Abstract
Loss of E-cadherin has been long considered to be a major hallmark of epithelial-mesenchymal transition (EMT) and has been reported in various cancers. P120 catenin regulates E-cadherin stability on the cell surface and also plays a role in intracellular signaling by modulating nuclear transcription. We recently characterized the nature of interactions between p120 catenin and Mucin 1 (MUC1) in pancreatic cancer. Expression of different p120 catenin isoforms with and without MUC1 induced distinct morphologies, cell adhesion, and dynamic properties of motility along with different metastatic properties in vivo. Re-expression of p120 catenin isoform 3A in the context of MUC1 expression in a p120 catenin-deficient cell line stabilized expression of E-cadherin. However, orthotopic implantation of tumors using this stable cell line produced large metastatic lesions to the liver, which exceeded the volume of the primary tumor, suggesting down regulation of E-cadherin is not required for tumor metastasis. Here we extend those studies by showing that ectopic expression of E-cadherin does not block in vitro invasion of the pancreatic cancer cells, and instead accelerated the rate of tumor invasion. Furthermore, results from 23 cases of human pancreatic primary tumor specimens revealed that most tumors exhibiting metastatic activity retained epithelial morphology and E-cadherin gene expression. Our results indicate that loss of E-cadherin and EMT are not required for metastasis and that an epithelial morphology can be maintained during the process of tumor cell movement.
Introduction
How cell movements occur within a cohesive tissue while maintaining cell-cell contact and barrier functions, a question that is of central importance in developmental, cellular and cancer biology, remains poorly understood.Citation1,2 The recent dogma of epithelial to mesenchymal transition (EMT) posits that tumor cells undertaking movement within tissues transiently down-regulate the adhesion molecule E-cadherin, perhaps substituting expression of another cadherin such as N-cadherin, which allows the cell to dissociate from neighbors and migrate through tissue, perhaps to lymphatic or blood vessels and on to a new location.
The process of EMT has been proposed to allow immotile epithelial cells with established cellular polarity to dissociate from each other and undergo a morphological transformation into cells with a fibroblast-like mesenchymal shape in order to engage in a program of cell motility that impacts cell migration. EMT controls morphogenetic events during embryonic development and contributes to gastrulation and tissue repair.Citation3 Recently, it was proposed that EMT occurs during tissue injury and is likely to promote cancer invasion and metastasis.Citation4 Thus, loss of E-cadherin is considered by some a sine qua non for EMT that precedes metastasis in human cancers, resulting in loss of epithelial morphology and gain of metastatic potential of the cells. Numerous studies on EMT have presented in vitro results and experiments in mouse models supporting this concept; however, strong evidence from studies of human tumor specimens to support this concept is currently lacking.Citation5,6
We recently reportedCitation7 that epithelial appearance, cell adhesion and motility of pancreatic cancer cells are not strictly limited by E-cadherin status at the cell surface. Instead, we showed that cells expressing high levels of stabilized E-cadherin at the surface exhibited a range of patterns of motility within monolayer cultures while maintaining E-cadherin-based adhesions to other cells. Stabilization of E-cadherin was observed upon expression of 2 structural-signaling molecules, p120 catenin (ctn) and MUC1, in a pancreatic tumor cell line (S2013) that was deficient in p120 catenin and low in expression of MUC1.Citation7 P120 catenin is known to stabilize E-cadherin at the surface of epithelial cells.Citation8 Given that the S2103 cells appear generally mesenchymal in culture, it was not surprising that we observed an increase in steady state E-cadherin localized to the cell surface, and a higher degree of epithelial cell morphology in this cell line upon expression of all 4 major isoforms of P120 ctn (whether or not MUC1 was co-expressed). Moreover, expression of combinations of different isoforms of p120 catenin and MUC1 stabilized E-cadherin and created cells that exhibited distinct and active patterns of motility in culture (motility independent of cell adhesion, motility within a monolayer while exchanging contacts with other cells, and unified motility while maintaining static epithelial contacts). Our publishedCitation7 time-lapse video microscopy revealed that the epithelial-appearing cells re-expressing p120 catenin 1A, 3A and 4A exhibited increased local motility of cells within monolayer cultures. These cells migrated within the monolayer and simultaneously exchanged membrane contacts with some cells while maintaining cell-cell adhesion. Expression of MUC1 (but not p120 catenin) increased local cell motility and the rate at which cells dissociated from the monolayer; however, expression of MUC1 in the context of all p120 catenin isoforms enforced an epithelial appearance on the cells commensurate with stabilizing high levels of E-cadherin. Simultaneous expression of p120 catenin isoform 4A and MUC1 enforced a remarkable highly epithelial morphology that showed little exchange of cellular contacts, but at the same time showed growth and motility in a unified, unidirectional manner that resembled collective cell migration.
