clinical signs of infection or any loss of body weight throughout the study (not shown), indicating that rNDVs were nonpathogenic in guinea pigs.
Figure 3. Immunization schedules. (A) Guinea pig immunization. Eighteen guinea pigs were divided into 6 groups (n=3 /group). Animals in each group were immunized with 2 doses of indicated recombinant virus on days 0 and 14 by i.n. route of administration and each dose consisted of 200 μl (100 μl in each nostril) of allantoic fluid. rLaSota (control group) and rLaSota/optGag (optGag group) received a dose of 106 PFU/ml of virus and rLaSota/gp160 and rLaSota/optGag (gp160+optGag group), rLaSota/gp140L and rLaSota/optGag (g140L+optGag group), or rLaSota/gp120 and rLaSota/optGag (g120+optGag group) received a mixture of 106 PFU/ml of virus. Blood, vaginal washes and fecal samples were collected on days 0, 7, 14, 21, 28, 42, 56, 70 and 90. All animals were sacrificed on day 90. (B) Mice immunization. Thirty six female BALB/c mice were divided in to 6 groups (n=6 /group). Animals in each group were immunized with 2 doses of virus on days 0 and 14 by the i.n. route of administration and each dose consisted of 50 μl (25 μl in each nostril) of allantoic fluid. rLaSota (control group) and rLaSota/optGag (optGag group) received a dose of 105 PFU/ml of virus and rLaSota/gp160 and rLaSota/optGag (gp160+optGag group), rLaSota/gp140L and rLaSota/optGag (g140L+optGag group), or rLaSota/gp120 and rLaSota/optGag (g120+optGag group) received a mixture of 105 PFU/ml of virus. All animals were sacrificed on day 56 and splenocytes were collected.
The induction of NDV-specific serum antibodies was measured on days 28 and 42 using an NDV-specific ELISA (). All six animal groups exhibited similar levels of NDV-specific IgG antibodies on these days (p values between different groups always >0.05), indicating that each of the viruses replicated to same level in the immunized animals
Figure 4. NDV-specific total IgG (panel A), HIV-1 gp120-specific total IgG, IgG1 and IgG2 (panel B-D), and HIV-1 Gag-specific total IgG (panel E) responses in guinea pig sera. The guinea pigs were immunized with the indicated rNDVs either alone or in a mixture of 2 by the i.n. route. (A) The guinea pig sera were analyzed for NDV-specific antibodies by commercial NDV ELISA kits (Synbiotics Corporation). Mean ELISA end-point titers of NDV-specific serum antibodies on days 28 and 42 are shown. (B–D) The guinea pig sera were analyzed by HIV-1 gp120 specific total IgG, IgG1 and IgG2 antibodies by isotype-specific ELISA with purified gp120. Mean ELISA end-point titers of gp120-binding serum antibodies of the indicated isotype on days 0, 7, 14, 21, 28, 42, 56, 70 and 90 are shown. (E) The guinea pig sera were analyzed for HIV-1 Gag-specific total IgG antibodies by ELISA with purified HIV-1 Gag p24. Mean ELISA end-point titers of Gag p24-binding serum antibodies on days 0, 7, 14, 21, 28, 42, 56, 70 and 90 are shown. Antibodies specific to gp120 and Gag p24 were not detected in any animal on any day in the control rLaSota group. The graph shows the geometric mean value ± SEM for 3 animals in each group. Arrows indicate time of rNDV immunizations on days 0 and 14. Statistically significant differences in data from serological analysis of different immunized guinea pig groups were evaluated by one-way analysis of variance (ANOVA) with the use of Prism 5.0 (Graph Pad Software Inc.., San Diego, CA) at a significance level of P < 0.05 (*) and P < 0.005 (**). P values of HIV-1 Env-specific total IgG, IgG1 and IgG2 response differences between different groups were < 0.05 (*). P value of HIV-1 Gag-specific total IgG response differences between different groups was < 0.005 (**).
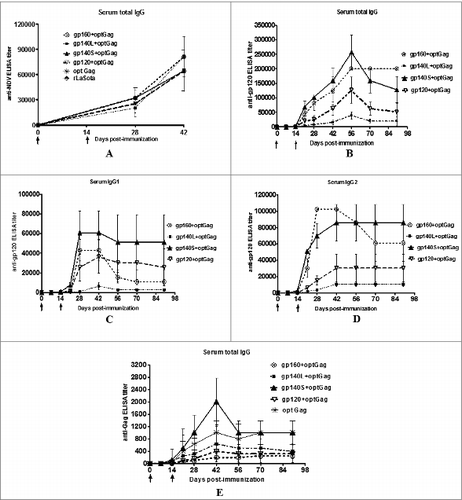
The induction of serum antibodies to HIV-1 Env was analyzed on days 7, 14, 21, 28, 42, 56, 70, and 90. Total serum IgG, IgG1 and IgG2a specific to BaL.1 gp120 was determined at each time point by ELISA (). Responses were detected on day 14 after the initial immunization and there was an increase in immune responses in all the groups following the boost on day 14. Total IgG response peaked on day 56 in all the groups. The IgG1 and IgG2a responses peaked between days 28 to 42 in all the groups. The highest gp120-specific total IgG titer was detected with the gp140S+optGag group followed by gp160+optGag and gp120+optGag groups, and the lowest titer was observed with gp140L+optGag group (P < 0.05 between different groups). The IgG1 response was highest in gp140S+optGag group followed by gp160+optGag, gp120+optGag, and gp140L+optGag groups (P < 0.05 between different groups). The IgG2a response was strongest in gp160+optGag group till day 42 followed by gp140S+optGag, gp120+optGag, and gp140L+optGag whereas it was strongest in gp140S+optGag group on days 70 and 90 (P < 0.05 between different groups). The serum IgG1:IgG2a was calculated to determine the Th1/Th2 balance. The ratio for gp160+optGag was 1:2.4 on day 28 and 1:5.6 on day 90, indicating a Th1-biased response. On the contrary, the ratios for gp140L+optGag, gp140S+optGag, and gp120+optGag varied from 1:0.2 to 1:0.8 on day 28, and 1:1 to 1.6 on day 90 post-immunization and thus showed Th2-biased response on day 28 which was shifted to mixed Th1/Th2 responses on day 90. In addition, the gp120-specific serum IgA in all the groups were assessed. All animals at all-time points were found to be negative (not shown).
