Abstract
RNA interference (RNAi) in insects is a gene regulatory process that also plays a vital role in the maintenance and in the regulation of host defenses against invading viruses. Small RNAs determine the specificity of the RNAi through precise recognition of their targets. These small RNAs in insects comprise small interfering RNAs (siRNAs), micro RNAs (miRNAs) and Piwi interacting RNAs (piRNAs) of various lengths. In this review, we have explored different forms of the RNAi inducers that are presently in use, and their applications for an effective and efficient fundamental and practical RNAi research with insects. Further, we reviewed trends in next generation sequencing (NGS) technologies and their importance for insect RNAi, including the identification of novel insect targets as well as insect viruses. Here we also describe a rapidly emerging trend of using plant viruses to deliver the RNAi inducer molecules into insects for an efficient RNAi response.
Introduction
RNA interference (RNAi) is a central regulatory process for gene expression and anti-viral defense in eukaryotes.Citation1-3 Small RNAs determine the specificity of the RNAi activities through precise base-pairing recognition of their complementary target RNAs.Citation1,4 These small RNAs comprise 3 distinct classes, short/small interfering RNAs (siRNAs), microRNAs (miRNAs) and Piwi-interacting RNAs (piRNAs), and are generated by independent biogenesis pathways. The sizes of siRNAs and miRNAs vary between 19-24 nucleotides in length and are generated from longer, more complex double-stranded RNAs (dsRNAs) by the action of ribonuclease III type Dicer enzymes. The third class of small RNAs, piRNAs are 24-30 nucleotides in length and are generated in a Dicer-independent manner. However, the 3 classes of RNAs associate with and load into Argonaute proteins (AGO) to mediate RNAi activities including target RNA cleavage, translational repression, RNA destabilization or epigenetic modifications.Citation5 RNAi is only relatively recently discovered but now is one of the most intensely studied areas in all of biology. Fundamental studies have provided new insight into complex, intracellular RNAi-mediated events, but the potential for practical application of RNAi approaches has generated great activity in fields ranging from agriculture to medicine. Here we give some background on RNAi studies in insects, but we also discuss ongoing and future efforts for practical application of RNAi for insect pest control.
Biogenesis of Small RNAs
MicroRNAs
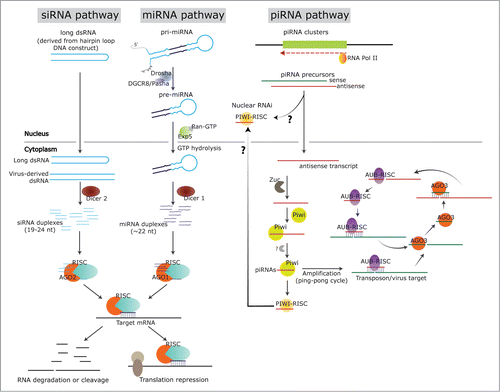
Mature miRNAs are single-stranded RNAs (ssRNAs) of ca. 22 nucleotides in length and are generated from endogenous primary transcripts by the action of 2 RNase III type nucleases, Drosha and Dicer ().Citation6 The mechanisms of miRNA biogenesis differ slightly in insects vs. plants, here we discuss insects. The primary transcripts (pri-miRNAs) are generated by RNA polymerase II (Pol-II) and contain a characteristic stem-loop structure. In one of the maturation steps of miRNA processing, the stem-loop of the pri-miRNA gets excised by RNase III type activity of Drosha while still in the nucleus. The cleavage by Drosha along with a co-factor Pasha (DGCR8) results in the generation of a precursor microRNA (pre-miRNA).Citation7,8 The pre-miRNAs are exported into the cytoplasm by the nuclear transport receptor family of proteins, exportin 5 by cooperative binding and association with GTP- bound form of the cofactor Ran.Citation9,10 The pre-miRNAs that are released by GTP hydrolysis in the cytoplasm, are further processed by Dicer enzymes into ca. 22 nucleotide microRNA duplexes.Citation11 Dicer (Dcr 1) cleavage is followed by the loading of miRNA duplexes into Argonaute proteins (). The 5′ terminal nucleotide was shown to influence the association of miRNAs or siRNAs (see below) with specific Argonaute proteins and thus the loading into RNA-induced silencing complex (RISC).Citation12 This difference in the 5′ terminal nucleotide leads to loading of miRNAs into different Argonaute proteins; a 5′ uridine containing miRNA loads into AGO1.
Figure 1. RNAi pathways in insects. The figure shows the biogenesis pathways for siRNAs, miRNAs and piRNAs in insects. In the siRNA pathway, the RNA transcript from a DNA construct encoding hairpin loop sequences, or from a virus-derived dsRNA will trigger siRNA generation in the cytoplasm, resulting from the recognition and dicing by Dicer 2. The siRNA duplexes will then load into the AGO 2 - RISC complex. The guide strand of siRNA is maintained in the RISC and will lead it to the target mRNA for degradation/cleavage or translation repression. For endogenous miRNAs, pri-miRNA is transcribed from genomic DNA, and processed by Drosha and cofactor Pasha (DGCR8) into the pre-miRNA. Next, the pre-miRNA will be transported into cytoplasm by exportin 5 and Ran-GTP, and cleaved by Dicer 1 into miRNA duplexes. Similar to siRNAs, the miRNA duplexes will incorporate into the AGO 1 - RISC complex, followed by target mRNA cleavage/degradation or translation repression. In the piRNA pathway, piRNA precursors are transcribed from piRNA clusters in the genomic DNA sequences. The antisense transcript could transport into cytoplasm, processed by series of proteins of the Piwi-group, in Zuc dependent or independent manner and incorporated into the PIWI-RISC complex, or into the AUB-RISC complex in the ping-pong cycle. The sense strand, which is targeted by the antisense strand in AUB-RISC, will then be cleaved and incorporated into AGO 3 to target the antisense strand; hence the ping-pong cycle.
Small interfering RNAs
Small interfering RNAs (siRNAs), like miRNAs, are dependent on Dicer processing and are ca. 19-24 nucleotides in length. The primary origins of siRNAs are from dsRNAs including virus-derived dsRNAs in the cytoplasm ().Citation13 These RNAs are derived from endogenous transcripts, sense-antisense transcript pairs, long stem loop structures, pseudogenes, inverted repeat structures and through bidirectional and antisense transcription.Citation6 The siRNAs are specifically associated with the Argonaute 2 (AGO2) protein.Citation14 In Drosophila, Heat-shock protein 90 (HSP90) is required for AGO 2 to receive the siRNA duplex from the RISC loading complex in RNAi suggesting an interaction between the 2 protein components.Citation15 The endo-siRNA pathways in plants and some other eukaryotes also have signal amplification polymerases, RNA dependent RNA polymerase (RdRP), that are absent in insects and most metazoans.Citation16 Small interfering endo-siRNAs are the result of processing by Dicer nuclease enzyme II (Dicer 2) compared to Dicer 1 for miRNAs.Citation17 Interestingly, the insect antiviral RNAi defense pathway also uses the same inherent siRNA pathway to detect viruses and to induce an antiviral response. RNAi pathway mutants for Dicer 2 and, AGO 2 in Drosophila were shown to be highly sensitive to RNA virus infection, which correlated with an increase in virus RNA accumulation.Citation18,19 Dicer 2 was shown to process viral dsRNAs in the cytoplasm into ca. 21 nucleotide virus-derived small RNAs (vsRNAs).Citation20 This highly efficient Dicer-2 protein of insects can act on a variety of substrates such as virus replication intermediates of ssRNA genomes or viral transcripts and dsRNAs resulting from overlapping transcripts of DNA viruses.Citation21 Several studies have recently confirmed the presence of vsRNAs in virus-infected insects and cell lines, thus confirming the role of Dicer protein in processing the virus dsRNAs.Citation22-24 The resulting siRNAs with 5’ adenosine load into AGO2 and those with a 5’ cytidine load into AGO5.Citation12 Similar to miRNAs, the duplex siRNAs are loaded into the RISC, followed by the retaining of the guide strand. RISC is then directed by the guide strand which determines the mRNA target.
Piwi-interacting RNAs
Piwi-interacting RNAs (piRNAs) are generally ca. 24 to 30 nucleotide long, endogenous, germ-cell-specific small RNAs that are distinct from miRNAs and siRNAs based on their size and means of biogenesis.Citation4 The primary function of piRNAs is to silence transposable elements (TEs) and their role is highly conserved among many eukaryotes.Citation4 They are derived from distinct transposable elements that are referred to as piRNA clusters, in a Dicer-independent manner.Citation25 These piRNAs from each locus are characterized by a complex mixture of sequences spanning large portions of the transposons. They were previously referred to as repeat- associated siRNAs (rasiRNAs) due to their association with the repeat elements.Citation6,26 The Piwi group of proteins mainly consists of 3 subclasses; PIWI, Aubergine (AUB) and AGO 3, and the small RNAs that associate with Piwi proteins are thus named piRNAs. These piRNAs from piRNA clusters are transcribed in the sense or antisense direction and the long single-stranded RNA serves as the basis for piRNA production.
There are divergent biogenesis pathways that are important for piRNA production. In one of the pathways, the long transposon transcript is sliced by zucchini (Zuc) nuclease that probably results in the generation of the 5′ ends of primary piRNAs (). In a ping-pong cycle, mature sense primary piRNAs guide Piwi clade proteins to complementary sequences on antisense transcripts from the same piRNA cluster. Piwi proteins, due to their slicer activity, cleave the target antisense transcript to generate a new 5′ end. These newly generated 5′ ends are bound by another Piwi protein. Subsequently, the 3′ ends are trimmed to the length of the mature piRNAs. In Drosophila, 2 Piwi proteins AUB and AGO 3 cooperate in the secondary piRNA production to generate sense and antisense piRNAs ().