Interestingly, although expression of p120 catenin and consequent stabilization of E-cadherin was predicted to decrease tumor growth and metastasis, instead we observed enhanced tumor growth properties and alterations in the pattern of metastatic spread. We attributed this to the possibility that different complexes formed by p120 ctn isoforms and MUC1 produce both structural effects at the cell surface and concomitant signaling events that contribute to localized motility, cell survival and other cell activities, given that we also observed stabilization of cytoplasmic and nuclear β catenin in these cells.
In a separate report,Citation9 we went on to explore the influence of MUC1 and p120 catenin on Wnt signaling through β catenin. Our results showed that in addition to stabilizing E-cadherin and β catenin, MUC1 and p120 catenin together caused significant effects on the transcription of Wnt target genes, including cyclin D1, which were distinct in the absence of either p120 catenin or MUC1. For cyclin D1, MUC1 and p120 catenin bound to and stabilized β catenin in the cell and at the cyclin D1 promoter; they also bound and caused de-repression of Kaiso, a transcriptional repressor known to be targeted by p120 catenin,Citation10 which together led to high levels of transcription of cyclin D1. High activity of the cyclin D1 promoter and elevated cyclin D1 protein also significantly increased cell proliferation.Citation9 These results demonstrate that the net effects of morphogenetic signaling in epithelial cells are determined by complexes of signaling proteins, which together integrate signals from different aspects of the cellular environment, differentiation status, and other cellular characteristics. The integration of these signals are important in directing the cell to undertake programs of survival, proliferation, motility, or metastasis. Thus, evaluation of expression of single markers such as E-cadherin, or even selected markers that are related to programs of EMT, are unlikely to fully reflect the invasive status of any given cell.
Results
Most pancreatic tumors express E-cadherin and exhibit widespread metastatic behavior
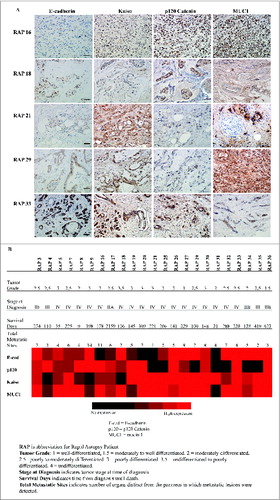
Given our observation that stabilization of E-cadherin did not adversely affect cell motility within monolayers of cell lines, and the importance of complexes of Kaiso/p120 ctn/MUC1 in modulating activity of the WNT signaling pathway and downstream effects on transcription, we elected to perform a comprehensive analysis of expression of p120 ctn, Kaiso, MUC1 and E-cadherin expression and localization in 23 cases of primary pancreatic cancer for which we had information on the metastatic spread of the primary tumor at time of death (from performing rapid autopsies on these cases). A principal purpose of this analysis was to evaluate the incidence of loss of E-cadherin and/or p120 ctn in human pancreatic tumors and to correlate expression of these to formation of metastatic tumors (). A secondary goal was to determine if there were correlations between metastatic activity and expression of p120 ctn, Kaiso, and MUC1. Based on the result ( and 1B), there was no correlation between expression of E-cadherin and either epithelial morphology (tumor differentiation status as assessed by tumor grade) or degree of metastatic spread. Only 17.4% (4 out of 23) of cases showed low to no expression of E-cadherin in primary pancreatic tumors, and only 2 cases (16 and 20) that lost E-cadherin expression showed evidence of metastasis to multiple sites. Only one instance (Case 21) was found to have lost both E-cadherin and p120 ctn, and this case did not show extensive metastasis (one extrapancreatic site). Case 31, which did not show expression of E-cadherin, p120 catenin or MUC1, showed evidence of metastasis to 4 sites and exhibited an extremely short survival time for 21 d post-diagnosis (). However, most cases showed abundant expression of adhesion molecule E-cadherin and p120 ctn, and these patients exhibit evidence of abundant metastasis (2–14 extrapancreatic sites). Thus, we conclude that there is no causal relationship between loss of expression of E-cadherin, p120 ctn or Kaiso and formation of metastatic pancreatic tumors. There was also no correlation between nuclear Kaiso staining and histological grading of tumor differentiation status in this invasive cancer cohort. Interestingly, patient 17 had a moderately differentiated tumor, median levels expression of p120 catenin, E-cadherin, MUC1 and Kaiso showed evidence of metastasis to 6 sites, but was along a term survivor 2159 d (). Overall and consistent with previous reports, we found that virtually all (22 out 23) of the metastatic pancreatic cancer cases expressed MUC1.
