Abstract
The aim of this study was to evaluate the one-month immune response to 2 different doses (10 and 20 μg) of recombinant hepatitis B vaccine in adults aged 20–46 y. Subjects who were negative for hepatitis B surface antigen (HBsAg), hepatitis B antibody (anti-HBs), and hepatitis B core antibody (anti-HBc) were recruited. The participants were divided into 2 groups: group I received 3 doses of 10 μg hepatitis B vaccine at 0, 1 and 3 months, and group II received 3 doses of 20 μg at the same time points. The anti-HBs levels were measured one month after the third vaccination. Among 739 subjects, 62 (9.70%) were positive for HBsAg, and 317 subjects were eligible. The anti-HBs seroprotection rates (anti-HBs ≥10 mIU/mL was considered to indicate seroprotection) after the third vaccination were 88.05% and 94.06% in group I and group II respectively, and the geometric mean titers were 91.69 and 290.23 mIU/mL respectively. The difference in the seroprotection rate was not significant (χ2 = 2.566, P > 0.05), but the GMT after the third dose was significantly lower for group I than for group II (F = 20.587, P < 0.05). Better responses were observed in young adults, especially in group I. In group I, the seroprotection rate and GMT were significantly higher in the 20–35 y group than in the 36–46 y group (P < 0.05); there was no significant difference compared to group II (P > 0.05). The hepatitis B vaccine has good immunological effect; the 20 μg dose can be used in adults aged 20–46 y and the 10 μg dose can be used in subjects aged 20–35 years, and it should be tested on a larger number of subjects before recommending it for adult routine vaccination.
Introduction
Hepatitis B virus (HBV) infection is an important public health problem all over the world.Citation1,2 It is estimated that more than 2 billion people worldwide are infected with HBV. Of these, approximately 360 million individuals are chronic carriers.Citation3,4 The number of HBV-related deaths is estimated to be 600000–1200000 each year worldwide.Citation5 HBV infection is especially serious in China, according to the National Seroepidemiological Survey conducted in 2006; the survey shows the hepatitis B surface antigen (HBsAg) carrier rates in the general population (1–59 years) to be 7.18%, and in the 15–59-year-old group to be 8.57%Citation6; that is, more than 93 million people have chronic infection.Citation7 As there is no satisfactory treatment for chronic hepatitis B infection and related diseases currently, hepatitis B vaccination is regarded as the most economical and effective method to prevent and control hepatitis B infection.Citation8,9
The hepatitis B vaccine has been available for 30 y and is recommended for use in all populations.Citation10 In China, the hepatitis B vaccine was incorporated into immunization planning management in 1992 and was incorporated into the national immunization plan in 2002. After more than 10 y of mass vaccination, the rate of HBV infection among Chinese children under the age of 15 y has declined to 2.08%, according to the 2006 survey.Citation11 With the improvement in hepatitis B prevention and control, more attention is currently being devoted to adult hepatitis B immunization. A US report in 2007 showed that the highest proportion of new hepatitis B infections occurs in the 25- to 44-year-old age group.Citation12 Vaccination for high-risk adults was started in the USA in 1998,Citation13 and adult hepatitis B vaccination was introduced to community primary health care and rehabilitation clinics in 2006.Citation14 The HBV infection rate is 8.57% for adults in China,Citation6 so adult hepatitis B vaccine vaccination is especially important in order to control the spread of hepatitis B in China.