AUB- and PIWI-associated piRNAs are mainly derived from antisense transcripts of retrotransposons, whereas AGO3- associated piRNAs are derived from sense transcripts. AUB- and PIWI-associated piRNAs show strong preferences for uracil at their 5′ terminal ends, while AGO3-associated piRNAs mostly have adenine at nucleotide 10, with no bias at their 5′ termini. In another divergent pathway, primary piRNAs that get loaded specifically on to PIWI, also generate secondary piRNAs that were found in nucleus and induce nuclear RNAi of targets (Fig.1). Both piRNAs and siRNAs are 2′-O-methylated by small RNA 2′-O-methyl transferase across eukaryotes. This methylation protects them from 3′ uridylation and degradation.Citation27
RNAi Activity as a Gene Regulation and Defense Strategy in Insects
RNAi activity in insects is achieved by all 3 classes of small RNAs, although they each show different specific activities. Some RNAi activities are important for natural gene regulation events and play important roles in insect development. For example, RISC, directed by the miRNA guide strand, locates target mRNAs containing specific nucleotide sequences complementary to the guide, binds to these sequences and blocks translation of the mRNA target. Recent functional studies have shown the regulation of ecdysone receptor (EcR) in an isoform specific manner by miRNAs in the Bombyx mori,Citation28 and studies in the hemipteran insect, Nilaparvata lugens, have shown the critical role of the conserved miRNAs miR-8-5p and miR-2a-3p, for ecdysone-induced chitin biosynthesis.Citation29
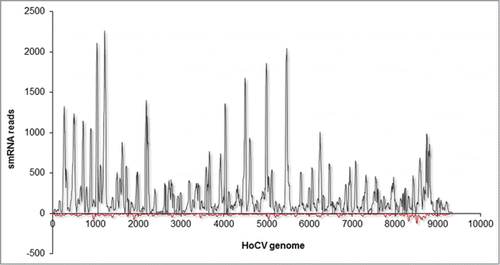
But RNAi activity in insects also includes several triggers and substrates that function to silence the expression of certain host endogenous genes along with parasite and pathogen invaders such as viruses and mobile genetic elements including transposons.Citation6,30 Insects respond to a variety of external stimuli, both biological and non-biological stresses, much like plants and other animals, through the generation of siRNAs and miRNAs. The generation of vsRNAs results because insects use RNAi activity as an anti-viral defense against both RNA and DNA viruses.Citation31-34 Abundant vsRNAs result during virus infections, and recent studies using next generation sequencing (NGS) approaches have demonstrated that it is possible to identify sufficient vsRNAs to assemble complete virus genomes.Citation23,35 However, it is important to note that the resulting populations of vsRNAs is not uniform across the infecting virus genomes, suggesting that certain regions of the infecting virus RNAs (either genomic or mRNAs) are targeted more than others. In a recent report, vsRNAs mapped to Homalodisca coagulata virus-1, (HoCV-1) and Homalodisca vitripennis reovirus (HoVRV) showed strikingly contrasting patterns for each of their genomic RNAs respectively. For example, in H. vitripennis, HoCV-1-specific vsRNAs were predominantly of positive strand polarity (), and hotspots of more abundant vsRNAs were observed in regions encoding the HoCV-1 RdRP, and the intergenic region between the 2 ORFs (). Such a bias for positive strands of genomic RNAs has also been reported for other viruses infecting plants and other invertebrate hosts.Citation35,36 The vsRNAs can be so abundant in virus infections that they can even be used as strategies to identify new viruses. Studies on small RNA analysis of soybean aphids resulted in the identification of the single-stranded positive-sense RNA virus (Aphis glycines virus, AGV) and 2 other known aphid viruses (Aphid lethal paralysis virus, ALPV and Rhopalosiphum padi virus, RHPV).Citation37 piRNAs also have been shown to play a pivotal role in the antiviral defense of mosquito cell lines against Rift valley fever virus (RVFV).Citation22
Figure 2. Mapping summary of vsRNA reads against HoCV-1 genomic RNA. The numbers of vsRNA reads mapped along the Y -axis and the HoCV-1 genomic RNA are represented across the X -axis. The gray colored peaks represent the positive-strand small RNAs while the red colored peaks represent the negative-strand small RNAs. This figure is adapted from Nandety et.,al 2013Citation23 with permission from Virology, Elsevier Limited. This is just one example, several publications describe the distribution of small RNA reads on different viruses.Citation22,24
RNAi Inducers
Experimental studies have demonstrated that RNAi activity can be specifically induced using dsRNAs, siRNAs and miRNAs that are homologous to specific target mRNAs, and a variety of approaches have been used to deliver RNAi inducers to target insects. DsRNAs are the most commonly used RNAi inducers and can be produced in bacteriaCitation38-40 or in vitro.Citation41-44 Also, siRNAs have been used to induce RNAi effects and down regulate target mRNAs in psyllids (Bactericerca cockerelli) and honeybees (Apis mellifera).Citation44,45 The siRNAs used in the above examples were produced through the digestion of dsRNAs in vitro by RNase III to generate 18 – 25 bp siRNAs. Artificial microRNAs (amiRNAs) can also potentially be used for inducing RNAi effects in insects. For both plants and animals, both miRNAs and amiRNAs have been used for gene function analysis, therapeutic approaches against diseases, and regulating gene expression.Citation7-10 Furthermore, a considerable number of studies have shown that miRNAs can affect insect morphology, behavior, immunity, and gene expression; some of those studies showed that stage-specific or tissue-specific expression of miRNAs during insect development in different insects.Citation46-49 Therefore, using miRNA mimics or amiRNAs offers potential for practical RNAi applications toward insect pests.
Target selection for efficient RNAi
The potential for using RNAi specifically and efficiently in insects depends on several parameters including: dose dependency of the RNAi inducers, the efficiency differences of RNAi inducers against targets in different tissues of insects, the types of RNAi inducers to be used (siRNAs, piRNAs or miRNAs), their mode of delivery, and the availability of targets for RNAi. The successful use of RNA interference with insects also requires the identification of best targets for RNAi and selection of the best interfering RNAs (siRNAs, piRNAs, miRNAs or dsRNAs).
Previous studies have demonstrated an efficient RNAi process through selection of targets that are inherent part of the insect development, metamorphosis, or gene targets that are involved in key metabolic processes. Recent studies have demonstrated the role of important gene targets such as actin and V-ATPase in mealybugs and psyllids.Citation44,50,51 Similarly, insect metamorphosis can be influenced by different classes and levels of juvenile hormones (JHs); the levels of JHs in turn can be influenced by JH metabolic enzymes (juvenile hormone esterase, JHE; juvenile hormone epoxide hydrolase, JHEH; juvenile hormone acid methyl transferase, JHAMT). Some of the recent studies in Tribolium showed enhanced RNAi effects when the targets were either juvenile hormoneCitation52,53 or juvenile hormone acid methyl transferase.Citation54 In Bemisia tabaci, RNAi effects were prominent in nymphal stages when the genes in the ecdysone pathway were silenced.Citation55
Some of the bottlenecks in the efficient use of RNAi for insects are due to the limitations with target choice and hence efficiency, and due to the lack of genetic resources in non-model insects. With the availability of NGS technologies such as RNA-seq and Miseq and associated bioinformatic processing of larger datasets, generation of complete transcriptomic/genomic data and gene expression data for non-model insects can be accomplished quickly and efficiently. The transcriptome is a complete set of RNA transcripts produced inside an organism at a particular time, and represents a genomic blueprint of that organism. Unlocking the complexity of the transcriptome is essential for interpreting the functional real world elements of a genome which can be applied for more effective downstream applications including developing genome-based biorational control strategies such as those based on RNA interference. Transcriptome sequencing in insects, particularly in non-model organisms, can result in the preliminary understanding of host genes and can reveal the information associated with molting, metamorphosis, and development, necessary for the design of targets for RNAi. The current approaches are very suitable for transcriptome resequencing or for transcriptome assemblies for which the reference genomes are available. In organisms, where the reference genomes are absent, partial assembly of reference genome can be developed based on the assembly of small reads obtained through RNAseq. Additionally transcript information and annotation of novel transcriptome data can be obtained through BLAST homology and annotations from Gene Ontology databases. Genetic resources for the design of novel RNAi targets were recently developed Sitodiplosis mosellana,Citation56 Manduca sexta,Citation57 A. glycines,Citation58 B. tabaci,Citation59 N. lugens,Citation60 Lygus hesperus,Citation61 Homalodisca vitripennis,Citation62 Halyomorpha halysCitation63 and the references there after.
After target selection, further consideration must be given to many factors including size of the RNAi inducer (short vs long), distal vs. proximal ends of the target match (5’, 3’ or in the central region of the target), etc. Also, the ability to continuously supply the RNAi inducer molecules into insects and their spread inside insects would also play vital role in ensuring the success of RNAi.
Delivery of RNAi inducers - in vitro Synthesized/Expressed RNAi Inducers
Several approaches have been used to deliver RNAi inducers to insects, and even cultured insect cells. Some insect cells can be grown in multi-welled plates allowing for testing many candidate RNAi inducers simultaneously,Citation27 and this can be a rapid way to obtain information on RNAi effects at the cellular level. In some cases, RNAi-specific cell phenotypes can even be observed in cultured cells.Citation43 For example, when cultured H. vitripennis Z-15 cells were transfected with dsRNAs targeting actin mRNA, actin filaments were greatly disturbed and cells showed an overall unhealthy appearance.Citation43
Microinjection into insect bodies has been widely used for RNAi studies in whole insects. Microinjection can ensure that the RNAi inducers are delivered to the tissue of choice, or more generally into the hemolymph. Furthermore, when specific volumes of known concentration are injected, the dosages used can be accurately compared, and different RNAi inducers can be mixed and simultaneously injected.Citation64,65 For example, the efficiency of RNAi to reduce mRNA target expression was higher in double injections compared to single injections in Rhodnius prolixus and N. lugens.Citation64,65
Because of its many benefits, microinjection of specific dsRNAs or siRNAs has been used to study the regulation and expression of genes in both model and pest insects including Drosophila melanogaster,Citation66-68 T. castaneumCitation69 B. mori,Citation70 B. tabaci,Citation71 B. cockerelli,Citation44 H. vitripennis,Citation72 and A. mellifera.Citation73 Each of these studies yielded very good information, more than can be discussed here, so only limited examples are presented. Mutti et al (2006) used microinjection to deplete a target salivary gland transcript Coo2 in the pea aphid (A. pisum). Aphids injected with anti-Coo2 siRNAs showed earlier mortality than control insects.Citation74 Microinjection of dsRNA into A. pisum, also induced mRNA degradation at about 40% for 2 targets, the Ap-crt mRNA encoding a calcium binding protein (calreticulin) and the gut specific Ap-cath-L mRNA encoding a cysteine protease, cathepsin-L.Citation75 Rong et al. (2013) showed through dsRNA injection, that β-N-acetylglucosaminidase (LmNAG1) in Locusta migratoria plays an essential role in insect molting.Citation76 Microinjection of dsRNAs against actin, chitin synthase 1 and V-ATPase in the citrus mealybug, Planococcus citri, showed that the corresponding mRNAs are good targets for RNAi.Citation50
In addition to microinjection, RNAi effects have also been achieved by soaking (C. elegans),Citation77 spraying (Ostrinia furnalalis),Citation78 and electroporation (Ixodes scapularis)Citation79 approaches to deliver RNAi inducers. In a recent study, El-Shesheny, et. al.Citation80 applied dsRNAs directly to the Diaphorina citri ventral epidermis by micro-application. The droplets containing dsRNAs were placed ventrally on the thorax region of D. citri nymphs. Treated nymphs showed target mRNA degradation and wing malformation. Like microinjection, these are excellent approaches for fundamental RNAi studies, but are unlikely to have practical application potential, and RNAi applications for pest control will most likely require other means for delivering the interfering RNAs.