Figure 1. Immunohistochemistry analysis of E-cadherin, p120 catenin, Kaiso and MUC1 in patient samples with metastatic pancreatic cancer. (A) Representative immunohistochemical images of primary pancreatic tumor tissue from patients with metastatic pancreatic cancer, stained for E-cadherin, p120 catenin, Kaiso, and MUC1. Scale bar, 5 μm. (B) Heat map shows the relative expression levels of each antigen analyzed in samples from 23 rapid autopsies of individual patients as detected by immunohistochemistry, with relative expression levels indicated as described previously.Citation25 Patient samples are annotated for tumor grade, stage and survival post-diagnosis.
Ectopic expression of E-cadherin enhances in vitro invasion of tumor cells
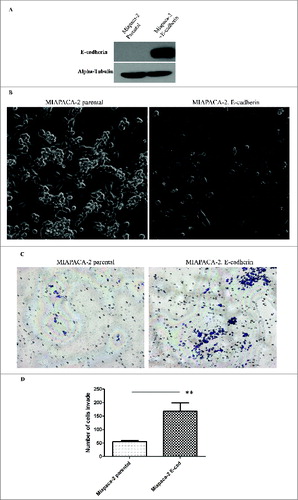
To further study the effect of overexpression of adhesion molecule E-cadherin on cell invasive properties, we examined potential of ectopically expressed E-cadherin to influence the invasive properties of the cell line Miapaca-2 (). We performed in vitro matrigel Boyden chamber assay and counted the invaded cells on the bottom of the Boyden chamber membrane after 36 hours. As compared to Miapaca-2 parental cells, which do not express detectable E-cadherin, Miapaca-2 cells with overexpression of E-cadherin showed a significantly higher degree of invasion through matrigel (). This result indicates that overexpression of E-cadherin failed to impede tumor cell invasion in the progression to metastasis. Instead, overexpression of E-cadherin in Miapaca-2 cells increased the invasive potential of the cells.
Figure 2. Ectopic expression of E-cadherin increases the Epithelial look of the cell but does not prevent in vitro invasion of tumor cells. (A) Western blot showing the expression level of E-cadherin in Miapaca-2 parental cells VS Miapaca-2 with E-cadherin overexpression. (B) Phase contrast photographs of control MIAPACA-2 cells and E-cadherin overexpressing MIAPACA-2 cells. (C) In vitro invasion assay of Miapaca-2 cells with/without E-cadherin. Experiment results are from 3 triplicates of 3 independent experiments. ** indicates the statistical analysis of the result shows significant difference. P < 0.01.
Discussion
Two of our recent publications and data presented here provide evidence that epithelial appearance, cell adhesion and E-cadherin status at the cell surface of pancreatic cancer cells do not predict disease progression to metastasis. In a few cases, immunohistochemical staining in patient samples show permanent or sustained loss of E-cadherin. The complete loss of expression of adhesion proteins such as E-cadherin within a tumor tissue may be caused by genetic or epigenetic alterations. Such loss of expression of adhesion and/or morphogenetic signaling molecules in tumors is distinct from the posited transient loss of E-cadherin during EMT, a concept generated from in vitro experiments where loss of E-cadherin is transient, followed by a reversal of the process, called mesynchemal to epithelial transition (MET), wherein cells re-express E-cadherin. Epithelial to mesenchymal transition is postulated to be initiated by loss of E-cadherin expression; however, whether or not loss of E-cadherin is a cause or consequence of EMT is controversial.Citation11 Taken together, our data suggest that expression of E-cadherin, which is predicted to be associated with EMT, does not predict either invasive behavior or metastatic phenotype.