We then measured the induction of HIV-1 Gag-specific serum antibodies in all the groups by ELISA (). A very low titer of anti-Gag antibodies was detected on day 14 in all the groups, and boosting on day 14 increased the titers only marginally. The titer peaked on day 42 in all the groups and the highest titer was observed with gp140S+optGag followed by optGag, gp140L+optGag, gp120+optGag and gp160+optGag group (P < 0.005 between different groups).
Mucosal immune responses.
Vaginal washes collected from each animal were evaluated by ELISA at different time points (). The titer of total IgG, IgG1 and IgG2a peaked between days 28 and 56, decreased between day 56 and 70 and increased again on day 90 (). The total IgG response was highest in the gp140S+optGag group followed by gp160+optGag gp120+optGag, and gp140L+optGag groups on days 28 and 42, whereas it was greatest in gp160+optGag group on days 56, 70, and 90 (P < 0.05 between different groups). The total IgG response in fecal samples of animals in all the groups were assayed but no detectable titer was found (not shown). The IgG1 responses were strongest with gp140S+optGag followed by gp120+optGag, gp160+optGag, and gp140L+optGag groups whereas the IgG2a responses were strongest with gp160+optGag followed by gp140S+optGag, gp120+optGag, and gp140L+optGag groups (P = 0 .26 and 0.33 for IgG1 and IgG2a, respectively, between different groups). The vaginal IgG1:IgG2a ratio varied from 1:2.5 to 1:4 at different time points for the gp160+optGag group, indicative of a Th1-biased response. In other groups, the vaginal Th1:Th2a ratio varied at different time points from 1:0.9 to 1:2, 1:0.35 to 1:2.3, and 1:0.7 to 1:4 for gp140L+optGag, gp140S+optGag, and gp120+optGag, respectively, indicating a mixed Th1/Th2 response. Similar to the serum antibody isotype, the isotype analysis of vaginal washes indicated an evidence of Th1-biased antibody response in gp160+optGag immunized group and mixed Th1/Th2 antibody response in all other groups.
Figure 5. HIV-1 gp120-specific total IgG (A), IgG1 (B), IgG2a (C) and IgA (D) antibodies in vaginal washes collected from guinea pigs, detected by isotype-specific ELISA with purified gp120. The guinea pigs were immunized with the indicated rNDVs by the i.n. route. Mean ELISA end-point titers of gp120-binding vaginal wash antibodies of the indicated isotype on days 0, 7, 14, 21, 28, 42, 56, 70 and 90 are shown. Antibodies specific to gp120 were not detected in any animal on any day in control rLaSota group. The graph shows the geometric mean value ± SEM for 3 animals in each group. Arrows indicate time of rNDV immunizations on days 0 and 14. Statistical differences between the groups were calculated by one-way ANOVA. P values of HIV-1 Env-specific total IgG, IgG1, IgG2 and IgA response differences between different groups were< 0.05(**), 0.26, 0.33 and 0.77, respectively.
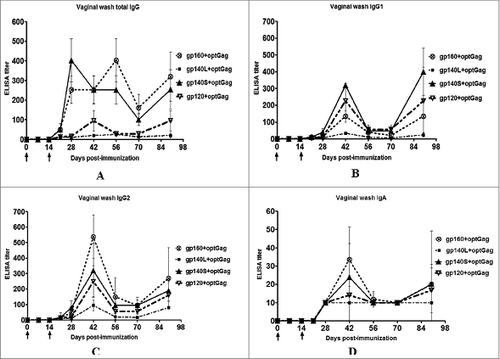
BaL.1 gp120-specific IgA responses were also measured in vaginal washes (). A low titer was detected in all groups. The titer peaked on day 42, decreased on day 56 and increased marginally on day 90. The highest response was detected in the gp160+optGag group followed by gp140S+optGag, gp120+optGag, and gp140L+optGag groups (P=0 .77 between different groups).
Neutralizing antibody (NAb) responses
Sera collected on days 28, 56, 70, and 90 from animals immunized with the gp160+optGag, gp140L+optGag, gp140S+optGag, gp120+optGag, and rLaSota were evaluated in the TZM.bl assay for their ability to neutralize homologous clade B tier 1 HIV-1 strains BaL.26 and heterologous clade B tier 1 HIV-1 strain MN.3 and heterologous clade B tier 2 HIV-1 strains RHPA4259.7 and TRO.11. NAb activity (expressed as ID50 value) against Bal.26 was detected in sera from all groups of animals, with the highest titer observed in gp160+optGag group followed by gp140S+optGag, gp140L+optGag, and gp120+optGag groups (). Although NAb titers against Bal.26 were higher in gp160+optGag and gp140S+optGag groups compared to gp140L+Gag and gp120+Gag groups, yet these differences are not statistically significant.
Figure 6. Virus neutralizing antibody activity (50%-inhibitory-concentration [ID50] titers) against homologous HIV-1 clade B tier 1 strains (BaL.26), heterologous clade B tier 1 strain MN.3 and heterologous clade B tier 2 strains (RHPA4257.7 and TRO.11) in sera from guinea pigs immunized with the indicated rNDVs. (A) Guinea pig sera obtained on days 28, 56, 70 and 90 were tested against BaL.26 pseudovirus by the TZM-bl assay (B) Guinea pig sera obtained on days 28, 56, 70 and 90 were tested against MN.3 pseudovirus by the TZM-bl assay (C) Guinea pig sera obtained on day 56 were tested against RHPA4259.7 and TRO.11 by the TZM-bl assay. Horizontal bars indicate the geometric mean titer. Pre-immune sera were used to establish baseline-neutralizing activity in each individual guinea pig, and these values were subtracted from the values shown. The neutralizing antibody activity against each virus in sera obtained from guinea pigs immunized with control rLaSota virus (empty vector) was < 20 and were not shown in the graphs. The samples that did not neutralize the target virus (titers <20) were given a value of 10 for plotting purposes; therefore, all samples registering an ID50 titer of 10 are negative. Statistical differences between the groups were calculated by unpaired t test (2-tailed) and only statistically significant numbers are shown underneath the horizontal line. *, ** and *** indicates statistically significant differences P < 0.05, P < 0.005 and P < 0.0005, respectively, between groups.