Oral delivery of RNAi inducers has been experimentally successful in many, but not all insects studied so far. A variety of approaches have been attempted including adding RNAi inducers to artificial diets, baits, and in some examples for plant-feeding insects, the plant has been used to deliver the RNAi inducers. While feeding RNAi inducers has great potential it is not as straightforward as is injection or other means of delivery. Inconsistent doses of RNAi inducers may be taken up by individual insects, frequency of feeding can be variable, and stability of the RNAi inducers in the oral delivery medium might be problematic. For example, for A. pisum, both salivary secretions and the hemolymph were able to degrade the dsRNAs; therefore, dsRNA-induced RNAi effects were not obtained through either ingestion or injection.Citation81
Oral delivery of RNAi inducers resulted in reduction of targeted mRNAs in several other agriculturally and medically important insects including Epiphyas postvittana,Citation82 Rhodnius prolixus,Citation64 Glossina morsitans morsitans,Citation83 Reticulitermes flavipes,Citation84 Plutella xylostella larvae,Citation85 Anopheles gambiae,Citation86 B. cockerelliCitation44 and D. citri.Citation87 Depending on the insect stage, in some cases it was possible to see phenotypic effects on the target insects and in others it was only possible to see target mRNA reductions. For example, the results from feeding tests with A. pisum showed that reduced expression of the hunchback (hb) mRNA encoded by a gap gene, (zinc finger transcription factor), which is a key player for insect segmentation, led to pea aphid lethality.Citation88 Similar artificial diet studies with B. cockerelli showed the efficiency of oral feeding dsRNAs and siRNAs by targeting endogenous mRNAs for the homologues of several essential genes including Actin, ATPase, Hsp70, and CLIC.Citation44
Whiteflies of the genus Bemisia are one of the most important global invasive agricultural insect pests in recent years.Citation55 The potential of these phloem-feeding hemipterans to develop resistance to chemical insecticides combined with their high reproduction rate has allowed for rapid expansion among many worldwide agricultural areas,Citation89 and RNAi has brought new hope for potential control strategies. In 2011, RNAi-induced mortality through oral delivery of dsRNA/siRNAs of 5 important target mRNAs, actin ortholog, ADP/ATP translocase, α-tubulin, ribosomal protein L9 (RPL9) and V-ATPase A for B. tabaci was reported.Citation90 In this study, reducing the expression of the corresponding mRNAs caused mortality after 6 days of feeding. Oral feeding of siRNAs showed a high mortality rate for RPL9 (84.61%) and V-ATPase A (85.62%), while the dsRNA treatment demonstrated the highest mortality rate for V-ATPase A with 97.5%.Citation90 In that bioassay, the mortality rate was similar in both treatments after reducing the expression of ADP/ATP translocase mRNA.Citation90 Interestingly, siRNAs were found stable in the insect diet for at least 7 days at room temperature.Citation90 Later, this group successfully engineered tobacco plants expressing a dsRNA precursor against the whitefly V-ATPaseA and the results illustrated that these modified plants formed siRNAs to downregulate the mRNA target and protect the plant.Citation91
Another successful example of oral delivery of dsRNA to induce RNAi was for Diabrotica virgifera virgifera. Snf7 is a vacuolar sorting protein belonging to the ESCRT (Endosomal Sorting Complex Required for Transport)–III complex involved in sorting of transmembrane proteins resulting in lysosome degradation through the endosomal-autophagic pathway.Citation92,93 RNAi of the WCR Snf7 ortholog (DvSnf7) led to deficiencies of mRNA and protein levels in the insect midgut and fat body resulting in de-ubiquitination and autophagy, and death of WCR larvae.Citation93 This result suggests this mRNA as a potential target for generation of transgenic plants for management of this economically important pest in the field.Citation93
Whether or not RNAi inducers acquired orally induce RNAi effects only in cells where they are taken up (e.g. midgut epithelial cells) is still an important question. Unlike in plants and C. elegans, insects are not known to exhibit a robust, systemic or distal RNAi response. Thus, if only the cells that are exposed to the RNAi inducer show RNAi effects, then care must be taken in interpreting results from oral feeding experiments.
Delivery of RNAi Inducers-Microbial-Mediated RNAi
In recent years several studies have identified ways to use microbial mediated-RNAi inducers to overcome some of the limitations mentioned above. A recombinant Escherichia coli was engineered to produce specific dsRNAs fed to C. elegans for inducing RNAi.Citation94,95 Similarly, E. coli was engineered to produce dsRNAs for successful RNAi of 5 different mRNA targets in the Colorado potato beetle, Leptinotarsa decemlineata.Citation96 The plasmids were transformed into a RNase III-deficient E. coli strain for dsRNA production and after ingesting the bacteria this resulted in significant mortality and loss of body weight in L. decemlineata. This system of microbial/bacterial delivery of dsRNAs worked efficiently in suppression of Penaeus merguiensis, densovirus (PmergDNV), which is a serious pathogen of the house cricket.Citation97 In that study, mortalities and viral loads in house crickets were significantly lower in treatments challenged with PmergDNV following exposure to bacterially expressed PmergDNV-dsRNAs.Citation97
For many plant-feeding insects, a more natural means of ingesting interfering RNAs would be by feeding on plants that generate the RNAi inducer. The use of transgenic plants engineered to produce dsRNAsCitation91,98 might be desirable in many cases, but transgenic plant generation requires time. As an alternative, several groups have investigated using recombinant plant viruses as tools for expressing RNAi inducersCitation50,51 in plants. The main advantage here is that RNAi activity is the primary plant defense against infecting viruses. Thus when plants respond to a virus infection by RNAi, siRNAs (vsRNAs) covering the entire virus genome are produced. Then if a plant virus is engineered to contain an insect RNAi inducer sequence, this also will be targeted and yield specific siRNAs (vsRNAs). Several plant viruses can be engineered to contain and express desired sequences in plants, and this can be done fairly easily and quickly. This is a commonly used research strategy in plant- microbe interaction studies and is referred to as host induced gene silencing (HIGS). If abundant RNAi inducer siRNAs were generated from the engineered virus, perhaps these could be acquired by insects feeding on these plants and then induce RNAi effects.
Three midgut-expressed MsCYPs mRNAs were targeted by RNAi activity in M. sexta through the use of recombinant Tobacco rattle virus engineered to produce RNAi inducers in Nicotiana attenuata.Citation99 Almost all plant-infecting viruses infect and move systemically in plants via the phloem, and thus plant viruses might also be useful for targeting phloem-feeding insects. To test this, recombinant Tobacco mosaic virus (TMV) was used to target the phloem feeding hemipteran, B. cockerelli.Citation51 When B. cockerelli were allowed to feed on tomato (Solanum lycopersicum), tomatillo (Physalis philadelphica) and tobacco (N. tabacum) plants infected with recombinant TMV harboring B. cockerelli actin and V-ATPase sequences, this resulted in a reduction of the corresponding B. cockerelli mRNAs.Citation51 Tomatillo plants infected with recombinant TMV also showed a decrease in B. cockerelli progeny production.Citation51 Similar approaches were applied in attempts to induce RNAi effects in N. benthamiana plants against the citrus mealybug (Planococcus citri), again by using recombinant TMV.Citation50 The results obtained from that study showed mortality of adult insects and dramatic reduced fecundity of P. citri after feeding on plants infected by the corresponding recombinant TMV50. These examples show experimentally the potential for using plant viruses to deliver RNAi inducers via non-transgenic plants, but obviously many plant viruses themselves are pathogens and this cure could be worse than the cause. However another plant virus has recently been used to assess whether this approach might have practical application. These authors engineered a common virus of citrus, Citrus tristeza virus (CTV) as a tool for expressing RNAs and proteins in citrus.Citation100 But what is most important is that the engineered CTV causes essentially no symptoms in citrus plants. This engineered CTV was then used to target one of the most destructive pests affecting citrus industry in the United States, D. citri, which is the vector of Candidatus Liberibacter asiaticus (CLas), causal agent of Huanglongbing (citrus greening). Citrus tristeza virus (CTV) engineered with truncated abnormal wing disc (Awd) RNA sequence of D. citri triggered degradation of the Awd mRNA after D. citri nymphs fed on these plants.Citation87 Furthermore, wing malformation and mortality of adult insects also was observed and attributed to RNAi.Citation87 This result is a very promising result, suggesting that even in woody perennial plants like citrus, such an approach could have practical application potential.
Delivery of RNAi Inducers-Plant Mediated RNAi
Potential application of transgenic plant-based RNAi approaches for pest insect management in plants has been widely discussed,Citation101,102 even leading to the suggestion of “insect-proof plants,”Citation103 and is very relevant today. A recent issue of Science (16 August 2013) featured on the cover the suggestion of “smarter pest control.” A special section within that issue (pages 728 – 765) was dedicated to our “pesticide planet” and discussed several current topics, one entitled “A Lethal Dose of RNA,”Citation104 and how new opportunities based on scientific research and our understanding of RNAi could help feed the world's growing population and simultaneously contribute to better sustainability in agriculture by decreasing our dependence on pesticides for pest and pathogen control. Clearly, RNAi approaches offer great potential for insect pest control, and by understanding the specificity and activity our hope is to take advantage of the opportunities to promote sustainable, environmentally sound applications of this technology.