Numerous studies have attempted to evaluate the expression levels of EMT markers including E-cadherin, vimentin, and regulatory transcription factors that are known to repress E-cadherin expression including snail, slug, and twist as a way to translate observations from in vitro experiments to human cancers.Citation12 Expression of these factors are predicted to be a harbinger the onset of EMT. However, these markers of EMT are also markers for the differentiation status of the cell, or they may represent maintenance of a stem cell state rather than a reflection of epithelial to mesenchymal transition and as such are likely reflective of biological processes other than EMT.Citation13,14
Histologic grading of most tumors incorporates grade (degree of differentiation), reflected by cell morphology and tissue architecture.Citation5 Given the high degree of heterogeneity of cell types within tumors and the overall configuration of the tumor microenvironment, cells that are deep within the tumor as opposed to those at the invasive edge may have different gene expression signatures and invasive or metastatic potential; however, it is also possible that single cells or small groups of cells from anywhere in a tumor may independently undertake invasive and metastatic behavior. The latter of these processes in which groups of cells move through tissues, termed collective epithelial migration, plays a key role in developmental processes; and is believed to contribute to tumor progression.Citation15 To undertake metastasis cancer cells must have high motility and the capacity to survive a journey that may include migration through different tissues, vascular or lymphatic invasion, and recolonization. There is longstanding evidence that the success rate of single circulating tumor cells to metastasize is very low.Citation16,17 There is evidence that carcinomas invade and metastasize through lymphatic vessels and blood vessels by collective/cohesive epithelial migration, in which grouped cells retain their cell-cell junctions and cell-matrix connections and move together.Citation18,19 Analysis of circulating tumor cells from blood samples and surgically removed samples often reveal circulating tumor cell clusters in addition to single migratory cells.Citation20 Circulating or migrating tumor cells need to undertake survival programs during and after migration through blood, lymphatic vessels or other conduits, followed by the need to proliferate and often reconfigure the microenvironment in a new location.Citation21 By what molecular mechanism does stabilization of E-cadherin influence migration? Collective migration that includes stabilized E-cadherin at cellular junctions, may provide increased survival signals or it may offer the capacity to maintain important signaling pathways that affect the viability and metastatic capacity of circulating tumor cell clusters as compared to single dissociated cells.Citation2,22 In addition, there is recent published evidence that E-cadherin contributes to mechanical sensing by groups of cells in a manner that assists in guidance mechanisms during collective migration or chemotaxis.Citation23
In summary, metastasis causes most cancer-related deaths. Loss of E-cadherin and EMT are not required for cell motility, invasion or metastasis in pancreatic cancer. The accumulation of genetic and epigenetic alterations, interactions with the microenvironment and activation of cellular programs that support motility, survival and replication are required for tumor cell metastasis. Collective epithelial migration of grouped cells that retain E-cadherin expression and cell-cell junctions is likely to be the major form of cancer cell migration that is understudied. Future studies of tumor cell invasive and metastatic properties should investigate aspects of cytoskeleton rearrangement, cell motility and maintenance of survival and proliferative cellular programs by groups of cells.
Material and Methods
Materials. Anti-MUC1 cytoplasmic tail antibody CT-2 was provided by Dr. Sandra Gendler (Mayo Clinic, Scottsdale, AZ). Rabbit polyclonal anti-p120 H-90 and anti-Kaiso were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-E-cadherin antibody was provided by Dr. Keith Johnson (University of Nebraska Medical Center, Omaha, Nebraska). Pancreatic tumor specimens were obtained with consent and Institutional Review Board (IRB) approval from surgically resected samples or from decedents through the Rapid Autopsy Program at the University of Nebraska Medical Center. All immunohistochemistry experiments were conducted using Dako EnVision kits (Carpinteria, CA).
Cell culture. Miapaca-2 cells were maintained in Dulbecco's Modified Eagle Medium (DMEM, Life Technologies, Inc.., Rockville, MD) supplemented with 10% heat-inactivated fetal bovine serum (FBS, Valley Biomedical, Winchester, VA), 1% penicillin/streptomycin (P/S), (Biowhittaker, Walkersville, MD) and 250 μg/ml G418.
In vitro invasion assay
Tumor cell invasion assays were performed according to previously described methods.Citation24 Briefly, 2×105 of Miapaca-2 cells (both parental and E-cadherin overexpression) were seeded with serum free medium on Matrigel-coated chamber and incubated for 36 hrs at 37°C. The chamber was immersed in 10% serum containing medum in a 24 well plate. After 36 hrs, noninvading cells on the upper surface of the matrigel-coated membrane were removed with cotton swabs. Cells that invaded through the membrane onto the other side of the membrane were fixed and stained with Diff-Quick cell stain kit (Siemens Healthcare Diagnostic). Cells that transversed the membrane were counted.