![Figure 6. Virus neutralizing antibody activity (50%-inhibitory-concentration [ID50] titers) against homologous HIV-1 clade B tier 1 strains (BaL.26), heterologous clade B tier 1 strain MN.3 and heterologous clade B tier 2 strains (RHPA4257.7 and TRO.11) in sera from guinea pigs immunized with the indicated rNDVs. (A) Guinea pig sera obtained on days 28, 56, 70 and 90 were tested against BaL.26 pseudovirus by the TZM-bl assay (B) Guinea pig sera obtained on days 28, 56, 70 and 90 were tested against MN.3 pseudovirus by the TZM-bl assay (C) Guinea pig sera obtained on day 56 were tested against RHPA4259.7 and TRO.11 by the TZM-bl assay. Horizontal bars indicate the geometric mean titer. Pre-immune sera were used to establish baseline-neutralizing activity in each individual guinea pig, and these values were subtracted from the values shown. The neutralizing antibody activity against each virus in sera obtained from guinea pigs immunized with control rLaSota virus (empty vector) was < 20 and were not shown in the graphs. The samples that did not neutralize the target virus (titers <20) were given a value of 10 for plotting purposes; therefore, all samples registering an ID50 titer of 10 are negative. Statistical differences between the groups were calculated by unpaired t test (2-tailed) and only statistically significant numbers are shown underneath the horizontal line. *, ** and *** indicates statistically significant differences P < 0.05, P < 0.005 and P < 0.0005, respectively, between groups.](/cms/asset/ff2be245-58bf-4f10-b04a-a3dddc4060b4/khvi_a_987006_f0006_b.gif)
NAb response against HIV-1 strain MN.3 in immunized guinea pigs was highest in gp140S+optGag group followed by gp160+optGag, gp120+optGag, and gp140L+optGag groups (). A comparison of mean ID50 values against MN.3 showed a significantly stronger NAb response in gp160+optGag group compared to gp140L+optGag (P < 0.005). On day 28, mean ID50 titres against MN.3 were also significantly higher in gp140S+optGag and gp160+optGag groups compared to gp140L+optGag and gp120+optGag groups, respectively (P < 0.05). Of note, the NAb titers in all the groups to BaL.26 and MN.3 remained high on day 90. Analysis of sera from all the groups on day 56 indicated a weak NAb activity against HIV-1 strains RHPA4259.7 and TRO.11 in TZM.bl assay ().
Cellular Immune Responses
Due to unavailability of antibody reagents for analyzing cellular immune responses in guinea pigs, the ability of rNDVs to elicit cellular immune responses against HIV-1 Env and Gag were determined in female BALB/c mice (n = 6 /group). IFN-γ-producing CD4+ T cells and CD8+ T cells against pool of Env peptides were highest in the mice that received gp140S+optGag followed by gp120+optGag, gp140L+optGag and gp160+optGag (). When number of IFN-γ-producing CD8+and CD4+T cells produced in mice immunized with gp160, gp140L, gp140S and gp120 along with Gag were compared with those produced in mice immunized with these envelope proteins alone in our previous study,Citation32 it was found that Env-specific IFN-γ-producing CD8+T cells were significantly enhanced in mice immunized with gp140S+optGag and tended to be higher in mice that received gp160+optGag and gp140L+optGag. Env-specific CD4+T cells were also increased in gp160+optGag and gp140L+optGag immunized groups. There is marginal decrease in number of CD4+T cells in gp140S+optGag group. The population of CD4 and CD8+ T cells were not effected in gp120+optGag immunized group.
Figure 7. HIV-1 Env- and Gag- specific CD4+ and CD8+ T cell response. Mice in groups of 6 were immunized with 105 PFU/ml of the indicated rNDV either alone or co-immunized with a mixture of 2 rNDVs by the i.n. route on days 0 and 14. On day 56, splenocytes were isolated, stimulated with either a pool of overlapping Env peptides or Gag-specific peptide and processed for intracellular cytokine staining for IFN-γ and CD4 and CD8. The symbols show the results of 3 experiments in each group where spleens from 2 mice were pooled in each experiment.
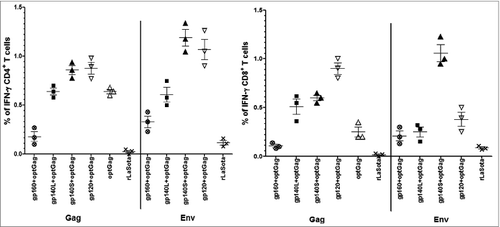
IFN-γ-producing CD4+ T cells and CD8+ T cells against Gag peptide were detected in all the co-immunized groups and group of mice immunized with rLasota/optGag alone. Surprisingly, the IFN-γ responses in CD8+ subpopulations were higher in mice immunized with rLaSota/gp120, rLaSota/gp140S and rLaSota/gp40L along with rLaSota/optGag compared to the mice immunized with rLaSota/optGag alone. Furthermore, IFN-γ responses in CD4+ subpopulations were higher in mice vaccinated with gp120+optGag and gp140S+optGag compared to the mice vaccinated with opt Gag alone. A very low level of Gag-specific IFN-γ-producing CD4+ T cells and CD8+ T cells was detected in gp160+optGag group.
Discussion
In the present study, we used a live, replication-competent NDV vector to express HIV-1 Gag. NDV (also known as APMV-1) has some of the key features that are desirable for use as a vaccine vector such as mucosal delivery of foreign antigen through intranasal route, high levels of gene expression for the recombinant product, stability of the insert with prolonged passages of vector, potent inducer of humoral, mucosal and cellular immune responses to the foreign antigens and safe in humans as it has been tested in high doses in adults as oncolytic agent. Further there is no preexisting immunity to the NDV in humans. Using NDV as a vector, booster immunization was not hampered by preexisting antibodies induced after first immunization in guinea pigs, rather immune responses were increased significantly after booster immunization.Citation31 Another advantage of NDV is that it can be used as prime-boost immunization with other serologically distinct APMV serotypes such APMV-2 to-10. NDV vector was based on the LaSota strain, which is a naturally-ocurring avirulent strain that is widely used in many countries as a live NDV vaccine in chickens for several years and thus poses no agricultural concerns.