Transgenic plant-mediated RNAi has proven to be successful against other plant pests and pathogens including nematodes,Citation105,106 bacteria,Citation107 and viruses.Citation108,109 Transgenic RNAi-specific approaches are even used commercially in U. S. papayas, squash and recently in plums.Citation110,111 The potential of transgenic plants targeting insects based on RNAi was first demonstrated in early studies against the western corn rootworm, D. virgifera.Citation98 Transformed corn (Zea mays) plants expressing the V-ATPase subunit A dsRNA exhibited a significant reduction in D. virgifera feeding damage.Citation98 Mao et al (2007) evaluated bollworm (Helicoverpa armigera) larvae growth on the N. benthamiana and Arabidopsis thaliana plants transformed with the P450 gene CYP6AE14 dsRNA. This mRNA is highly expressed in the H. armigera midgut and is important for larval growth and tolerance to cotton gossypol (a polyphenol compound playing an anti-herbivore role in cotton). The results showed that levels of CYP6AE14 mRNA were decreased and larval growth was reduced.Citation112 They further targeted H. armigera larvae by feeding them on CYP6AE14-derived hpRNA transgenic cotton.Citation113 Genetically modified cotton plants experienced less feeding damage and larval growth was significantly retarded but they were not killed.Citation113 Recently, cotton plants showed greater protection from H. armigera when plants were engineered to express both dsCYP6AE14 and full length cotton cysteine protease. Plant cysteine proteases help to enhance uptake of dsRNA through increasing insect peritrophic matrix permeability.Citation113 Zhu et al. also reported an improvement in pest resistance in engineered tobacco plants expressing dsRNA of ecdysone receptor (EcR) from the cotton bollworm, H. armigera.Citation114 EcR and ultraspiracle (USP) are 2 components of a nuclear receptor complex of the steroid hormone, 20-hydroxyecdysone (20E), which controls molting and metamorphosis. Therefore, EcR and USP are required for insect development. Zhu et al. showed that not only was pest resistance improved after feeding of H. armigera larvae on the transgenic tobacco plants expressing EcR dsRNA, but also the level of the EcR mRNA was significantly decreased causing molting defects and eventually death.Citation114 An interesting observation from that study was the resistance of the same transgenic tobacco plants to another pest, Spodoptera exigua.Citation114 The reason behind of that later resistance was the similarity of EcR gene sequence in S. exigua H. armigera. This is an example of off-target effects (more details in a separate section later).
Transgenic RNAi-based approaches also have shown good potential against some phloem-feeding hemipteran insects. N. lugens is an important phloem feeder pest of rice (Oryza sativa L) and is the vector for some important rice-infecting viruses. In N. lugens, the expression levels of 3 mRNAs of the hexose transporter gene NlHT1, the carboxypeptidase gene Nlcar, and the trypsin-like serine protease gene Nltry in the N. lugens midgut; were suppressed after N. lugens fed on transgenic rice plants; however, insect mortality was not observed.Citation115
The green peach aphid, M. persicae, has one of the broadest host ranges among crop pests and is an important vector for many plant viruses. RNAi studies were performed targeting 2 different M. persicae mRNAs. Rack1 encodes an essential multifunctional scaffold protein and its mRNA is predominantly expressed in the gut, and the M. persicae C002 (MpC002), is highly expressed in the salivary glands.Citation116 The expression of these 2 mRNAs was decreased by up to 60% after M. persicae fed on transgenic N. benthamiana and A. thaliana plants expressing dsRNAs corresponding to the targeted mRNAs, and M. persicae on these plants produced less progeny and some lethality was observed.Citation116 Furthermore, Mao and Zeng, reported plant-mediated RNAi against the mRNA for the gap gene, hunchback (hb) in M. persicae.Citation117 They demonstrated a reduction of the hb mRNA level in Myzus persicae nymphs after feeding on hb dsRNA-expressing tobacco affecting the insect fecundity.Citation117
In addition to using dsRNA in plant mediated RNAi targeting insects, amiRNAs are also being used as a strategy to induce RNAi. Recently, amiRNAs were used to confer aphid resistance in tobacco plants (N. tabacum cv. Xanthi).Citation118 Nine genes of the aphid (Myzus persicae) were selected as silencing targets and their hpRNA-expressing vectors were constructed and transformed into tobacco plants. The results showed that amiRNA could be another approach to develop for conducting RNAi in insects.
Potential for Off-target Effects
The potential for unwanted off-target effects is an issue that has been discussed, particularly in regards to practical applications of RNAi.Citation104,110,111,119 This issue is important and must be approached scientifically, particularly for RNAi-based approaches for insect control in crop plants. However, as RNAi activities occur naturally every day, everywhere, we must consider potential off-target effects against the background of naturally-occurring RNAi activities. Off-target effects can result if the interfering RNAs affect more than just the intended RNA target. This could be within the given insect, when the interfering RNA affects heterologous RNAs (Type I), or perhaps when the interfering RNA induces RNAi effects in non-target insects (or other organisms; Type II). The latter are generally thought to be of more concern from a risk point of view.
There are several potential ways that interfering RNAs might give rise to off-target effects. For example, if the mRNA to be targeted by RNAi activity is a highly conserved sequence among different species (e.g., actin), then the probability for potential off-target effects is likely to be higher than if the target mRNA is unique, or if the specific region of the mRNA targeted is unique. Some small RNAs can have up to hundreds of potential target sequences in a genomeCitation120,121 and off-target effects can be triggered due to the partially complementary sequence of the mRNA 3’UTR.Citation122-124 This mechanism of off-target effects has been suggested to be similar to that of miRNA silencing,Citation125,126 and can be further complicated as some miRNAs can have 4 to 5 mismatched base pairs with their target mRNA. There are other features of siRNAs that can affect the binding with target mRNAs, such as compositional features, thermodynamic, and structural features. The loading of small RNAs into different Argonaute proteins (AGO1, AGO3 and AGO4) besides AGO2 can also contribute toward different thermodynamic stability between the siRNA and its mRNA target, and target mRNA cellular abundance and turnover rate can influence siRNA efficacy.Citation4,127
Some suggestions for designing dsRNA and siRNA/miRNA RNAi inducers have been proposed to help avoid potential off-target effects.Citation128,129 Among them, several software are available online, such as SnapGragon, BLOCK-iT™ RNAi Designer, dsCheck, siDirect, and E-RNAi, etc. Some of the softwares are optimized for Drosophila melanogaster or other organisms. Each can be used for different purposes. For example, SnapDragon, dsCheck, and E-RNAi are web-based tools for the design of long dsRNAs for specific gene knockdown, and avoid the potential off-target effects; whereas, BLOCK-iT and siDirect are for selecting effective small RNA sequences with target specificity for multiple organism or a specific organism. Therefore, to have an efficient and specific RNAi, the correct program with the right algorithm for each siRNA design is important.
When the form of RNAi inducer used is dsRNA, then RNAi activity upon this dsRNA will occur yielding a range of siRNAs (), which might influence the potential for off target effects. Upon uptake by an insect, the dsRNAs are recognized as foreign and RNAi activity results. However, RNAi-based processing of dsRNAs does not occur at distinct, phased ∼21 nt intervals. Rather the dsRNA sequence can be cleaved by Dicer beginning at essentially any nucleotide, thereby yielding a population of overlapping siRNAs ranging in size and sequence corresponding to the target sequence. This is further compounded by other size siRNAs generated by RNAi activity. The qualitative differences are small, sometimes just a single nucleotide, but this can be important and the population complexity is great.
Figure 3. RNA dependent RNA polymerase amplification of siRNAs. The figure shows the signal amplification by RdRP and processing of siRNAs in plants. In this mechanism, Dicer processed primary siRNAs from an exogenous dsRNA guide the target mRNA cleavage. The cleaved mRNA will then be used as template for RdRP to produce dsRNA and targeted by Dicer, initiating the amplification cycle. This type of robust amplification is absent in hemipteran insects but present in plants and C. elegans.
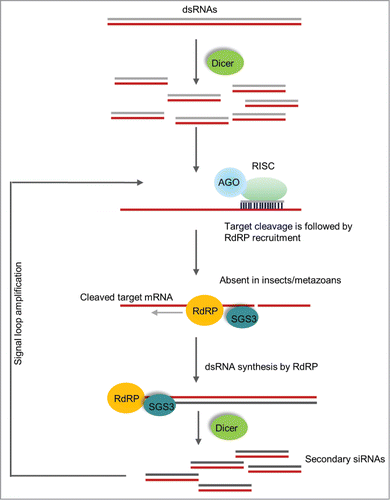
When plants are engineered to produce dsRNAs, RNAi activity will result in the plant, thus yielding a population of siRNAs corresponding to those dsRNAs. The dsRNA per se, is not then the only RNAi inducer likely to be present in the plant. Furthermore, at least in plants RNAi activity includes RNA-dependent RNA polymerase-based amplification to yield secondary siRNAs (), resulting in skewed populations of siRNAs. In addition, the resulting siRNAs will not be equal in abundance, there are “hot spots” which amplify more, giving rise to specific siRNAs of much greater abundance for a given region. This has potential for greater homology of siRNAs/dsRNAs to different target mRNAs, possibly increasing the complexity for off-target effects. If the dsRNAs are directly acquired by the target insect, then RNAi activity within that insect will yield the population of siRNAs and this could give rise to potential Type I off-target effects. These may or may not be of real concern. However, Type II off-target effects could conceivably result if non-target insects acquire the dsRNAs. For example, transgenic maize plants engineered for RNAi-based resistance to D. virgifera also showed RNAi effects against several other beetle species in which the sequence conservation exists,Citation98 supporting the arguments above.
Future Directions
We have covered some aspects of RNAi relative to insects, and even some possibilities and considerations relative to practical applications of RNAi approaches for insect pest control in plants. We also presented some examples of new approaches including using plant viruses as potential delivery vehicles in crop-mediated RNAi applications against insect pests. This strategy could be particularly attractive for use in woody plants such as grapes and/or citrus, where transgenic plant approaches would be very difficult due to the time required for developing all the needed plants and the great number of different plant genotypes. For example in overall citrus production there are many varieties (e.g. grapefruit, oranges, lemons, limes etc.) in a given area, and for old established orchards, or vineyards for grapes, there can be 100 year old plants that need to be protected (e.g., grapes: Cabernet sauvignon). Plant transgenic approaches in such instances would be very difficult. But it might be possible to use recombinant plant viruses to deliver RNAi inducers in existing, non-transgenic plants. However, this approach has its own challenges. Phloem sap feeding hemipteran insects acquire nutrients from phloem. Therefore, RNAi inducers must be available in the phloem, but evidence already shows that phloem feeding hemipterans can be targeted by this approach.Citation50,51,87 And, if viruses are to be used in agriculture, they must not induce a negative phenotype (disease) in the plant, they must be engineered to not spread beyond the treated plants but must still be able to move in phloem of treated plants, and the recombinant virus must retain and express the RNAi inducing sequence while infecting the plant. Although this approach offers great potential, clearly more studies are needed.