In China, vaccination for adults has not been generally carried out, and there are no standard vaccination dosages for adults. The 20 μg hepatitis B vaccine has widely used all over the world while the recommended standard dose for adults is 3 times 20 μg.Citation15,16 However, the hepatitis B vaccine dose for adults is 10 μg in China. Therefore, it is necessary to find a suitable dosage for the Chinese population. The aim of this study was to evaluate the immunological effect of 2 different doses (10 and 20 μg) of domestic hepatitis B vaccine given at 0, 1 and 3 months in healthy adults aged 20–46 y
Results
Subject characteristics
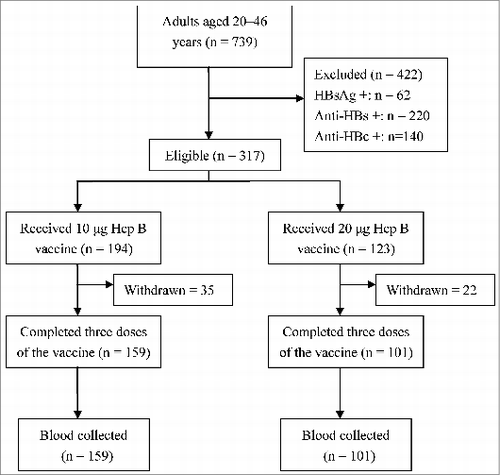
A total of 739 participates were screened. With regard to the baseline detection rates, 62 (9.70%), 220 (34.43%) and 140 (21.91%) of the subjects were positive for HBsAg, anti-HBs and anti-HBc. Therefore, these 422 subjects were excluded from the vaccination program. shows the selection flow chart. There were a total of 317 eligible subjects, 194 in group I (10 μg) and 123 in group II (20 μg). One month after the third dose of vaccination, 260 blood samples were collected; 44 individuals did not complete the immunization schedule and 13 were lost to follow-up. Finally, 159 and 101 individuals from group I and group II, respectively, were considered for evaluation in the study. The average age was 33.81 y (S.D = 6.80 years) and 34.47 y (S.D = 7.88 years) for group I and group II respectively. There were no statistically significant differences in age (t = −0.695, P > 0.05) or gender (χ2 = 3.679, P > 0.05). Age and sex distribution of the study participants is shown in .
Table 1. Age and sex distribution of the study subjects
Antibody response
A total of 235 adults had protective antibodies after the third dose of HepB vaccine, with a total seroprotection rate of 90.38%; the GMT value of the anti-HBs titers was 143.45 mIU/mL. The seroprotection rate and GMT in group II were higher than those in group II. The difference in seroprotection rate between the 2 groups was not significant (χ2 = 2.566, P > 005), but the difference in GMT was significant (F = 20.587, P < 0.05). The antibody responses one month after the third vaccination are shown in .
Table 2. Antibody response one month after the third vaccination
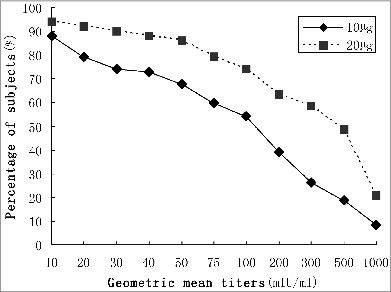
and show the distribution of anti-HBs titers after the third dose of hepatitis B vaccine: 61.63% of the subjects were good responders (anti-HBs titer > 100 mIU/mL), 54.09% and 74.26% for group I and group II respectively. The difference in the distribution of the anti-HBs titer was significant (χ2 = 14.910, P < 0.05).
Table 3. Distribution of the anti-HBs titer after the third dose of hepatitis B vaccine
The antibody response was always higher in the younger age group (20–35 years), especially in group I. The seroprotection rate and GMT value of anti-HBs in group I was significantly higher in the younger age group. The GMT values of anti-HBs titers for the younger age group and older age group (36–46 years) in group II are 351.02 mIU/mL and 237.19 mIU/mL respectively (P < 0.05). The anti-HBs seroprotection rate and GMT in different age groups are shown in .
Table 4. Age-wise distribution of the anti-HBs seroprotection rate and GMT
Discussion
In 1993, WHO had recommended that the hepatitis B vaccine be introduced to the expanded program of immunization (EPI),Citation17,18 and it resulted in a significant reduction in the HBsAg carrier rate from 10% in 1992 to 2.08% in 2006 among children under 15 years.Citation6 In the present study, the prevalence of HBsAg among subjects aged 20–46 y was 9.70%, which confirms that HBV infection is endemic in the Chinese population. Therefore, hepatitis B vaccination in adults to prevent HBV infection is especially important.
The standard schedule for hepatitis B vaccine was 0, 1 and 6 months, and it was widely used all over the world. Due to the large time span of immunization, some people can not complete the full vaccinations.Citation19 In a study by Kollar et al.Citation20 it reported that only about 50% of the people can complete the 3-dose vaccination plan. The 0–1–6 months schedule is easily to carry out in children. And the mobility of adults is bigger than children, so to shorten the time interval between vaccinations may improve the compliance of adults.Citation21 There were a lot of flexibility immunization schedules in other countries, such as accelerated series 0/1/2-month and 0/7/21-day et al. In our study, the hepatitis B vaccine was administered at 0, 1 and 3 months, and 0–1–3 months schedule was widely used in other scholars’ studies. Chen SY et al.Citation22 study compared the 2 different schedules 0–1–3 months and 0–1–6 months, the result showed that the anti-HBs seroprotection rates were both high in the 2 schedules. Xu F et al.Citation23 study showed the same result. Belloni et al.Citation24 study compared the 2 schedules and found that the antibody levels showed no significant difference one year after the full vaccinations. Therefore, we thought that 0–1–3 months schedule was a simple and effective immune schedule.