The goal of this study was to evaluate whether co-administration of rNDV expressing HIV Gag along with rNDVs expressing different forms of HIV Env would increase the breadth of immune responses against HIV in guinea pigs and mice. Our results showed that co-immunization of Gag and Env immunogens expressed by rNDV increased Env-specific total IgG responses in serum compared with rNDVs expressing Env alone.Citation32 However, the mean values of serum IgG1 and IgG2a responses elicited after co-immunization of soluble Env forms, rLaSota/140S and rLaSota/120 along with rLaSota/optGag were slightly decreased compared to immunization with these recombinants alone, but the differences were not statistically significant (p values >0.05) suggesting that the observed differences were not due to antigenic competition. The reduction in serum IgG1 and IgG2a responses after co-administration of rLasota/gp140L and rLaSota/optGag compared to rLaSota/140L alone was statistically significant. The exact reason of this lower immune response is not known but there is a possibility that this truncated mutant may be defective in folding or the protein expressed by this mutant may be unstable and rapidly degraded. The mucosal IgG immune responses elicited after co-administration of rNDVs expressing Env and Gag were either similar or enhanced compared to responses generated after immunization of Env alone in our previous study.Citation32 The pattern of anti-Env mucosal immune responses produced against combination of Env and Gag antigens were similar to the immune responses produced against individual Env antigen that the immune responses peaked between days 28–42, decreased between days 56–70 and again increased on day 90. The Th1 and Th2 profiles of humoral and mucosal immune responses developed after immunization of combination of rNDVs expressing different forms of Env protein and Gag protein were also similar to immune response induced after vaccination with Env alone. These results suggest that co-immunization of NDV expressed Gag along with Env antigens does not change the immunogenicity of Env antigen. Further, our results showed that most of the immune responses induced by NDV vectors expressing Gag and Env proteins peaked at 6 weeks rather than usual 2 weeks post final boosting and stayed for at least 13 weeks. The results are interesting because it shows the longevity of the immune responses due to the use of NDV vector, which could be due to persistent low level of replication of NDV. Alternatively, NDV is highly immunogenic and even a low level of replication could result in potent induction of interferon and stimulation of dendritic cell maturation. The IgG antibody titer decreased in vaginal washes on day 56 and 70 and unexpectedly, its titer bounced back on day 90 post immunization. This observation is similar to our previous findings.Citation32 A possible explanation for this antibody surge could be a result of the memory B cell response or the antibody production from the long-lived plasma cells in bone marrow.Citation33
We found a very low anti-Gag IgG response in sera of guinea pigs immunized with either rLaSota/optGag alone or in combination with rNDVs expressing different forms of Env. Similar observation were reported by Xu et al.Citation34 previously as they found a low level of Gag specific IgG after prime boost vaccination with recombinant mumps virus and vesicular stomatitis virus vectors in rhesus macaques. A likely explanation for the lower response to Gag is that this protein is mainly localized intracellularly which reduces availability of Gag for B cell presentation. Even though Gag protein is myristylated and could form virus like particles,Citation35 a critical mass of Gag protein required for antibody induction may not have been achieved.
A possible complication of multivalent vaccines containing 2 or more HIV-1 antigens is the potential of one antigen to suppress or overwhelm the cellular immune response over other antigens. One aim of this study was to evaluate the cellular immune responses against Env and Gag proteins in mice co-immunized with rNDVs expressing different forms of Env along with Gag. The results demonstrate that Gag-specific IFN-γ-producing CD8+ T cells were increased 2- to 3.5-fold after immunization with rLaSota/gp140L, rLaSota/gp140S and rLaSota/gp120 expressing soluble forms of Env protein together with rLasota/optGag compared to level of cells induced with rLasota/optGag alone. Gag-specific IFN-γ-producing CD4+ T cells were also increased marginally (1.3-fold) after immunization with rLaSota/gp140S and rLaSota/gp120 together with rLasota/optGag compared to level of cells produced with rLasota/optGag alone. It is possible that the shedding of soluble Env forms from viruses or virus infected cells could be increasing the antigenicity of these proteins by raising its effective concentration and hence able to increase CD4+ or CD8+ cytokine response; which might be outweighing the competition between Gag and soluble forms of Env for MHC class I epitope presentation. However, the mean value of Gag specific CD4+ and CD8+ T cells decreased after immunization of rLaSota/gp160 together with rLaSota/optGag compared to immunization with rLaSota/opt Gag alone. The gp160 mediated suppression of CD4+ and CD8+ T cell responses against Gag could be due to various reasons as reported in various findings reported previously (1) competition of expression resulting in immunodominance of Env over Gag;Citation36 (2) suppression of expression via type1 IFN;Citation37 (3) Env-mediated suppression of dendritic cell activation and maturation;Citation38 and (4) epitope competition for H-2d MHC class I presentation especially in case of CD8+ T cell suppression.Citation39,40 Further, inclusion of rLasota/optGag with rLaSota/gp160, rLaSota/gp140L and rLaSota/gp140S enhanced Env-specific IFN-γ-producing CD4+ and CD8+ T cells compared to the Env expressing rNDVs inoculated alone in our previous study.Citation32 These findings are unexpected and this phenomenon might be explained by higher level of activation due to increased cytokine production and proliferation in the doubly immunized mice. The results presented in this study suggest that it is possible to manipulate cell mediated immune response through co-immunization of rNDVs expressing Gag and different forms of Env proteins. Our study was done with a gag peptide covering only one Kd restricted CTL epitope (AMQMLKETI amino acids from position 197–205), it would be meaningful to look for the immune responses against the complete Gag protein using peptide pool covering the entire Gag region.
In summary, we have shown that immunization of rNDVs expressing Gag along with different forms of Env protein in guinea pigs elicits potent and long lasting systemic and mucosal immune responses against Env. Inclusion of rLasota/optGag with rLaSota/gp160, rLaSota/gp140L and rLaSota/gp140S enhanced Env-specific IFN-γ-producing CD8+ T cells compared to the Env expressing rNDV inoculated alone. Immunization of mice with Gag along with gp160 and gp140L also resulted in increase of Env-specific CD4+ T cells. Additionally, inclusion of rNDV expressing Gag with rNDVs expressing soluble forms of Env proteins, gp140 and gp120, also enhanced Gag-specific IFN-γ-producing CD8+ and CD4+ T cell responses. The present study indicates that the development of vaccine strategies based on combination of HIV Gag and Env proteins expressed by rNDVs is feasible and may broaden the humoral and cellular responses against different HIV strains.
Materials and Methods
Cells, viruses, antibodies and protein. Human epidermoid carcinoma (HEp-2), chicken embryo fibroblast (DF1), Vero, and 293T/17 cell lines were obtained from the American Type Culture Collection (ATCC). The TZM-bl cell line was obtained from the NIH AIDS Research and Reference Reagent Program (NIH ARRRP), Division of AIDS, NIAID, NIH. HEp-2, DF1 and TZM-bl cells were grown in Dulbecco's modified Eagle medium (DMEM) containing 10% fetal bovine serum (FBS) and maintained in DMEM with 5% FBS. Vero cells were grown and maintained in minimum essential medium (MEM) containing 10% FBS. 293T/17 cells were grown and maintained in Opti-MEM I reduced serum medium containing 10% FBS. Recombinant and wild-type NDV strains were grown in 9-day-old specific-pathogen-free (SPF) embryonated chicken eggs. Purified recombinant HIV-1 BaL gp120 protein was obtained from NIH ARRRP. A pool of HIV gp120 monoclonal antibodies (mAbs) was kindly provided by Dr. Anthony DeVico, University of Maryland School of Medicine, UMB, Baltimore, MD. Purified recombinant HIV-1 Gag p24 protein and Gag p24 specific mAbs were obtained from Abcam.