But what new alternative approaches are there? Can insects be targeted directly, perhaps by using insect- specific viruses to induce RNAi in the target insects? Viruses are the most abundant microbes on our planetCitation133 and various viruses are found in all types of organisms, including insects. Insects are known to be hosts to a variety of viruses belonging to different taxa including baculoviruses, picornaviruses, parvoviruses, entomopoxviruses, ascoviruses, iridoviruses and others,Citation134 and probably many more remain undiscovered. We know that insects mount effective RNAi responses against infecting viruses, so if insect viruses could be engineered to contain specific target insect RNAi inducers, will the infected insect mount an RNAi response against this virus (and the insects’ own RNA)? There are some reports already suggesting that this strategy might have potential for at least some insects.Citation135 Part of the problem, but also the beauty of this approach is that viruses have limited and specific host ranges. Therefore if the correct insect virus can be used, the potential for at least Type II off-target effects will be lessened, as a virus that might be used for an aphid such as R. padi, will not be useful for a psyllid such as D. citri.
Viruses naturally infect insects and thus are already present in the environment in non-recombinant forms, but there are important challenges for using recombinant viruses. For example, can insect viruses be engineered to contain, efficiently express and retain the inserted RNAi inducer sequence? Replication, packaging cycles often lead to recombination events that excise sequences not needed by the virus, thus the RNA inducer sequence might be lost over time. This is a potential problem for long term establishment of the recombinant virus in the population, but also means that the recombinant virus will evolve back to wild type and the recombinant virus is not permanently in the population, which might be desirable in some cases. These are questions/concerns which need to be addressed to see if insect viruses can be successful as gene delivery vehicles to induce RNAi and eventually control of crop pests as well as plant viruses. RNAi is clearly a very important topic for fundamental study but also with great practical potential. A variety of approaches are potentially available and we anticipate an even more rapid accumulation of new data and successes. We hope that RNAi approaches can be used for environmentally sound, efficacious insect control.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
We would like to thank the California Department of Food and Agriculture Pierce's Disease Research Program, the California Citrus Research Board, the Florida Citrus Research and Development Foundation and the U. S. Department of Agriculture, NIFA grants program for funding projects in our lab.
References
- Ding SW. RNA-based antiviral immunity. Nat Rev Immunol 2010; 10:632-44; PMID:20706278; http://dx.doi.org/10.1038/nri2824
- Ding SW, Lu R. Virus-derived siRNAs and piRNAs in immunity and pathogenesis. Curr Opin Virol 2011; 1:533-44; PMID:22180767; http://dx.doi.org/10.1016/j.coviro.2011.10.028
- Pelechano V, Steinmetz LM. Gene regulation by antisense transcription. Nat Rev Genet 2013; 14:880-93; PMID:24217315; http://dx.doi.org/10.1038/nrg3594
- Siomi MC, Sato K, Pezic D, Aravin AA. PIWI-interacting small RNAs: the vanguard of genome defence. Nat Rev Mol Cell Biol 2011; 12:246-58; PMID:21427766; http://dx.doi.org/10.1038/nrm3089
- Siomi H, Siomi MC. On the road to reading the RNA-interference code. Nature 2009; 457:396-404; PMID:19158785; http://dx.doi.org/10.1038/nature07754
- Kim VN, Han J, Siomi MC. Biogenesis of small RNAs in animals. Nat Rev Mol Cell Biol 2009; 10:126-39; PMID:19165215; http://dx.doi.org/10.1038/nrm2632
- Lee Y, Jeon K, Lee JT, Kim S, Kim VN. MicroRNA maturation: stepwise processing and subcellular localization. EMBO J 2002; 21:4663-70; PMID:12198168; http://dx.doi.org/10.1093/emboj/cdf476
- Mallory AC, Elmayan T, Vaucheret H. MicroRNA maturation and action–the expanding roles of ARGONAUTEs. Curr Opin Plant Biol 2008; 11:560-6; PMID:18691933; http://dx.doi.org/10.1016/j.pbi.2008.06.008
- Bohnsack MT, Czaplinski K, Gorlich D. Exportin 5 is a RanGTP-dependent dsRNA-binding protein that mediates nuclear export of pre-miRNAs. RNA 2004; 10:185-91; PMID:14730017; http://dx.doi.org/10.1261/rna.5167604
- Lund E, Guttinger S, Calado A, Dahlberg JE, Kutay U. Nuclear export of microRNA precursors. Science 2004; 303:95-8; PMID:14631048; http://dx.doi.org/10.1126/science.1090599
- Lee YS, Nakahara K, Pham JW, Kim K, He Z, Sontheimer EJ, Carthew RW. Distinct roles for Drosophila Dicer-1 and Dicer-2 in the siRNA/miRNA silencing pathways. Cell 2004; 117:69-81; PMID:15066283; http://dx.doi.org/10.1016/S0092-8674(04)00261-2
- Seitz H, Tushir JS, Zamore PD. A 5’-uridine amplifies miRNA/miRNA* asymmetry in Drosophila by promoting RNA-induced silencing complex formation. Silence 2011; 2:4; PMID:21649885; http://dx.doi.org/10.1186/1758-907X-2-4
- Okamura K, Lai EC. Endogenous small interfering RNAs in animals. Nat Rev Mol Cell Biol 2008; 9:673-8; PMID:18719707; http://dx.doi.org/10.1038/nrm2479
- Liu J, Carmell MA, Rivas FV, Marsden CG, Thomson JM, Song JJ, Hammond SM, Joshua-Tor L, Hannon GJ. Argonaute2 is the catalytic engine of mammalian RNAi. Science 2004; 305:1437-41; PMID:15284456; http://dx.doi.org/10.1126/science.1102513
- Miyoshi T, Takeuchi A, Siomi H, Siomi MC. A direct role for Hsp90 in pre-RISC formation in Drosophila. Nat Struct Mol Biol 2010; 17:1024-6; PMID:20639883; http://dx.doi.org/10.1038/nsmb.1875
- Chapman EJ, Carrington JC. Specialization and evolution of endogenous small RNA pathways. Nat Rev Genet 2007; 8:884-96; PMID:17943195; http://dx.doi.org/10.1038/nrg2179
- Chung WJ, Okamura K, Martin R, Lai EC. Endogenous RNA interference provides a somatic defense against Drosophila transposons. Curr Biol 2008; 18:795-802; PMID:18501606; http://dx.doi.org/10.1016/j.cub.2008.05.006
- Galiana-Arnoux D, Dostert C, Schneemann A, Hoffmann JA, Imler JL. Essential function in vivo for Dicer-2 in host defense against RNA viruses in drosophila. Nat Immunol 2006; 7:590-7; PMID:16554838; http://dx.doi.org/10.1038/ni1335
- van Rij RP, Saleh MC, Berry B, Foo C, Houk A, Antoniewski C, Andino R. The RNA silencing endonuclease Argonaute 2 mediates specific antiviral immunity in Drosophila melanogaster. Genes Dev 2006; 20:2985-95; PMID:17079687; http://dx.doi.org/10.1101/gad.1482006
- Marques JT, Wang JP, Wang X, de Oliveira KP, Gao C, Aguiar ER, Jafari N, Carthew RW. Functional specialization of the small interfering RNA pathway in response to virus infection. PLoS Pathog 2013; 9:e1003579; PMID:24009507; http://dx.doi.org/10.1371/journal.ppat.1003579
- Bronkhorst AW, van Rij RP. The long and short of antiviral defense: small RNA-based immunity in insects. Curr Opin Virol 2014; 7C:19-28; http://dx.doi.org/10.1016/j.coviro.2014.03.010
- Leger P, Lara E, Jagla B, Sismeiro O, Mansuroglu Z, Coppee JY, Bonnefoy E, Bouloy M. Dicer-2- and Piwi-mediated RNA interference in Rift Valley fever virus-infected mosquito cells. J Virol 2013; 87:1631-48; PMID:23175368; http://dx.doi.org/10.1128/JVI.02795-12
- Nandety RS, Fofanov VY, Koshinsky H, Stenger DC, Falk BW. Small RNA populations for two unrelated viruses exhibit different biases in strand polarity and proximity to terminal sequences in the insect host Homalodisca vitripennis. Virology 2013; 442:12-9; PMID:23642540; http://dx.doi.org/10.1016/j.virol.2013.04.005
- Wu Q, Luo Y, Lu R, Lau N, Lai EC, Li WX, Ding SW. Virus discovery by deep sequencing and assembly of virus-derived small silencing RNAs. Proc Natl Acad Sci U S A 2010; 107:1606-11; PMID:20080648; http://dx.doi.org/10.1073/pnas.0911353107
- Gunawardane LS, Saito K, Nishida KM, Miyoshi K, Kawamura Y, Nagami T, Siomi H, Siomi MC. A slicer-mediated mechanism for repeat-associated siRNA 5’ end formation in Drosophila. Science 2007; 315:1587-90; PMID:17322028; http://dx.doi.org/10.1126/science.1140494
- Saito K, Nishida KM, Mori T, Kawamura Y, Miyoshi K, Nagami T, Siomi H, Siomi MC. Specific association of Piwi with rasiRNAs derived from retrotransposon and heterochromatic regions in the Drosophila genome. Genes Dev 2006; 20:2214-22; PMID:16882972; http://dx.doi.org/10.1101/gad.1454806
- Meister G. Argonaute proteins: functional insights and emerging roles. Nat Rev Genet 2013; 14:447-59; PMID:23732335; http://dx.doi.org/10.1038/nrg3462
- Jiang J, Ge X, Li Z, Wang Y, Song Q, Stanley DW, Tan A, Huang Y. MicroRNA-281 regulates the expression of ecdysone receptor (EcR) isoform B in the silkworm, Bombyx mori. Insect Biochem Mol Biol 2013; 43:692-700; PMID:23707601; http://dx.doi.org/10.1016/j.ibmb.2013.05.002
- Chen J, Liang Z, Liang Y, Pang R, Zhang W. Conserved microRNAs miR-8-5p and miR-2a-3p modulate chitin biosynthesis in response to 20-hydroxyecdysone signaling in the brown planthopper, Nilaparvata lugens. Insect Biochem Mol Biol 2013; 43:839-48; PMID:23796434; http://dx.doi.org/10.1016/j.ibmb.2013.06.002