Immunohistochemistry
We obtained specimen slides of 4-μm sections with formalin-fixed tissues from rapid autopsies. These specimens are all pancreatic primary tumor from 23 different deceased patients. We stained the tissue by using the methods as described previously.Citation25 Briefly, tissue were deparaffinized with xylene and rehydrated with a decreased gradient of alcohol. Antigen retrieval was performed with boiled 10 mM citrate buffer (pH6) for 10 mins. Add 3% H2O2 to tissue samples for no more than 5 min. Then wash with PBS. Slides were blocked with 1–5% bovine serum albumin. Incubate the slides with primary antibodies and secondary antibodies. Add DAB substrate to tissue samples. Immerse slides into counter stain solution (Hematoxylin Dye) and dehydration with an increasing alcohol gradient ending with xylene. Seal the slides with mounting media.
Tissue analysis and scoring of Immunohistochemistry
Histologic sections were annotated by 2 independent pathologists. Tumor differentiation grade at autopsy were scored 0–4. 0 = undetermined. 1 = well-differentiated, 1.5 = moderately to well differentiated. 2 = moderately differentiated. 2.5 =poorly to moderately differentiated. 3 = poorly differentiated. 3.5 = undifferentiated to poorly differentiated. 4 = undifferentiated. Relative antigen expression levels were semiquantified on the basis of the percentage of cells of the same cell type staining positive for each antigen.Citation25 A scale of 0 to 3 was used to indicate the relative percentage of cells positive, with 0 being no detectable expression and 3 indicating that 67% or more of the total cell population expressed the antigen. Scores were converted into heat maps for better visualization. Pictures were taken using a Nikon Eclipse 90i Microscope.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
Funding was provided by the National Cancer Institute of the National Institutes of Health with the following grants: CA57362, CA127297, CA111294, CA72712, CA116199, CA098258, CA36727, and CA09476.
References
- Pilot F, Lecuit T. Compartmentalized morphogenesis in epithelia: from cell to tissue shape. Dev Dyn 2005; 232:685-94; PMID:15712202; http://dx.doi.org/10.1002/dvdy.20334
- Ewald AJ, Brenot A, Duong M, Chan BS, Werb Z. Collective epithelial migration and cell rearrangements drive mammary branching morphogenesis. Dev Cell 2008; 14:570-81; PMID:18410732; http://dx.doi.org/10.1016/j.devcel.2008.03.003
- Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell 2009; 139:871-90; PMID:19945376; http://dx.doi.org/10.1016/j.cell.2009.11.007
- Yang J, Weinberg RA. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell 2008; 14:818-29; PMID:18539112; http://dx.doi.org/10.1016/j.devcel.2008.05.009
- Chui MH. Insights into cancer metastasis from a clinicopathologic perspective: Epithelial-Mesenchymal Transition is not a necessary step. Int J Cancer 2013; 132:1487-95; PMID:22833228; http://dx.doi.org/10.1002/ijc.27745
- Steinestel K, Eder S, Schrader AJ, Steinestel J. Clinical significance of epithelial-mesenchymal transition. Clin Transl Med 2014; 3:17; PMID:25050175; http://dx.doi.org/10.1186/2001-1326-3-17
- Liu X, Yi C, Wen Y, Radhakrishnan P, Tremayne JR, Dao T, Johnson KR, Hollingsworth MA. Interactions between MUC1 and p120 catenin regulate dynamic features of cell adhesion, motility, and metastasis. Cancer Res 2014; 74:1609-20; PMID:24371222; http://dx.doi.org/10.1158/0008-5472.CAN-13-2444
- Daniel JM, Reynolds AB. The catenin p120(ctn) interacts with Kaiso, a novel BTB/POZ domain zinc finger transcription factor. Mol Cell Biol 1999; 19:3614-23; PMID:10207085
- Liu X, Caffrey TC, Steele MM, Mohr A, Singh PK, Radhakrishnan P, Kelly DL, Wen Y, Hollingsworth MA. MUC1 regulates cyclin D1 gene expression through p120 catenin and beta-catenin. Oncogenesis 2014; 3:e107; PMID:24979278; http://dx.doi.org/10.1038/oncsis.2014.19