Construction and characterization of recombinant NDVs expressing HIV-1 Gag. Full-length HIV-1 wild-type (wt) Gag (1548 nucleotides) was amplified by PCR using a plasmid (obtained from NIH ARRRP) containing a HIV Gag gene (GenBank accession number AY713409) that was derived from clade B strain BaL. The forward primer 5′-AGCTTTGTTTAAACTTAGAAAAAATACGGGTAGAACGCCGCCACCatgggtgcgagagcgtcagta-3′ and reverse primer 5′-AGCTTTGTTTAAACttattgtga-cgaggggtcgttg-3′ contained PmeI sites (italicized), NDV gene end and gene start transcriptional signals (underlined), an NDV T intergenic nucleotide (boldface), an additional nucleotide in order to maintain the genome length as a multiple of 6 necessary for efficient replicationCitation41 [italicized and bold], a 6-nucleotide Kozak sequence for efficient translation (bold, underlined), and Gag-specific sequence (small case). A potential gene end sequence for NDV polymerase 5′-369TAAGAAAAA377–3′ that was present in Gag cDNA was mutated by changing the nucleotides present at third-codon positions without changing the encoded protein sequence. Full-length HIV-1 human codon-optimized (opt) Gag cDNA (1548 nucleotides) was amplified by PCR using a plasmid containing HIV-1 opt Gag gene (synthesized by GenScript). Both the genes were modified by PCR to add NDV transcription signals and cloned in cDNA of full length antigenome of NDV vaccine strain LaSota (). The integrity of both the genes were confirmed by sequence analysis. Recombinant viruses (designated rLaSota/wtGag and rLaSota/optGag) were recovered as described previouslyCitation42 and were grown in 9-day-old embryonated SPF chicken eggs.Citation43,44 The expression of wt Gag and opt Gag proteins by rLaSota/wtGag and rLaSota/optGag was examined by Western blot analysis. Briefly, DF1 cells were infected with rLasota/wtGag and rLaSota/optGag and rLaSota at a multiplicity of infection (MOI) of 0.01 PFU. The cells were harvested at 48 h post-infection and lysed using radioimmunoprecipitation assay buffer. The cell culture medium supernatants were collected at 48 h post-infection and were concentrated 10x by passing through Amicon filters. The cell lysate and cell culture medium supernatants were analyzed by Western blotting using a 1:10 dilution of a pool of gp120-specific mAbs. The expression of opt Gag by the recombinant virus was further examined in Vero cells by immunofluorescence assay.
To determine multicycle growth kinetics of rLaSota/optGag and rLaSota, DF1 cells in duplicate wells of 6-well plates were infected with each virus at an MOI of 0.01 PFU. After 1 h of adsorption, the cells were washed with DMEM and then incubated with DMEM containing 5% FBS and 5% allantoic fluid. The cell culture supernatant samples were collected and replaced with an equal volume of fresh medium at 8 h intervals until 64 h post-infection. The titers of virus in the samples were quantified by plaque assay in DF1 cells.
The pathogenicity of rLasota/optGag and rLaSota in chickens was evaluated by an internationally established in vivo test: the mean death time (MDT) test in 9-day-old SPF embryonated chicken eggs. The MDT test was performed by a standard procedure.Citation45 Briefly, a series of fold10- dilutions of fresh allantoic fluid from eggs infected with the test virus were made in sterile PBS, and 0.1 ml of each dilution was inoculated into the allantoic cavity of each of 5 eggs. The eggs were incubated at 37°C and examined 4 times daily for 7 d The time that each embryo was first observed dead was recorded. The highest dilution that killed all embryos was considered the minimum lethal dose. The MDT was recorded as the time (in h) for the minimum lethal dose to kill the embryos. The MDT has been used to classify NDV strains as velogenic (MDT < 60 h), mesogenic (MDT 60 to 90 h), and lentogenic (MDT > 90 h).
Construction and characterization of recombinant NDVs expressing HIV-1 Env. Four NDV recombinant viruses expressing (i) the full-length, 852-aa gp160 protein (virus rLaSota/gp160); (ii) a 679-aa gp140 protein (gp140L, virus rLaSota/gp140L) that contained the complete 30-aa MPER at its C-terminus and terminated with the sequence DKWAS immediately adjacent to the transmembrane domain; (iii) a shorter, 663-aa gp140 protein (gp140S, virus rLaSota/gp140S) that contained a partial, 14-aa long MPER at its C-terminus and terminated with the sequence WYIKI; and (iv) a 560-aa gp120 protein (virus rLaSota/gp120) that terminated with the cleavage site sequence REKR were used in this study. The construction and characterization of these recombinants have been previously describedCitation32
Guinea pig immunizations. All of the animals (guinea pigs and mice) used in this study were housed in isolator cages in our Bio Safety Level-2+ facility and cared for in accordance with established guidelines, and the experimental procedures were performed with approval from Institutional Animal Care and Use Committee of the University of Maryland. Female Hartley guinea pigs weighing approximately 375 g each were obtained from Charles River Laboratories. A total of 18 guinea pigs were divided into 6 groups of 3 animals each that were immunized with a dose of 106 PFU/ml of rLaSota (control group) and rLaSota/optGag (optGag group) and a mixture of 106 PFU/ml each of rLaSota/gp160 and rLaSota/optGag (gp160+optGag group), rLaSota/gp140L and rLaSota/optGag (g140L+optGag group), or rLaSota/gp120 and rLaSota/optGag (g120+optGag group). Animals were immunized by intranasal (i.n.) route on days 0 and 14 with 200 μl (100 μl in each nostril) of allantoic fluid containing the indicated virus. All animals were sacrificed 76 d after the second boost (i.e. 90 d following the first immunization). Blood was collected on day 0 (pre-bleed) and on days 7, 14, 21, 28, 35, 42, 56, 70 and 90. Sera were prepared and stored at −70°C. Vaginal washes and fecal samples were collected in parallel with the blood samples. To collect vaginal washes, animal feeding needles (Fisher Scientific) were used to flush 100 μl of PBS containing protease inhibitor cocktail (Sigma) 4 to 6 times into vaginal cavity. Vaginal washes were spun at 10,000 rpm for 15 min to remove cellular debris and supernatants were collected and stored at −70°C. Fecal sample were collected in PBS containing antibiotics, vortexed and incubated at 37°C for 20 min and spun at 4,000 rpm for 10 min. Supernatants were collected and stored at −70°C