- Kingsolver MB, Huang Z, Hardy RW. Insect antiviral innate immunity: pathways, effectors, and connections. J Mol Biol 2013; 425:4921-36; PMID:24120681; http://dx.doi.org/10.1016/j.jmb.2013.10.006
- Bronkhorst AW, Miesen P, van Rij RP. Small RNAs tackle large viruses: RNA interference-based antiviral defense against DNA viruses in insects. Fly (Austin) 2013; 7
- Bronkhorst AW, van Cleef KW, Vodovar N, Ince IA, Blanc H, Vlak JM, Saleh MC, van Rij RP. The DNA virus Invertebrate iridescent virus 6 is a target of the Drosophila RNAi machinery. Proc Natl Acad Sci U S A 2012; 109:E3604-13; PMID:23151511; http://dx.doi.org/10.1073/pnas.1207213109
- Jayachandran B, Hussain M, Asgari S. RNA interference as a cellular defense mechanism against the DNA virus baculovirus. J Virol 2012; 86:13729-34; PMID:23055564; http://dx.doi.org/10.1128/JVI.02041-12
- Kemp C, Mueller S, Goto A, Barbier V, Paro S, Bonnay F, Dostert C, Troxler L, Hetru C, Meignin C, et al. Broad RNA interference-mediated antiviral immunity and virus-specific inducible responses in Drosophila. J Immunol 2013; 190:650-8; PMID:23255357; http://dx.doi.org/10.4049/jimmunol.1102486
- Parameswaran P, Sklan E, Wilkins C, Burgon T, Samuel MA, Lu R, Ansel KM, Heissmeyer V, Einav S, Jackson W, et al. Six RNA viruses and forty-one hosts: viral small RNAs and modulation of small RNA repertoires in vertebrate and invertebrate systems. PLoS Pathog 2010; 6:e1000764; PMID:20169186; http://dx.doi.org/10.1371/journal.ppat.1000764
- Donaire L, Wang Y, Gonzalez-Ibeas D, Mayer KF, Aranda MA, Llave C. Deep-sequencing of plant viral small RNAs reveals effective and widespread targeting of viral genomes. Virology 2009; 392:203-14; PMID:19665162; http://dx.doi.org/10.1016/j.virol.2009.07.005
- Liu S, Vijayendran D, Bonning BC. Next generation sequencing technologies for insect virus discovery. Viruses 2011; 3:1849-69; PMID:22069519; http://dx.doi.org/10.3390/v3101849
- Timmons L, Fire A. Specific interference by ingested dsRNA. Nature 1998; 395:854; PMID:9804418; http://dx.doi.org/10.1038/27579
- Timmons L, Court DL, Fire A. Ingestion of bacterially expressed dsRNAs can produce specific and potent genetic interference in Caenorhabditis elegans. Gene 2001; 263:103-12; PMID:11223248; http://dx.doi.org/10.1016/S0378-1119(00)00579-5
- Surakasi VP, Mohamed AA, Kim Y. RNA interference of beta1 integrin subunit impairs development and immune responses of the beet armyworm, Spodoptera exigua. J Insect Physiol 2011; 57:1537-44; PMID:21856307; http://dx.doi.org/10.1016/j.jinsphys.2011.08.006
- Clemens JC, Worby CA, Simonson-Leff N, Muda M, Maehama T, Hemmings BA, Dixon JE. Use of double-stranded RNA interference in Drosophila cell lines to dissect signal transduction pathways. Proc Natl Acad Sci U S A 2000; 97:6499-503; PMID:10823906; http://dx.doi.org/10.1073/pnas.110149597
- Kennerdell JR, Carthew RW. Use of dsRNA-mediated genetic interference to demonstrate that frizzled and frizzled 2 act in the wingless pathway. Cell 1998; 95:1017-26; PMID:9875855; http://dx.doi.org/10.1016/S0092-8674(00)81725-0
- Rosa C, Kamita SG, Dequine H, Wuriyanghan H, Lindbo JA, Falk BW. RNAi effects on actin mRNAs in Homalodisca vitripennis cells. J RNAi Gene Silencing 2010; 6:361-6; PMID:20628496
- Wuriyanghan H, Rosa C, Falk BW. Oral delivery of double-stranded RNAs and siRNAs induces RNAi effects in the potato/tomato psyllid, Bactericerca cockerelli. PloS One 2011; 6:e27736; PMID:22110747; http://dx.doi.org/10.1371/journal.pone.0027736
- Mussig L, Richlitzki A, Rossler R, Eisenhardt D, Menzel R, Leboulle G. Acute disruption of the NMDA receptor subunit NR1 in the honeybee brain selectively impairs memory formation. J Neurosci 2010; 30:7817-25; PMID:20534830; http://dx.doi.org/10.1523/JNEUROSCI.5543-09.2010
- Aravin AA, Lagos-Quintana M, Yalcin A, Zavolan M, Marks D, Snyder B, Gaasterland T, Meyer J, Tuschl T. The small RNA profile during Drosophila melanogaster development. Dev Cell 2003; 5:337-50; PMID:12919683; http://dx.doi.org/10.1016/S1534-5807(03)00228-4
- Behura SK, Whitfield CW. Correlated expression patterns of microRNA genes with age-dependent behavioural changes in honeybee. Insect Mol Biol 2010; 19:431-9; PMID:20491979
- Rao Z, He W, Liu L, Zheng S, Huang L, Feng Q. Identification, expression and target gene analyses of Micrornas in Spodoptera litura. PloS One 2012; 7:e37730; PMID:22662202; http://dx.doi.org/10.1371/journal.pone.0037730
- Skalsky RL, Vanlandingham DL, Scholle F, Higgs S, Cullen BR. Identification of microRNAs expressed in two mosquito vectors, Aedes albopictus and Culex quinquefasciatus. BMC genomics 2010; 11:119; PMID:20167119; http://dx.doi.org/10.1186/1471-2164-11-119
- Khan AM, Ashfaq M, Kiss Z, Khan AA, Mansoor S, Falk BW. Use of recombinant tobacco mosaic virus to achieve RNA interference in plants against the citrus mealybug, Planococcus citri (Hemiptera: Pseudococcidae). PloS One 2013; 8:e73657; PMID:24040013; http://dx.doi.org/10.1371/journal.pone.0073657
- Wuriyanghan H, Falk BW. RNA Interference towards the Potato Psyllid, Is Induced in Plants Infected with Recombinant Tobacco mosaic virus (TMV). PloS One 2013; 8:e66050; PMID:23824081; http://dx.doi.org/10.1371/journal.pone.0066050
- Abdel-Latief M, Hoffmann KH. Functional activity of allatotropin and allatostatin in the pupal stage of a holometablous insect, Tribolium castaneum (Coleoptera, Tenebrionidae). Peptides 2014; 53:172-84; PMID:24140809; http://dx.doi.org/10.1016/j.peptides.2013.10.007
- Xu J, Sheng Z, Palli SR. Juvenile hormone and insulin regulate trehalose homeostasis in the red flour beetle, Tribolium castaneum. PLoS Genet 2013; 9:e1003535; PMID:23754959; http://dx.doi.org/10.1371/journal.pgen.1003535
- Minakuchi C, Namiki T, Yoshiyama M, Shinoda T. RNAi-mediated knockdown of juvenile hormone acid O-methyltransferase gene causes precocious metamorphosis in the red flour beetle Tribolium castaneum. FEBS J 2008; 275:2919-31; PMID:18435763; http://dx.doi.org/10.1111/j.1742-4658.2008.06428.x
- Luan JB, Ghanim M, Liu SS, Czosnek H. Silencing the ecdysone synthesis and signaling pathway genes disrupts nymphal development in the whitefly. Insect Biochem Mol Biol 2013; 43:740-6; PMID:23748027; http://dx.doi.org/10.1016/j.ibmb.2013.05.012
- Gong ZJ, Wu YQ, Miao J, Duan Y, Jiang YL, Li T. Global Transcriptome Analysis of Orange Wheat Blossom Midge, Sitodiplosis mosellana (Gehin) (Diptera: Cecidomyiidae) to Identify Candidate Transcripts Regulating Diapause. PloS One 2013; 8:e71564
- Pauchet Y, Wilkinson P, Vogel H, Nelson DR, Reynolds SE, Heckel DG, ffrench-Constant RH. Pyrosequencing the Manduca sexta larval midgut transcriptome: messages for digestion, detoxification and defence. Insect Mol Biol 2010; 19:61-75; PMID:19909380; http://dx.doi.org/10.1111/j.1365-2583.2009.00936.x
- Liu S, Chougule NP, Vijayendran D, Bonning BC. Deep sequencing of the transcriptomes of soybean aphid and associated endosymbionts. PloS One 2012; 7:e45161; PMID:22984624; http://dx.doi.org/10.1371/journal.pone.0045161
- Wang XW, Luan JB, Li JM, Bao YY, Zhang CX, Liu SS. De novo characterization of a whitefly transcriptome and analysis of its gene expression during development. BMC Genomics 2010; 11:400; PMID:20573269; http://dx.doi.org/10.1186/1471-2164-11-400
- Peng X, Zha W, He R, Lu T, Zhu L, Han B, He G. Pyrosequencing the midgut transcriptome of the brown planthopper, Nilaparvata lugens. Insect Mol Biol 2011; 20:745-62; PMID:21919985; http://dx.doi.org/10.1111/j.1365-2583.2011.01104.x
- Hull JJ, Geib SM, Fabrick JA, Brent CS. Sequencing and de novo assembly of the western tarnished plant bug (Lygus hesperus) transcriptome. PloS One 2013; 8:e55105; PMID:23357950; http://dx.doi.org/10.1371/journal.pone.0055105
- Nandety RS, Kamita SG, Hammock BD, Falk BW. Sequencing and De Novo Assembly of the Transcriptome of the Glassy-Winged Sharpshooter (Homalodisca vitripennis). PloS One 2013; 8:e81681; PMID:24339955; http://dx.doi.org/10.1371/journal.pone.0081681
- Ioannidis P, Lu Y, Kumar N, Creasy T, Daugherty S, Chibucos MC, Orvis J, Shetty A, Ott S, Flowers M, et al. Rapid transcriptome sequencing of an invasive pest, the brown marmorated stink bug Halyomorpha halys. BMC Genomics 2014; 15:738; PMID:25168586; http://dx.doi.org/10.1186/1471-2164-15-738
- Araujo RN, Santos A, Pinto FS, Gontijo NF, Lehane MJ, Pereira MH. RNA interference of the salivary gland nitrophorin 2 in the triatomine bug Rhodnius prolixus (Hemiptera: Reduviidae) by dsRNA ingestion or injection. Insect Biochem Mol Biol 2006; 36:683-93; PMID:16935217; http://dx.doi.org/10.1016/j.ibmb.2006.05.012
- Liu S, Ding Z, Zhang C, Yang B, Liu Z. Gene knockdown by intro-thoracic injection of double-stranded RNA in the brown planthopper, Nilaparvata lugens. Insect Biochem Mol Biol 2010; 40:666-71; PMID:20599616; http://dx.doi.org/10.1016/j.ibmb.2010.06.007
- Bischoff V, Vignal C, Duvic B, Boneca IG, Hoffmann JA, Royet J. Downregulation of the Drosophila immune response by peptidoglycan-recognition proteins SC1 and SC2. PLoS Pathog 2006; 2:e14; PMID:16518472; http://dx.doi.org/10.1371/journal.ppat.0020014