- Vermeulen JF, van de Ven RA, Ercan C, van der Groep P, van der Wall E, Bult P, Christgen M, Lehmann U, Daniel J, van Diest PJ, et al. Nuclear Kaiso expression is associated with high grade and triple-negative invasive breast cancer. PLoS One 2012; 7:e37864; PMID:22662240; http://dx.doi.org/10.1371/journal.pone.0037864
- Nilsson GM, Akhtar N, Kannius-Janson M, Baeckstrom D. Loss of E-cadherin expression is not a prerequisite for c-erbB2-induced epithelial-mesenchymal transition. Int J Oncol 2014; 45:82-94; PMID:24807161
- Hugo HJ, Kokkinos MI, Blick T, Ackland ML, Thompson EW, Newgreen DF. Defining the E-cadherin repressor interactome in epithelial-mesenchymal transition: the PMC42 model as a case study. Cells Tissues Organs 2011; 193:23-40; PMID:21051859; http://dx.doi.org/10.1159/000320174
- Korita PV, Wakai T, Ajioka Y, Inoue M, Takamura M, Shirai Y, Hatakeyama K. Aberrant expression of vimentin correlates with dedifferentiation and poor prognosis in patients with intrahepatic cholangiocarcinoma. Anticancer Res 2010; 30:2279-85; PMID:20651380
- Rukstalis JM, Habener JF. Snail2, a mediator of epithelial-mesenchymal transitions, expressed in progenitor cells of the developing endocrine pancreas. Gene Expr Patterns 2007; 7:471-9; PMID:17185046; http://dx.doi.org/10.1016/j.modgep.2006.11.001
- Liotta LA, Saidel MG, Kleinerman J. The significance of hematogenous tumor cell clumps in the metastatic process. Cancer Res 1976; 36:889-94; PMID:1253177
- Fidler IJ. Biological behavior of malignant melanoma cells correlated to their survival in vivo. Cancer Res 1975; 35:218-24; PMID:1109790
- Fidler IJ, Talmadge JE. Evidence that intravenously derived murine pulmonary melanoma metastases can originate from the expansion of a single tumor cell. Cancer Res 1986; 46:5167-71; PMID:3756870
- Ruiter DJ, van Krieken JH, van Muijen GN, de Waal RM. Tumour metastasis: is tissue an issue? Lancet Oncol 2001; 2:109-12; PMID:11905791; http://dx.doi.org/10.1016/S1470-2045(00)00229-1
- Yu M, Bardia A, Wittner BS, Stott SL, Smas ME, Ting DT, Isakoff SJ, Ciciliano JC, Wells MN, Shah AM, et al. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science 2013; 339:580-4; PMID:23372014; http://dx.doi.org/10.1126/science.1228522
- Aceto N, Bardia A, Miyamoto DT, Donaldson MC, Wittner BS, Spencer JA, Yu M, Pely A, Engstrom A, Zhu H, et al. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell 2014; 158:1110-22; PMID:25171411; http://dx.doi.org/10.1016/j.cell.2014.07.013
- Tarin D, Price JE, Kettlewell MG, Souter RG, Vass AC, Crossley B. Mechanisms of human tumor metastasis studied in patients with peritoneovenous shunts. Cancer Res 1984; 44:3584-92; PMID:6744281
- Rorth P. Collective cell migration. Annu Rev Cell Dev Biol 2009; 25:407-29; PMID:19575657; http://dx.doi.org/10.1146/annurev.cellbio.042308.113231
- Cai D, Chen SC, Prasad M, He L, Wang X, Choesmel-Cadamuro V, Sawyer JK, Danuser G, Montell DJ. Mechanical feedback through E-cadherin promotes direction sensing during collective cell migration. Cell 2014; 157:1146-59; PMID:24855950; http://dx.doi.org/10.1016/j.cell.2014.03.045
- Radhakrishnan P, Grandgenett PM, Mohr AM, Bunt SK, Yu F, Chowdhury S, Hollingsworth MA. Expression of core 3 synthase in human pancreatic cancer cells suppresses tumor growth and metastasis. Int J Cancer 2013; 133:2824-33; PMID:23754791
- Remmers N, Anderson JM, Linde EM, DiMaio DJ, Lazenby AJ, Wandall HH, Mandel U, Clausen H, Yu F, Hollingsworth MA. Aberrant expression of mucin core proteins and o-linked glycans associated with progression of pancreatic cancer. Clin Cancer Res 2013; 19:1981-93; PMID:23446997; http://dx.doi.org/10.1158/1078-0432.CCR-12-2662