Measuring gp120-specific total IgG, IgG1, IgG2a and IgA and Gag p24-specific total IgG antibodies in sera, vaginal washes and fecal samples by ELISA. HIV-1 Env-specific antibody titers were determined by isotype-specific ELISA. Ninety-six-well Maxisorp ELISA plates (Nunc), coated overnight with 100 μl/well of 1 μg/ml purified recombinant HIV-1 BaL gp120 protein in sodium carbonate/bicarbonate buffer (pH 9.8), were blocked first with 3% skimmed milk in water for 30 sec and then with 2% sucrose in water for 30 sec. Plates were dried for 2 h at 37°C. Serial dilutions of sera or vaginal washes or fecal samples from immunized guinea pigs were prepared in dilution buffer (Synbiotics Carporation), added to the plates, and incubated for 2 h at room temperature. The plates were washed 3 times with plate washing solution (Synbiotics Carporation) and incubated for 1 h with a 1:1,000 dilution of an isotype-specific secondary antibody; namely, horseradish peroxidase (HRP)-conjugated goat anti-guinea pig IgG (KPL), goat anti-guinea pig IgG1, goat anti-guinea pig IgG2a (Novus Biologicals), or sheep anti-guinea pig IgA (Immunology Consultants Laboratory). The plates were washed 3 times and developed with ABTS (2,2′-Azinobis [3-ethylbenzothiazoline-6-sulfonic acid]-diammonium salt) peroxidase substrate solution (Synbiotics Carporation), stopped by the addition of peroxidase stop solution, and analyzed at 405 nm with ELx800 ELISA plate reader (BioTek). HIV-1 Gag-specific total IgG antibody titers in serum were determined in ELISA plates coated with purified recombinant HIV-1 Gag p24 protein as described above. The ELISA endpoint titers were defined as the highest reciprocal serum dilution at which the mean OD value of duplicate wells were >fold2- above the mean OD value plus 2 SD of serum or vaginal wash or fecal sample from negative control animals. Commercial NDV ELISA kits (Synbiotics Corporation) was used to detect antibodies against the NDV antigens.
Neutralization assays. Neutralizing antibody activity was measured in 96-well culture plates by using Tat-regulated Luc reporter gene expression to quantify reductions in virus infection in TZM-bl cells. Assays in TZM-bl cells were performed with HIV Env-pseudotyped viruses as described previously.Citation46 TZM-bl cells were used for neutralization of clade B tier 1 HIV-1 strains BaL.26 and MN.3 and heterologous clade B tier 2 HIV-1 strains RHPA4259.7 and TRO.11. Briefly, neutralization assays were performed with serial dilutions of heat-inactivated (56°C, 1 hr) samples. Serum samples were diluted over a range of 1:20 to 1:43740 in cell culture medium and were pre-incubated with virus (∼150,000 relative light unit equivalents) for 1 hr at 37°C before addition of cells. Following 48 hr incubation, cells were lysed and luciferase activity was determined using a microtiter plate luminometer and BriteLite Plus Reagent (Perkin Elmer). Neutralization titers are the sample dilution at which relative luminescence units (RLU) were reduced by 50% compared to RLU in virus control wells after subtraction of background RLU in cell control wells. For each animal in this study, pre- and post-immune serum samples were assayed side-by-side. As is often the case with guinea pig sera, a low-level background signal was present in many pre-immune serum samples; the background has been subtracted from the neutralization titers presented.
Detection of Env- and Gag-specific IFN-γ producing cells by intracellular cytokine staining (ICS). Six-week-old female BALB/c mice (Charles River Laboratories) in groups of 6 animals each were immunized by the i.n. route on days 0 and 14 with a dose of 105 PFU/ml of rLaSota (control group) and rLaSota/optGag (optGag group) and a mixture of 105 PFU/ml each of rLaSota/gp160 and rLaSota/optGag (gp160+optGag group), rLaSota/gp140L and rLaSota/optGag (gp140L+optGag group), or rLaSota/gp120 and rLaSota/optGag (gp120+optGag group) in 50 μl (25 μl in each nostril) of allantoic fluid. Splenocytes were collected on day 56 and stimulated for 12 h with either 10 μg/ml of HIV-1 consensus subtype B 10-mer overlapping Env peptide pools (NIH ARRRP) or HIV-1 Gag p24 Peptide, AMQMLKETI (NIH ARRRP) or medium alone. Cells were incubated for 6 h with 10 μg/ml brefeldin A (Sigma). After blocking Fcγ receptors (rat anti-mouse CD16/32; BD Biosciences), cells were stained with Alexa Fluor 488-conjugated anti-mouseCD3ϵ and APC-Cy™7 conjugated anti-CD4 and per CP-CyTM5 conjugated anti-mouse CD8 for 30 min at 4°C. The cells were fixed, permeabilized (Cytofix/Cytoperm Plus, BD Biosciences) and stained with PE anti–IFN-γ mAbs for 30 min at 4°C (BD Biosciences). Cells were analyzed by flow cytometry and Flowjo software was used for data analysis. The frequencies of cells positive for IFN-γ+ and CD4 or CD8 were determined. Data are representative of 3 experiments where spleens from 2 mice were pooled in each experiment.
Statistical analysis. Statistical differences between the groups were calculated by one-way ANOVA and unpaired t test (2-tailed) with the use of Prism 5.0 (Graph Pad Software Inc.., SanDiego, CA) with a significance level of P < 0.05.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Author Contributions
Conceived and designed the experiments: SKK, CCL, DCM, XZ and SKS. Performed the experiments: SKK, SP, SS and CCL. Analyzed the data: SKK, SP, SS and CCL. Wrote the manuscript: SKK and SKS.
Acknowledgments
We thank Yunsheng Wang, Daniel Rockemann, and other lab members for their technical assistance and help. We thank Girmay Gebreluul and Yonas Araya for their help with handling of guinea pigs.
Funding
This research was supported NIH R21 grant AI-093198 awarded to SKS and by NIAID Primate Central Immunology Laboratory Contract HHSN27201100016C awarded to DCM. The views expressed herein neither necessarily reflect the official policies of the Department of Health and Human Services; nor does mention of trade names, commercial practices, or organizations imply endorsement by the US. Government.