- Miller SC, Brown SJ, Tomoyasu Y. Larval RNAi in Drosophila? Dev Genes Evol 2008; 218:505-10; PMID:18663472; http://dx.doi.org/10.1007/s00427-008-0238-8
- Roignant JY, Carre C, Mugat B, Szymczak D, Lepesant JA, Antoniewski C. Absence of transitive and systemic pathways allows cell-specific and isoform-specific RNAi in Drosophila. Rna 2003; 9:299-308; PMID:12592004; http://dx.doi.org/10.1261/rna.2154103
- Parthasarathy R, Palli SR. Molecular analysis of juvenile hormone analog action in controlling the metamorphosis of the red flour beetle, Tribolium castaneum. Arch Insect Biochem Physiol 2009; 70:57-70; PMID:19072925; http://dx.doi.org/10.1002/arch.20288
- Hossain M, Shimizu S, Matsuki M, Imamura M, Sakurai S, Iwami M. Expression of 20-hydroxyecdysone-induced genes in the silkworm brain and their functional analysis in post-embryonic development. Insect Biochem Mol Biol 2008; 38:1001-7; PMID:18835445; http://dx.doi.org/10.1016/j.ibmb.2008.08.006
- Ghanim M, Kontsedalov S, Czosnek H. Tissue-specific gene silencing by RNA interference in the whitefly Bemisia tabaci. Insect BiochemMol Biol 2007; 37:732-8; PMID:17550829; http://dx.doi.org/10.1016/j.ibmb.2007.04.006
- Rosa C, Kamita SG, Falk BW. RNA interference is induced in the glassy winged sharpshooter Homalodisca vitripennis by actin dsRNA. Pest Manag Sci 2012:995-1002; PMID:22345053; http://dx.doi.org/10.1002/ps.3253
- Li-Byarlay H, Li Y, Stroud H, Feng S, Newman TC, Kaneda M, Hou KK, Worley KC, Elsik CG, Wickline SA, et al. RNA interference knockdown of DNA methyl-transferase 3 affects gene alternative splicing in the honey bee. Proc Natl Acad Sci U S A 2013; 110:12750-5; PMID:23852726; http://dx.doi.org/10.1073/pnas.1310735110
- Mutti NS, Park Y, Reese JC, Reeck GR. RNAi knockdown of a salivary transcript leading to lethality in the pea aphid, Acyrthosiphon pisum. J Insect Sci 2006; 6:1-7; PMID:20233093; http://dx.doi.org/10.1673/031.006.3801
- Jaubert-Possamai S, Le Trionnaire G, Bonhomme J, Christophides GK, Rispe C, Tagu D. Gene knockdown by RNAi in the pea aphid Acyrthosiphon pisum. BMC Biotechnol 2007; 7:63; PMID:17903251; http://dx.doi.org/10.1186/1472-6750-7-63
- Rong S, Li DQ, Zhang XY, Li S, Zhu KY, Guo YP, Ma EB, Zhang JZ. RNA interference to reveal roles of beta-N-acetylglucosaminidase gene during molting process in Locusta migratoria. Insect Sci 2013; 20:109-19; PMID:23955831; http://dx.doi.org/10.1111/j.1744-7917.2012.01573.x
- Maeda I, Kohara Y, Yamamoto M, Sugimoto A. Large-scale analysis of gene function in Caenorhabditis elegans by high-throughput RNAi. Curr Biol 2001; 11:171-6; PMID:11231151; http://dx.doi.org/10.1016/S0960-9822(01)00052-5
- Wang Y, Zhang H, Li H, Miao X. Second-generation sequencing supply an effective way to screen RNAi targets in large scale for potential application in pest insect control. PloS One 2011; 6:e18644; PMID:21494551; http://dx.doi.org/10.1371/journal.pone.0018644
- Karim S, Troiano E, Mather TN. Functional genomics tool: gene silencing in Ixodes scapularis eggs and nymphs by electroporated dsRNA. BMC Biotechnol 2010; 10:1; PMID:20074328; http://dx.doi.org/10.1186/1472-6750-10-1
- El-Shesheny I, Hajeri S, El-Hawary I, Gowda S, Killiny N. Silencing abnormal wing disc gene of the Asian citrus psyllid, Diaphorina citri disrupts adult wing development and increases nymph mortality. PloS One 2013; 8:e65392; PMID:23734251; http://dx.doi.org/10.1371/journal.pone.0065392
- Christiaens O, Swevers L, Smagghe G. DsRNA degradation in the pea aphid (Acyrthosiphon pisum) associated with lack of response in RNAi feeding and injection assay. Peptides 2014; 53:307-314; PMID:24394433
- Turner CT, Davy MW, MacDiarmid RM, Plummer KM, Birch NP, Newcomb RD. RNA interference in the light brown apple moth, Epiphyas postvittana (Walker) induced by double-stranded RNA feeding. Insect Mol Biol 2006; 15:383-91; PMID:16756557; http://dx.doi.org/10.1111/j.1365-2583.2006.00656.x
- Walshe DP, Lehane SM, Lehane MJ, Haines LR. Prolonged gene knockdown in the tsetse fly Glossina by feeding double stranded RNA. Insect Mol Biol 2009; 18:11-9; PMID:19016913; http://dx.doi.org/10.1111/j.1365-2583.2008.00839.x
- Zhou X, Wheeler MM, Oi FM, Scharf ME. RNA interference in the termite Reticulitermes flavipes through ingestion of double-stranded RNA. Insect Biochem Mol Biol 2008; 38:805-15; PMID:18625404; http://dx.doi.org/10.1016/j.ibmb.2008.05.005
- Bautista MA, Miyata T, Miura K, Tanaka T. RNA interference-mediated knockdown of a cytochrome P450, CYP6BG1, from the diamondback moth, Plutella xylostella, reduces larval resistance to permethrin. Insect Biochem Mol Biol 2009; 39:38-46; PMID:18957322; http://dx.doi.org/10.1016/j.ibmb.2008.09.005
- Zhang S, Shukle R, Mittapalli O, Zhu YC, Reese JC, Wang H, Hua BZ, Chen MS. The gut transcriptome of a gall midge, Mayetiola destructor. J Insect Physiol 2010; 56:1198-206; PMID:20346948; http://dx.doi.org/10.1016/j.jinsphys.2010.03.021
- Hajeri S, Killiny N, El-Mohtar C, Dawson WO, Gowda S. Citrus tristeza virus-based RNAi in citrus plants induces gene silencing in Diaphorina citri, a phloem-sap sucking insect vector of citrus greening disease (Huanglongbing). J Biotechnol 2014; 76:42-49; PMID:24572372; http://dx.doi.org/10.1016/J.Jbiotec.2014.02.010
- Mao J, Zeng F. Feeding-based RNA interference of a gap gene is lethal to the pea aphid, Acyrthosiphon pisum. PloS One 2012; 7:e48718; PMID:23144942; http://dx.doi.org/10.1371/journal.pone.0048718
- Karatolos N, Denholm I, Williamson M, Nauen R, Gorman K. Incidence and characterisation of resistance to neonicotinoid insecticides and pymetrozine in the greenhouse whitefly, Trialeurodes vaporariorum Westwood (Hemiptera: Aleyrodidae). Pest Manag Sci 2010; 66:1304-7; PMID:20799247; http://dx.doi.org/10.1002/ps.2014
- Upadhyay SK, Chandrashekar K, Thakur N, Verma PC, Borgio JF, Singh PK, Tuli R. RNA interference for the control of whiteflies (Bemisia tabaci) by oral route. J Biosci 2011; 36:153-61; PMID:21451256; http://dx.doi.org/10.1007/s12038-011-9009-1
- Thakur N, Upadhyay SK, Verma PC, Chandrashekar K, Tuli R, Singh PK. Enhanced Whitefly Resistance in Transgenic Tobacco Plants Expressing Double Stranded RNA of v-ATPase A Gene. PloS One 2014; 9:e87235; PMID:24595215; http://dx.doi.org/10.1371/journal.pone.0087235
- Bolognesi R, Ramaseshadri P, Anderson J, Bachman P, Clinton W, Flannagan R, Ilagan O, Lawrence C, Levine S, Moar W, et al. Characterizing the mechanism of action of double-stranded RNA activity against western corn rootworm (Diabrotica virgifera virgifera LeConte). PloS One 2012; 7:e47534; PMID:23071820; http://dx.doi.org/10.1371/journal.pone.0047534
- Ramaseshadri P, Segers G, Flannagan R, Wiggins E, Clinton W, Ilagan O, McNulty B, Clark T, Bolognesi R. Physiological and cellular responses caused by RNAi- mediated suppression of Snf7 orthologue in western corn rootworm (Diabrotica virgifera) larvae. PloS One 2013; 8:e54270; PMID:23349844; http://dx.doi.org/10.1371/journal.pone.0054270
- Kamath RS, Ahringer J. Genome-wide RNAi screening in Caenorhabditis elegans. Methods 2003; 30:313-21; PMID:12828945; http://dx.doi.org/10.1016/S1046-2023(03)00050-1
- Kamath RS, Fraser AG, Dong Y, Poulin G, Durbin R, Gotta M, Kanapin A, Le Bot N, Moreno S, Sohrmann M, et al. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature 2003; 421:231-7; PMID:12529635; http://dx.doi.org/10.1038/nature01278
- Zhu F, Xu J, Palli R, Ferguson J, Palli SR. Ingested RNA interference for managing the populations of the Colorado potato beetle, Leptinotarsa decemlineata. Pest Manag Sci 2011; 67:175-82; PMID:21061270; http://dx.doi.org/10.1002/ps.2048
- La Fauce K, Owens L. Suppression of Penaeus merguiensis densovirus following oral delivery of live bacteria expressing dsRNA in the house cricket (Acheta domesticus) model. J Invertebr Pathol 2013; 112:162-5; PMID:23201454; http://dx.doi.org/10.1016/j.jip.2012.11.006
- Baum JA, Bogaert T, Clinton W, Heck GR, Feldmann P, Ilagan O, Johnson S, Plaetinck G, Munyikwa T, Pleau M, et al. Control of coleopteran insect pests through RNA interference. Nat Biotechnol 2007; 25:1322-6; PMID:17982443; http://dx.doi.org/10.1038/nbt1359
- Kumar P, Pandit SS, Baldwin IT. Tobacco rattle virus vector: A rapid and transient means of silencing manduca sexta genes by plant mediated RNA interference. PloS One 2012; 7:e31347; PMID:22312445; http://dx.doi.org/10.1371/journal.pone.0031347
- Folimonov AS, Folimonova SY, Bar-Joseph M, Dawson WO. A stable RNA virus-based vector for citrus trees. Virology 2007; 368:205-16; PMID:17651777; http://dx.doi.org/10.1016/j.virol.2007.06.038
- Belles X. Beyond Drosophila: RNAi in vivo and functional genomics in insects. Annu Rev Entomol 2010; 55:111-28; PMID:19961326; http://dx.doi.org/10.1146/annurev-ento-112408-085301
- Xue XY MY, Tao XY, Huang YP, Chen XY. New approaches to agricultural insect pest control based on RNA interference. In: Jockusch EL, ed. Advances in Insect Physiology. UK: Academic Press, 2012:73-117.