Several studies have used the 2 doses, that is, 10 μg and 20 μg, of hepatitis B vaccine. In a study by Kulkarni et al.,Citation25 a group of adults aged 19–57 y received 3 20-μg doses of recombined hepatitis B vaccine at 0, 1 and 6 months; one month after the third vaccination, the seroprotection rate was 96% and the GMT was 443 mIU/ml. In another study by Joshi et al.,Citation26 120 healthy adults received 20 μg Engerix-B and Shanvac-B at 0, 1 and 2 months, which provided seroprotection rates of 91.4% and 96.4% respectively and geometric mean titers of 140 and 419 mIU/ml respectively after the third dose. Another study by Xu F et al. Citation23 used 2 different dosages 10 μg and 20 μg in a 0–1–3 months schedule, which obtained seroprotection rate of 98.36% and 97.86% respectively and had no significant difference. In the present study, 10 μg and 20 μg of the hepatitis B vaccine were administered at 0, 1 and 3 months; the seroprotection rates one month after the third dose were 88.05% and 94.06% respectively, and the geometric mean titers were 91.69 and 290.23 mIU/ml respectively. The difference in seroprotection rates between the 2 groups was not significant; thus, these 2 doses, 10 μg and 20 μg, are effective in healthy adults. In addition, the GMTs reported in our study are lower compared to other studies that used the 0/1/6-month schedule; this is probably because the interval between the last 2 doses of the primary series is different between the studies.Citation27,28 When the vaccine was administered as an accelerated series at 0, 1 and 2 months, the seroprotection rate at the third month was 96.4% and the GMT was 60.2 mIU/mL, as shown in an earlier study that used 20 μg Engerix B vaccine.Citation29
An anti-HBs level greater than 10 mIU/ml is considered to provide protection against hepatitis B infection, and an anti-HBs level exceeding 100 mIU/ml is considered to indicate long-term immunity.Citation30 Qian W et al.Citation31 reported seroprotection provided by 10 μg or 20 μg doses of hepatitis B vaccine persists for 23 y in more than half of vaccinated individuals. The duration of immunity after hepatitis B vaccine vaccination is unknown, but it is generally considered that the higher the antibody titers after vaccination, the longer the protection will last.Citation32,33 In the present study, the GMT values after administration of the 20 μg dose was higher than those after the 10 μg dose. This result is similar to those of previous studies.Citation34,35 Thus, based on these findings, 20 μg may be the appropriate dose that provides longer protection.
In this study, young adults showed a better response. In agreement with this finding, increasing age was shown to be an important factor that affects immunogenicity, as evidenced by the decline in the seroprotection rate and GMT in the higher age groups.Citation35 This age-based difference was more obvious in the 10 μg group: the seroprotection rates are 93.55% and 88.3% in subjects aged 20–35 y and 36–46 y respectively; the differences were significant. It is similar to that reported in earlier studies: Ren JJ et al.Citation36 reported seroprotection rates of 92% and 80% in the 16–34 y and 35–49 y groups respectively. Another studyCitation37 found seroprotection rates of only 60% at the age of 40 y and more. These findings indicate that good immunity in people who are vaccinated at a young age. In the 20 μg group, the seroprotection rate and GMTs also declined with increase in age, but the difference in different age groups was not significant. Adults aged 36–46 y who received 20 μg HepB had a significantly higher seroprotection rate compared to those of the same age group given 10 μg HepB. This is consistent with the report that a higher dose should render a higher response.Citation35
In our study, the younger group (20–35 years) had a similar seroprotection rate after the 10 μg and 20 μg HepB vaccination: the seroprotection rate was 93.55% for the 10 μg dose and 94.23% for the 20 μg dose, and the GMT was 123.76 and 351.02 mIU/mL respectively. This observation is similar to the study of Schiff GM et al.,Citation38 where Engerix B doses of 10 μg and 20 μg were used in adolescents; they reported seroprotection rates of 97% and 99% for the 10 μg dose and 20 μg dose respectively. Similar rates were also reported by Ran SS et al.Citation39 and Velu V et al.Citation40 It was recommended that low-dose hepatitis B vaccine studies be conducted to reduce the cost of vaccination in endemic areas of low-income countries.Citation41 A lower dose would significantly reduce the cost of the vaccination. Our study shows that administering a 10 μg dose of hepatitis B vaccine in young adults will be clinically effective and an economic alternative to the 20 μg dose.