References
- Mascola JR, Stiegler G, VanCott TC, Katinger H, Carpenter CB, Hanson CE, Beary H, Hayes D, Frankel SS, Birx DL, et al. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat Med 2000; 6:207-10; PMID:10655111
- Balazs AB, Chen J, Hong CM, Rao DS, Yang L, Baltimore D. Antibody-based protection against HIV infection by vectored immunoprophylaxis. Nature 2012; 481:81-4; http://dx.doi.org/10.1038/nature10660
- Baba TW, Liska V, Hofmann-Lehmann R, Vlasak J, Xu W, Ayehunie S, Cavacini LA, Posner MR, Katinger H, Stiegler G, et al. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat Med 2000; 6:200-6; PMID:10655110
- Excler JL, Robb ML, Kim JH. HIV-1 vaccines: Challenges and new perspectives. Hum Vaccin Immunother 2014; 10; PMID:24637946; http://dx.doi.org/10.4161/hv.28462
- Zolla-Pazner S. A critical question for HIV vaccine development: which antibodies to induce? Science 2014; 345:167-8; PMID:25013066; http://dx.doi.org/10.1126/science.1256526
- Su B, Moog C. Which Antibody Functions are Important for an HIV Vaccine? Front Immunol 2014; 5:289; PMID:24995008; http://dx.doi.org/10.3389/fimmu.2014.00289
- Barouch DH, Liu J, Li H, Maxfield LF, Abbink P, Lynch DM, Iampietro MJ, SanMiguel A, Seaman MS, Ferrari G, et al. Vaccine protection against acquisition of neutralization-resistant SIV challenges in rhesus monkeys. Nature 2012; 482:89-93; PMID:22217938; http://dx.doi.org/10.1038/nature10766
- Borrow P, Lewicki H, Hahn BH, Shaw GM, Oldstone MB. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J Virol 1994; 68:6103-10; PMID:8057491
- Rosenberg ES, Billingsley JM, Caliendo AM, Boswell SL, Sax PE, Kalams SA, Walker BD. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science 1997; 278:1447-50; PMID:9367954
- Koup RA, Safrit JT, Cao Y, Andrews CA, McLeod G, Borkowsky W, Farthing C, Ho DD. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol 1994; 68:4650-5; PMID:8207839
- McDermott AB, Koup RA. CD8(+) T cells in preventing HIV infection and disease. Aids 2012; 26:1281-92; PMID:22441256
- Blackbourn DJ, Mackewicz CE, Barker E, Hunt TK, Herndier B, Haase AT, Levy JA. Suppression of HIV replication by lymphoid tissue CD8+ cells correlates with the clinical state of HIV-infected individuals. Proc Natl Acad Sci U S A 1996; 93:13125-30; PMID:8917555
- Frahm N, Kiepiela P, Adams S, Linde CH, Hewitt HS, Sango K, Feeney ME, Addo MM, Lichterfeld M, Lahaie MP, et al. Control of human immunodeficiency virus replication by cytotoxic T lymphocytes targeting subdominant epitopes. Nat Immunol 2006; 7:173-8; PMID:16369537
- Hersperger AR, Migueles SA, Betts MR, Connors M. Qualitative features of the HIV-specific CD8+ T-cell response associated with immunologic control. Curr Opin HIV AIDS 2011; 6:169-73; PMID:21399496; http://dx.doi.org/10.1097/COH.0b013e3283454c39
- Greene JM, Lhost JJ, Burwitz BJ, Budde ML, Macnair CE, Weiker MK, Gostick E, Friedrich TC, Broman KW, Price DA, et al. Extralymphoid CD8+ T cells resident in tissue from simian immunodeficiency virus SIVmac239{Delta}nef-vaccinated macaques suppress SIVmac239 replication ex vivo. J Virol 2010; 84:3362-72; PMID:20089651; http://dx.doi.org/10.1128/JVI.02028-09.
- Hansen SG, Ford JC, Lewis MS, Ventura AB, Hughes CM, Coyne-Johnson L, Whizin N, Oswald K, Shoemaker R, Swanson T, et al. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature 2011; 473:523-7; PMID:21562493; http://dx.doi.org/10.1038/nature10003
- Schmitz JE, Kuroda MJ, Santra S, Sasseville VG, Simon MA, Lifton MA, Racz P, Tenner-Racz K, Dalesandro M, Scallon BJ, et al. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 1999; 283:857-60; PMID:9933172
- Hansen SG, Piatak M, Jr., Ventura AB, Hughes CM, Gilbride RM, Ford JC, Oswald K, Shoemaker R, Li Y, Lewis MS, et al. Immune clearance of highly pathogenic SIV infection. Nature 2013; 502:100-4; PMID:24025770; http://dx.doi.org/10.1038/nature12519
- Edwards BH, Bansal A, Sabbaj S, Bakari J, Mulligan MJ, Goepfert PA. Magnitude of functional CD8+ T-cell responses to the gag protein of human immunodeficiency virus type 1 correlates inversely with viral load in plasma. J Virol 2002; 76:2298-305; PMID:11836408
- Julg B, Williams KL, Reddy S, Bishop K, Qi Y, Carrington M, Goulder PJ, Ndung'u T, Walker BD. Enhanced anti-HIV functional activity associated with Gag-specific CD8 T-cell responses. J Virol 2010; 84:5540-9; PMID:20335261; http://dx.doi.org/10.1128/JVI.02031-09
- Kiepiela P, Ngumbela K, Thobakgale C, Ramduth D, Honeyborne I, Moodley E, Reddy S, de Pierres C, Mncube Z, Mkhwanazi N, et al. CD8+ T-cell responses to different HIV proteins have discordant associations with viral load. Nat Med 2007; 13:46-53; PMID:17173051
- Kawada M, Tsukamoto T, Yamamoto H, Iwamoto N, Kurihara K, Takeda A, Moriya C, Takeuchi H, Akari H, Matano T. Gag-specific cytotoxic T-lymphocyte-based control of primary simian immunodeficiency virus replication in a vaccine trial. J Virol 2008; 82:10199-206; PMID:18667518; http://dx.doi.org/10.1128/JVI.01103-08
- Liu J, O'Brien KL, Lynch DM, Simmons NL, La Porte A, Riggs AM, Abbink P, Coffey RT, Grandpre LE, Seaman MS, et al. Immune control of an SIV challenge by a T-cell-based vaccine in rhesus monkeys. Nature 2009; 457:87-91; PMID:18997770; http://dx.doi.org/10.1038/nature07469