- Gordon KH, Waterhouse PM. RNAi for insect-proof plants. Nat Biotechnol 2007; 25:1231-2; PMID:17989682; http://dx.doi.org/10.1038/nbt1107-1231
- Kupferschmidt K. A lethal dose of RNA. Science 2013; 341:732-3; PMID:23950525; http://dx.doi.org/10.1126/science.341.6147.732
- Huang G, Allen R, Davis EL, Baum TJ, Hussey RS. Engineering broad root-knot resistance in transgenic plants by RNAi silencing of a conserved and essential root-knot nematode parasitism gene. Proc Natl Acad Sci U S A 2006; 103:14302-6; PMID:16985000; http://dx.doi.org/10.1073/pnas.0604698103
- Steeves RM, Todd TC, Essig JS, Trick HN. Transgenic soybeans expressing siRNAs specific to a major sperm protein gene suppress Heterodera glycines reproduction. Funct Plant Biol 2006; 33:991-9; http://dx.doi.org/10.1071/FP06130
- Escobar MA, Civerolo EL, Summerfelt KR, Dandekar AM. RNAi-mediated oncogene silencing confers resistance to crown gall tumorigenesis. Proc Natl Acad Sci U S A 2001; 98:13437-42; PMID:11687652; http://dx.doi.org/10.1073/pnas.241276898
- Shimizu T, Yoshii M, Wei T, Hirochika H, Omura T. Silencing by RNAi of the gene for Pns12, a viroplasm matrix protein of Rice dwarf virus, results in strong resistance of transgenic rice plants to the virus. Plant Biotechnol J 2009; 7:24-32; PMID:18761654; http://dx.doi.org/10.1111/j.1467-7652.2008.00366.x
- Simon-Mateo C, Garcia JA. Antiviral strategies in plants based on RNA silencing. Biochim Biophys Acta 2011; 1809:722-31; PMID:21652000; http://dx.doi.org/10.1016/j.bbagrm.2011.05.011
- Fuchs M, Gonsalves D. Safety of virus-resistant transgenic plants two decades after their introduction: lessons from realistic field risk assessment studies. Ann Rev Phytopathol 2007; 45:173-202; PMID:17408355; http://dx.doi.org/10.1146/annurev.phyto.45.062806.094434
- Gonsalves D. Transgenic papaya: development, release, impact and challenges. Adv Virus Res 2006; 67:317-54; PMID:17027684; http://dx.doi.org/10.1016/S0065-3527(06)67009-7
- Mao YB, Cai WJ, Wang JW, Hong GJ, Tao XY, Wang LJ, Huang YP, Chen XY. Silencing a cotton bollworm P450 monooxygenase gene by plant-mediated RNAi impairs larval tolerance of gossypol. Nat Biotechnol 2007; 25:1307-13; PMID:17982444; http://dx.doi.org/10.1038/nbt1352
- Mao YB, Xue XY, Tao XY, Yang CQ, Wang LJ, Chen XY. Cysteine protease enhances plant-mediated bollworm RNA interference. Plant Mol Biol 2013; 83:119-29; PMID:23460027; http://dx.doi.org/10.1007/s11103-013-0030-7
- Zhu JQ, Liu S, Ma Y, Zhang JQ, Qi HS, Wei ZJ, Yao Q, Zhang WQ, Li S. Improvement of pest resistance in transgenic tobacco plants expressing dsRNA of an insect-associated gene EcR. PloS One 2012; 7:e38572; PMID:22685585; http://dx.doi.org/10.1371/journal.pone.0038572
- Zha W, Peng X, Chen R, Du B, Zhu L, He G. Knockdown of midgut genes by dsRNA-transgenic plant-mediated RNA interference in the hemipteran insect Nilaparvata lugens. PloS One 2011; 6:e20504; PMID:21655219; http://dx.doi.org/10.1371/journal.pone.0020504
- Pitino M, Coleman AD, Maffei ME, Ridout CJ, Hogenhout SA. Silencing of aphid genes by dsRNA feeding from plants. PloS One 2011; 6:e25709; PMID:21998682; http://dx.doi.org/10.1371/journal.pone.0025709
- Mao J, Zeng F. Plant-mediated RNAi of a gap gene-enhanced tobacco tolerance against the Myzus persicae. Transgenic Res 2014; 23:145-52; PMID:23949691; http://dx.doi.org/10.1007/s11248-013-9739-y
- Guo H, Song X, Wang G, Yang K, Wang Y, Niu L, Chen X, Fang R. Plant-Generated Artificial Small RNAs Mediated Aphid Resistance. PloS One 2014; 9:e97410; PMID:24819752; http://dx.doi.org/10.1371/journal.pone.0097410
- Lundgren JG, Duan, J. J. RNAi-based insecticidal crops: Potential effects on nontarget species. Bioscience 2013; 63:657-65; http://dx.doi.org/10.1525/bio.2013.63.8.8
- Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 2005; 120:15-20; PMID:15652477; http://dx.doi.org/10.1016/j.cell.2004.12.035
- Lim LP, Lau NC, Garrett-Engele P, Grimson A, Schelter JM, Castle J, Bartel DP, Linsley PS, Johnson JM. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature 2005; 433:769-73; PMID:15685193; http://dx.doi.org/10.1038/nature03315
- Jackson AL, Bartz SR, Schelter J, Kobayashi SV, Burchard J, Mao M, Li B, Cavet G, Linsley PS. Expression profiling reveals off-target gene regulation by RNAi. Nat Biotechnol 2003; 21:635-7; PMID:12754523; http://dx.doi.org/10.1038/nbt831
- Jackson AL, Burchard J, Schelter J, Chau BN, Cleary M, Lim L, Linsley PS. Widespread siRNA “off-target” transcript silencing mediated by seed region sequence complementarity. RNA 2006; 12:1179-87; PMID:16682560; http://dx.doi.org/10.1261/rna.25706
- Birmingham A, Anderson EM, Reynolds A, Ilsley-Tyree D, Leake D, Fedorov Y, Baskerville S, Maksimova E, Robinson K, Karpilow J, et al. 3’ UTR seed matches, but not overall identity, are associated with RNAi off-targets. Nat Methods 2006; 3:199-204; PMID:16489337; http://dx.doi.org/10.1038/nmeth854
- Grimson A, Farh KK, Johnston WK, Garrett-Engele P, Lim LP, Bartel DP. MicroRNA targeting specificity in mammals: determinants beyond seed pairing. Mol Cell 2007; 27:91-105; PMID:17612493; http://dx.doi.org/10.1016/j.molcel.2007.06.017
- Aleman LM, Doench J, Sharp PA. Comparison of siRNA-induced off-target RNA and protein effects. RNA 2007; 13:385-95; PMID:17237357; http://dx.doi.org/10.1261/rna.352507
- Ishizu H, Siomi H, Siomi MC. Biology of PIWI-interacting RNAs: new insights into biogenesis and function inside and outside of germlines. Genes Dev 2012; 26:2361-73; PMID:23124062; http://dx.doi.org/10.1101/gad.203786.112
- Ui-Tei K. Optimal choice of functional and off-target effect-reduced siRNAs for RNAi therapeutics. Front Genet 2013; 4:107; PMID:23781232; http://dx.doi.org/10.3389/fgene.2013.00107
- Ma Y, Creanga A, Lum L, Beachy PA. Prevalence of off-target effects in Drosophila RNA interference screens. Nature 2006; 443:359-63; PMID:16964239; http://dx.doi.org/10.1038/nature05179
- Green EW, Fedele G, Giorgini F, Kyriacou CP. A Drosophila RNAi collection is subject to dominant phenotypic effects. Nat Methods 2014; 11:222-3; PMID:24577271; http://dx.doi.org/10.1038/nmeth.2856
- Fellmann C, Lowe SW. Stable RNA interference rules for silencing. Nat Cell Biol 2014; 16:10-8; PMID:24366030; http://dx.doi.org/10.1038/ncb2895
- Sato K, Siomi MC. Piwi-interacting RNAs: biological functions and biogenesis. Essays Biochem 2013; 54:39-52; PMID:23829526; http://dx.doi.org/10.1042/bse0540039
- Weitz JS, Wilhelm SW. Ocean viruses and their effects on microbial communities and biogeochemical cycles. F1000 Biol Rep 2012; 4-17; PMCID: PMC3434959; http://dx.doi.org/10.3410/B4-17
- Asgari S, Johnson KN. Insect Virology. Horizon Scientific Press, 2010
- Naito Y, Ui-Tei K. siRNA Design Software for a Target Gene-Specific RNA Interference. Front Genet 2012; 3:102; PMID:22701467; http://dx.doi.org/10.3389/fgene.2012.00102