There are several limitations in this study need to be acknowledged. Due to the short observation time; we can't evaluate the long-time effects of the vaccination. Additionally, we failed to acquire the data of body mass index (BMI) which may affect the immunity effect. Besides, the generalizability of these results is limited as a result of possible volunteer bias in this study. This study has a limited number of subjects, it is too low to draw conclusions on the recommended dosage and it should be tested on a larger number of subjects before recommending it for adult routine vaccination.
In conclusion, hepatitis B is endemic to China and therefore hepatitis B vaccination is necessary for adults. As our results show, the 10 μg dose of the hepatitis B vaccine is economic in subjects aged 20–35 years; moreover, the 20 μg dose is more appropriate in adults aged 20–46 y
Materials and Methods
Subjects
This study was performed in Deqing County in Hangzhou City and Xianju County in Taizhou City, Zhejiang Province, China; 3 communities were selected in each county as research sites. Subjects aged 20–46 y were selected as sample subjects and underwent serological screening. This study was approved by the Institutional Review Board of the Zhejiang Center for Disease Control and Prevention (Hangzhou, China), and written informed consent was obtained from every participant before they were included in the study. The specific inclusion and exclusion criteria are as follows:
Inclusion criteria
(1) 20–46 y of age; (2) willingness to participate in the follow-up study and to undergo blood sampling after vaccination; (3) absence of diseases; (4) absence of HBsAg, anti-HBs and anti-HBc IgM.
Exclusion criteria
(1) presence of HBsAg, anti-HBs or anti-HBc; (2) history of allergies or severe reaction to vaccination; (3) presence of immune dysfunction disease; (4) known or anticipated immune dysfunction; (5) previous immune suppressive therapy; (6) high risk of becoming immunologically compromised; (7) any kind of vaccination within the previous 4 weeks; (8) treatment with observational or experimental drugs during the past 4 weeks; (9) acute illness within the past 7 days; (10) infection that required treatment with antibacterial or antiviral therapy within the past 7 days; (11) fever within the past 3 d (armpit temperature, ≥38°C).
Vaccines
The subjects were divided into 2 groups, group I and group II. The following vaccines were administered: (1) group I: hepatitis B vaccine (dosage 10 μg; Shenzhen Kangtai Biological Products Co. Ltd., China); (2) group 2: hepatitis B vaccine (dosage 20 μg; Shenzhen Kangtai Biological Products Co. Ltd., China). These vaccines were stored at 4°C. Use and storage of the vaccine were carried out under the supervision of responsible medical and research staff who participated in the study. The vaccine was administered to the deltoid muscle, and after administration of each dose of vaccine, the volunteers were followed up for adverse events for 30 min.
Methods
All the subjects who fulfilled the inclusion criteria underwent serological screening for HBsAg, anti-HBs and anti-HBc. The study participants were administered 1 mL of the vaccine at 0, 1 and 3 months. Subjects were asked to fill in a pre-designed questionnaire, “Research Questionnaire on Adult Immunization Strategy of Hepatitis B,” which included personal information about the participants (age as on the date the first dose was administered using the subjects’ dates of birth on the ID, which was determined to be accurate). We collected 3 ml of blood samples from each participant who received 3 doses of the vaccine and preserved them for anti-HBs quantification.
Laboratory testing
The frozen separated serum samples were sent to ADICON Clinical Laboratories Inc.in Hangzhou for HBsAg, anti-HBs and anti-HBc quantification by chemiluminescence immunoassay (CLIA). Samples with anti-HBs levels ≥1000 mIU/ml were diluted for further testing, while samples with antibody titer levels >15000 mIU/ml were excluded from further analysis. An Architect-i2000 (Abbott, US) analyzer was used to perform the CLIA. The reagent lot number for the HBsAg tests was 26057LF00; HBsAg S/N ≥0 .05 mIU/mL was considered to indicate the presence of HBsAg. The reagent lot number for the anti-HBc test was 25548lI00, and an anti-HBc antibody level ≥1 mIU/ml was considered as a positive result. The reagent lot number was 27235LF00 for the anti-HBs screening test and 32040LF00 for the anti-HBs test 1 month after the third vaccination. An antibody titer of at least 1 mIU/mL or more was interpreted as seroconversion; a titer above 10 mIU/mL was considered to indicate seroprotection; and a titer above 100 mIU/mL was considered to indicate a good response.