- Bukreyev A, Huang Z, Yang L, Elankumaran S, St Claire M, Murphy BR, Samal SK, Collins PL. Recombinant newcastle disease virus expressing a foreign viral antigen is attenuated and highly immunogenic in primates. J Virol 2005; 79:13275-84; PMID:16227250
- Nakaya Y, Nakaya T, Park MS, Cros J, Imanishi J, Palese P, Garcia-Sastre A. Induction of cellular immune responses to simian immunodeficiency virus gag by two recombinant negative-strand RNA virus vectors. J Virol 2004; 78:9366-75; PMID:15308731
- Martinez-Sobrido L, Gitiban N, Fernandez-Sesma A, Cros J, Mertz SE, Jewell NA, Hammond S, Flano E, Durbin RK, Garcia-Sastre A, et al. Protection against respiratory syncytial virus by a recombinant Newcastle disease virus vector. J Virol 2006; 80:1130-9; PMID:16414990
- DiNapoli JM, Kotelkin A, Yang L, Elankumaran S, Murphy BR, Samal SK, Collins PL, Bukreyev A. Newcastle disease virus, a host range-restricted virus, as a vaccine vector for intranasal immunization against emerging pathogens. Proc Natl Acad Sci U S A 2007; 104:9788-93; PMID:17535926
- DiNapoli JM, Yang L, Samal SK, Murphy BR, Collins PL, Bukreyev A. Respiratory tract immunization of non-human primates with a Newcastle disease virus-vectored vaccine candidate against Ebola virus elicits a neutralizing antibody response. Vaccine 2010; 29:17-25; PMID:21034822
- DiNapoli JM, Nayak B, Yang L, Finneyfrock BW, Cook A, Andersen H, Torres-Velez F, Murphy BR, Samal SK, Collins PL, et al. Newcastle disease virus-vectored vaccines expressing the hemagglutinin or neuraminidase protein of H5N1 highly pathogenic avian influenza virus protect against virus challenge in monkeys. J Virol 2010; 84:1489-503; PMID:19923177; http://dx.doi.org/10.1128/JVI.01946-09
- Nakaya T, Cros J, Park MS, Nakaya Y, Zheng H, Sagrera A, Villar E, Garcia-Sastre A, Palese P. Recombinant Newcastle disease virus as a vaccine vector. J Virol 2001; 75:11868-73; PMID:11689668
- Khattar SK, Samal S, Devico AL, Collins PL, Samal SK. Newcastle disease virus expressing human immunodeficiency virus type 1 envelope glycoprotein induces strong mucosal and serum antibody responses in Guinea pigs. J Virol 2011; 85:10529-41; PMID:21849467
- Khattar SK, Samal S, LaBranche CC, Montefiori DC, Collins PL, Samal SK. Comparative immunogenicity of HIV-1 gp160, gp140 and gp120 expressed by live attenuated newcastle disease virus vector. PloS one 2013; 8:e78521; PMID:24098600
- Pulendran B, Ahmed R. Immunological mechanisms of vaccination. Nat Immunol 2011; 12:509-17; PMID:21739679
- Xu R, Nasar F, Megati S, Luckay A, Lee M, Udem SA, Eldridge JH, Egan MA, Emini E, Clarke DK. Prime-boost vaccination with recombinant mumps virus and recombinant vesicular stomatitis virus vectors elicits an enhanced human immunodeficiency virus type 1 Gag-specific cellular immune response in rhesus macaques. J Virol 2009; 83:9813-23; PMID:19625392
- Gottlinger HG, Sodroski JG, Haseltine WA. Role of capsid precursor processing and myristoylation in morphogenesis and infectivity of human immunodeficiency virus type 1. Proc Natl Acad Sci U S A 1989; 86:5781-5; PMID:2788277
- Toapanta FR, Craigo JK, Montelaro RC, Ross TM. Reduction of anti-HIV-1 Gag immune responses during co-immunization: immune interference by the HIV-1 envelope. Curr HIV Res 2007; 5:199-209; PMID:17346134
- Hovav AH, Santosuosso M, Bivas-Benita M, Plair A, Cheng A, Elnekave M, Righi E, Chen T, Kashiwagi S, Panas MW, et al. X4 human immunodeficiency virus type 1 gp120 down-modulates expression and immunogenicity of codelivered antigens. J Virol 2009; 83:10941-50; PMID:19692474
- Martinelli E, Cicala C, Van Ryk D, Goode DJ, Macleod K, Arthos J, Fauci AS. HIV-1 gp120 inhibits TLR9-mediated activation and IFN-{α} secretion in plasmacytoid dendritic cells. Proc Natl Acad Sci U S A 2007; 104:3396-401; PMID:17360657
- Lopez D, Samino Y, Koszinowski UH, Del Val M. HIV envelope protein inhibits MHC class I presentation of a cytomegalovirus protective epitope. J Immunol 2001; 167:4238-44
- Bockl K, Wild J, Bredl S, Kindsmuller K, Kostler J, Wagner R. Altering an artificial Gagpolnef polyprotein and mode of ENV co-administration affects the immunogenicity of a clade C HIV DNA vaccine. PloS one 2012; 7:e34723; PMID:22509350
- Calain P, Roux L. The rule of six, a basic feature for efficient replication of Sendai virus defective interfering RNA. J Virol 1993; 67:4822-30; PMID:8392616
- Huang Z, Krishnamurthy S, Panda A, Samal SK. High-level expression of a foreign gene from the most 3'-proximal locus of a recombinant Newcastle disease virus. J Gen Virol 2001; 82:1729-36; PMID:11413385
- Huang Z, Krishnamurthy S, Panda A, Samal SK. Newcastle disease virus V protein is associated with viral pathogenesis and functions as an α interferon antagonist. J Virol 2003; 77:8676-85; PMID:12885886
- Krishnamurthy S, Huang Z, Samal SK. Recovery of a virulent strain of newcastle disease virus from cloned cDNA: expression of a foreign gene results in growth retardation and attenuation. Virology 2000; 278:168-82; PMID:11112492
- Alexander DJ. Newcastle disease and other avian Paramyxoviridae infection Ames, IA: Iowa State University Press, 1997
- Li M, Gao F, Mascola JR, Stamatatos L, Polonis VR, Koutsoukos M, Voss G, Goepfert P, Gilbert P, Greene KM, et al. Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J Virol 2005; 79:10108-25; PMID:16051804