Data analysis
A database using EpiData3.2 (EpiData; Norway and Denmark) was established, and double data entry was performed. Statistical analysis was performed using SPSS 13.0 and Excel 2003. The arithmetic values of anti-HBs were log transformed, and the antilog of the mean log values was calculated for the geometric mean titer (GMT). Because we were unable to detect anti-HBs antibody titer levels <0.01 mIU/ml and >15000 mIU/ml, we assigned a value of 0.001 mIU/ml and 15000 mIU/ml respectively to these subjects when calculating the GMT of anti-HBs. We used a 2-tailed test to analyze the data, with an α value of 0.05 considered to be significant. A chi-square test or Fisher chi-square test was used to analyze quantitative data: a U test was used for data that followed a normal distribution, and a rank-sum test was used for data that showed a skewed distribution.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank the Xian Ju and Pu Tuo Center for Disease Control and Prevention (CDC) and their relevant personnel for their contribution to this study.
Funding
Project supported by the National Scientific and Technological Major Project of China (No. 2013ZX10004–904) and Scientific Research Fund of Medical Technology and Education in Zhejiang Province (No. 2013C25114).
References
- Ganem D, Prince AM. Hepatitis B virus infection-natural history and clinical consequences. N Engl J Med 2004; 350(11):1118-29; PMID:15014185; http://dx.doi.org/10.1056/NEJMra031087
- Lee WM. Hepatitis B virus infection. N Engl J Med 1997; 337:1733-45; PMID:9392700; http://dx.doi.org/10.1056/NEJM199712113372406
- Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat 2004; 11(2):97-107; PMID:14996343; http://dx.doi.org/10.1046/j.1365-2893.2003.00487.x
- Ganczak M, Szych Z, Korzen M. Preoperative vaccination for HBV at Polish hospitals as a possible public health tool to limit the spread of the epidemic: a cross-sectional study. Vaccine 2009; 27(30):3969-74; http://dx.doi.org/10.1016/j.vaccine.2009.04.042
- Goldstein ST, Zhou F, Hadler SC, Bell BP, Mast EE, Margolis HS. A mathematical model to estimate global hepatitis B disease burden and vaccination impact. Int J Epidemiol 2005; 34(6):1329-39; http://dx.doi.org/10.1093/ije/dyi206
- Liang X, Bi S, Yang W, Wang L, Cui G, Cui F, Zhang Y, Liu J, Gong X, Chen Y, et al. Epidemiological serosurvey of hepatitis B in China–declining HBV prevalence due to hepatitis B vaccination. Vaccine 2009; 27(47):6550-7; PMID:19729084; http://dx.doi.org/10.1016/j.vaccine.2009.08.048
- Lu FM, Zhuang H. Management of hepatitis B in China. Chin Med 2009; 122:3-4.
- Chang MH, Hadzic D, Rouassant SH, Jonas M, Kohn IJ, Negro F, Roberts E, Sibal A; Asian Pan-Pacific Society for Pediatric Gastroenterology, Hepatology and Nutrition. Acute and chronic hepatitis: working group report of the second world congress of pediatric gastroenterology, hepatology and nutrition. J Pediatr Gastroenterol Nutr 2004; 39:584-8; PMID:15184756; http://dx.doi.org/10.1097/00005176-200406002-00002
- Weinbaum CM, Mast EE, Ward JW. Recommendations for identification and public health management of persons with chronic hepatitis B virus infection. Hepatology 2009; 49(5 Suppl):S35-44.
- World Health Organization. Hepatitis B vaccines. Wkly Epidemiol Rec 2009; 84:405-20; PMID:19817017
- Liang X, Bi S, Yang W, Wang L, Cui G, Cui F, Zhang Y, Liu J, Gong X, Chen Y, Liu J, Gong X, Chen Y, Wang F, et al. Evaluation of the impact of hepatitis B vaccination among children born during 1992–2005 in China. J Infect Dis 2009; 200:39-47; PMID:19469708; http://dx.doi.org/10.1086/599332
- Mast EE, Weinbaum CM, Fiore AE, Alter MJ, Bell BP, Finelli L, Rodewald LE, Douglas JM Jr, Janssen RS, Ward JW, et al. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) Part II: immunization of adults. MMWR Morb Mortal Wkly Rep 2007; 56(42):1114; PMID:17159833
- Keating GM, Noble S. Recombinant hepatitis B vaccine (Engerix-B): a review of its immunogenicity and protective efficacy against hepatitis B. Drugs 2003; 63(10):1021-51; PMID:12699402; http://dx.doi.org/10.2165/00003495-200363100-00006
- Idilman R, De MN, Colantoni A, Nadir A, Van Thiel DH. The effect of high dose and short interval HBV vaccination in individuals with chronic hepatitis C. Am J Gastroenterol 2002; 97(2):435-9; http://dx.doi.org/10.1111/j.1572-0241.2002.05482.x
- Immunization Practices Advisory Committee. Hepatitis B virus: a comprehensive strategy for eliminating transmission in the United States through universal childhood vaccination. Recommendations of the Immunization Practices Advisory Committee (ACIP). MMWR Recomm Rep 1991; 40(RR–13):1–25; PMID: 1835756
- American Academy of Pediatrics Committee on Infectious Diseases. Universal hepatitis B immunization. Pediatrics 1992; 89(4 Pt 2):795-800; PMID: 1557285
- Daniels D, Grytdal S, Wasley A, Centers for Disease C, Prevention. Surveillance for acute viral hepatitis—United States, 2007. MMWR Surveill Summ 2009; 58(3):1-27; PMID:19478727
- Mast EE, Mahoney FJ, Alter MJ, Margolis HS. Progress toward elimination of hepatitis B virus transmission in the United States. Vaccine 1998; 16 Suppl:S48-51.
- Levie K, Beran J, Collard F, Nguyen C. Long term (24 months) follow-up of a hepatitis A and B vaccine, comparing a two and three dose schedule in adolescents aged 12-15 years. Vaccine 2002; 20(19-20):2579-84; http://dx.doi.org/10.1016/S0264-410X(02)00153-6
- Kollar LM, Rosenthal SL, Biro FM. Hepatitis B vaccine series compliance in adolescents. Pediatr Infect Dis J 1994; 13(11):1006-8.
- Chen S. Hepatitis B vaccine immunization status and problems. World Chin J Digestol 2006; 14(27):2661-7.
- Chen S, Wang X, Dong X, Xu H, Wang F, Tang Z, Wang X, Fan J. Effects of two immunization schedules of Recombinant yeast-derived hepatitis vaccine for adults. Chin Prev Med 2013; 2(14):96-8.
- Xu F, He F, Zhou B, Zhao Q, Yan R. Observation on immunization effect of hepatitis B vaccine in adults by different dosages and schedules. Dis Surveillance 2013; 1(28):38-41.
- Belloni C, Pistorio A, Tinelli C, Komakec J, Chirico G, Rovelli D, Gulminetti R, Comolli G, Orsolini P, Rondini G. Early immunisation with hepatitis B vaccine: a five-year study. Vaccine 2000, 18(14): 1307-11.
- Kulkarni PS, Raut SK, Patki PS, Phadke MA, Jadhav SS, Kapre SV, Dhorje SP, Godse SR. Immunogenicity of a new, low-cost recombinant hepatitis B vaccine derived from Hansenula polymorpha in adults. Vaccine 2006; 24(17):3457-60; PMID:16530299; http://dx.doi.org/10.1016/j.vaccine.2006.02.008
- Joshi N, Kumar A, Sreenivas DV, Palan S, Nagarjuna Kumar YR. Safety and immunogenicity of indigenous recombinant hepatitis B vaccine (Shanvac-B) in comparison with commercially available vaccine. Indian J Gastroenterol 2000; 19(2):71-3; PMID:10812819
- Gilca V, De Serres G, Boulianne N, Murphy D, Ouakki M, De Wals P, Trudeau G, Massé R, Dionne M. Long-term persistence of immunity after vaccination of pre-adolescents with low doses of a recombinant hepatitis B vaccine. Hum Vaccin Immunother 2013; 9(8):1685-90; PMID:23744506; http://dx.doi.org/10.4161/hv.25015
- Girisha KM, Kamat JR, Nataraj G. Immunological response to two hepatitis B vaccines administered in two different schedules. Indian J Pediatr 2006; 73(6):489-91; http://dx.doi.org/10.1007/BF02759892
- Leroux-Roels G, Haelterman E, Maes C, Levy J, De Boever F, Licini L, David MP, Dobbelaere K, Descamps D. Randomized trial of the immunogenicity and safety of the Hepatitis B vaccine given in an accelerated schedule coadministered with the human papillomavirus type 16/18 AS04-adjuvanted cervical cancer vaccine. Clin Vaccine Immunol 2011; 18(9):1510-8; PMID:21734063; http://dx.doi.org/10.1128/CVI.00539-10
- Hadler SC, de Monzon MA, Lugo DR, Perez M. Effect of timing of hepatitis B vaccine doses on response to vaccine in Yucpa Indians. Vaccine 1989; 7(2):106-10; http://dx.doi.org/10.1016/0264-410X(89)90046-7
- Wu Q, Zhuang GH, Wang XL, Hou TJ, Shah DP, Wei XL, Wang LR, Zhang M. Comparison of long-term immunogenicity (23 years) of 10 mug and 20 mug doses of hepatitis B vaccine in healthy children. Hum Vaccin Immunother 2012; 8(8):1071-6; http://dx.doi.org/10.4161/hv.20656
- McMahon BJ, Bruden DL, Petersen KM, Bulkow LR,Parkinson AJ, Nainan O, Khristova M, Zanis C, Peters H, Margolis HS. Antibody levels and protection after hepatitis B vaccination: results of a 15-year follow-up. Ann Intern Med 2005; 142:333-41; PMID:15738452; http://dx.doi.org/10.7326/0003-4819-142-5-200503010-00008
- McMahon BJ, Dentinger CM, Bruden D, Zanis C, Peters H, Hurlburt D, Hurlburt D, Bulkow L, Fiore AE, Bell BP, et al. Antibody levels and protection after hepatitis B vaccine: results of a 22-year follow-up study and response to a booster dose. J Infect Dis 2009; 200:1390-6; PMID:19785526; http://dx.doi.org/10.1086/606119
- Poovorawan Y, Pongpunlert W, Theamboonlers A, Vimolgej L, Chumdermpadetsuk S, Vandepapeliere P, Safary A. Randomized, single-blind comparison of the immunogenicity and reactogenicity of 20 micrograms and 10 micrograms doses of hepatitis B vaccine in adolescents. Southeast Asian J Trop Med Public Health 1993; 24(2):255-9; PMID:8266226
- ul-Haq N, Hasnain SS, Umar M, Abbas Z, Valenzuela-Silva C, Lopez-Saura P. Immunogenicity of 10 and 20 microgram hepatitis B vaccine in a two-dose schedule. Vaccine 2003; 21(23):3179-85; http://dx.doi.org/10.1016/S0264-410X(03)00232-9
- Ren JJ, Dai XW, Jiang ZG, Shen LZ, Chen YD, Li Q, Ren W, Liu Y, Yao J, Li LJ. Immunological effects of a 10-mug dose of domestic hepatitis B vaccine in adults. J Zhejiang Univ Sci B 2012; 13(11):948-54; PMID:23125088; http://dx.doi.org/10.1631/jzus.B1200179
- Bennett RG, Powers DC, Remsburg RE, Scheve A, Clements ML. Hepatitis B virus vaccination for older adults. J Am Geriatr Soc 1996; 44(6):699-703; PMID:8642163
- Schiff GM, Sherwood JR, Zeldis JB, Krause DS. Comparative study of the immunogenicity and safety of two doses of recombinant hepatitis B vaccine in healthy adolescents. J Adolesc Health 1995; 16(1):12-7; http://dx.doi.org/10.1016/1054-139X(94)00105-N
- Rana SS, Singhal R, Gupta RK, Sharma D, Kar P. Immunogenicity of low-dose and conventional-dose recombinant hepatitis B vaccines in healthy adolescents in India. Singapore Med J 2004; 45(9):427-9.
- Velu V, Nandakumar S, Shanmugam S, Thyagarajan SP. Persistence of anti-HBs titers after two different doses of Genevac B, a recombinant hepatitis B vaccine, in healthy adolescents. Indian J Gastroenterol 2007; 26(1):48.
- Kim SY, Salomon JA, Goldie SJ. Economic evaluation of hepatitis B vaccination in low-income countries: using cost-effectiveness affordability curves. Bull World Health Organ 2007; 85(11):833-42.