Abstract
The proteome in all cells is manufactured via the intricate process of translation by multimolecular factories called ribosomes. Nevertheless, these ribonucleoprotein particles, the largest of their kind, also have an elaborate assembly line of their own. Groundbreaking discoveries that bacterial ribosomal subunits can be self-assembled in vitro jumpstarted studies on how ribosomes are constructed. Until recently, ribosome assembly has been investigated almost entirely in vitro with bacterial small subunits under equilibrium conditions. In light of high-resolution ribosome structures and a more sophisticated toolkit, the past decade has been defined by a burst of kinetic studies in vitro and, importantly, also a shift to examining ribosome maturation in living cells, especially in eukaryotes. In this review, we summarize the principles governing ribosome assembly that emerged from studies focusing on ribosomal proteins and their interactions with rRNA. Understanding these paradigms has taken center stage, given the linkage between anomalous ribosome biogenesis and proliferative disorders.
Introduction
Ribosomes are integral players in linking genotypes to phenotypes by manufacturing the proteome in any given cell. Each of these molecular machines is composed of 2 structural units, forming a megadalton-scale multi-protein/RNA complex. The small ribosomal subunit (30S in prokaryotes and 40S in eukaryotes) is the decoder that reads the genetic blueprint encoded in mRNAs. The large ribosomal subunit (50S in prokaryotes and 60S in eukaryotes) is the protein generator that catalyzes peptide bond formation through its peptidyltransferase ribozyme activity. In the past decade, immense progress has been achieved to elucidate atomic-resolution structures of ribosomal subunits and whole ribosomes spanning all domains of life.Citation1-10 Ribosomes contain 50–80 ribosomal proteins (r-proteins) and up to 6 rRNA species that are assembled into a universally conserved core structure, albeit with variable composition and increased complexity in eukaryotes.Citation11 A unified nomenclature for ribosomal proteins belonging to the same family was recently proposed.Citation12 In the yeast, Saccharomyces cerevisiae, the small subunit is composed of 33 r-proteins and an 18S rRNA, while the large subunit contains 46 r-proteins and 3 rRNA species (5S, 5.8S, and 25S rRNAs). R-proteins are mostly organized on the solvent-surface of the ribosome, but they also harbor long extensions that penetrate deep into the rRNA core. Interestingly, the intersubunit interface where the functional activities reside is mostly devoid of r-proteins, strongly indicating that rRNA is a key player in the decoding and catalytic capabilities of the ribosome. Each ribosomal subunit has characteristic structural landmarks: the small subunit displays a body, platform, head, and beak, while the large subunit has a more massive, rounded body, with central and lateral protuberances (acidic stalks) (, bottom panel). Ribosome structures have revealed detailed insights into the mechanism of translation.Citation13-15 Importantly, given the exquisitely complex structures of these ribonucleoprotein (RNP) nanomachines and their intimate connection to cell growth, it has become increasingly important to understand how the individual r-protein and rRNA components come together to assemble the polypeptide factories of the cell.
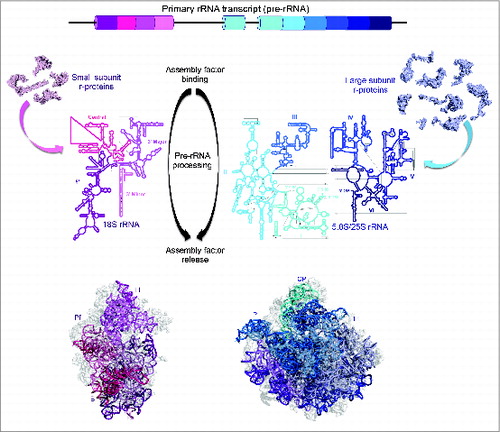
Figure 1. Overview of ribosome biogenesis. (Top) Mature rRNA sequences (cylinders) are transcribed as part of a long primary transcript (pre-rRNA) and are separated by spacer sequences (lines). (Middle) Mature rRNA sequences are produced by removal of the spacer sequences through a series of pre-rRNA processing steps. Ribosome assembly is facilitated by co-transcriptional binding of ribosomal proteins as well as the association and dissociation of trans-acting assembly factors. In yeast, mature 18S and 5.8S/25S rRNAs are divided into 4 and 6 secondary structure domains, respectively. (Bottom) These domains fold up into tertiary structures to form mature ribosomal subunits. The small subunit has characteristic body (B), platform (Pf), and head (H) substructures while the large subunit has a larger rounded body, a central protuberance, the L1 stalk (L1), and the phospho-stalk (P).
Heroic efforts of Nomura, Nierhaus, and colleagues pioneered investigations on ribosome assembly long before the first ribosome structures were solved. They showed that functional ribosomal subunits of E. coli could be reconstituted in vitro using purified r-proteins and rRNAs.Citation16-21 Since then, assembly of these RNP complexes has been extensively studied in vitro, mostly with the 30S ribosomal subunits because the 50S subunit is inherently more complex.Citation22 It is now clear that ribosome assembly proceeds via a series of conformational changes in rRNA that are coupled with binding of ribosomal proteins. In both prokaryotic and eukaryotic cells, however, these events occur alongside transcription of the primary rRNA transcript (pre-rRNA) from which spacer sequences are removed by irreversible nucleolytic events to generate mature rRNAs. In prokaryotes these pre-rRNA processing steps are not compartmentalized. In contrast, processing of pre-rRNAs in eukaryotes is spatially regulated. In yeast, for example, transcription of the primary transcript and initial stages of assembly, including the generation of separate precursors for 18S and 5.8S/25S rRNAs, take place in the nucleolus. Downstream pathways of pre-rRNA processing and subunit maturation occur in the nucleoplasm and cytoplasm (). Furthermore, coordinated association and dissociation of approximately dozens (in bacteria) up to several hundred (in eukaryotes) trans-acting RNA and protein assembly factors also play a role in the complex process of ribosome biogenesis ().Citation23-26
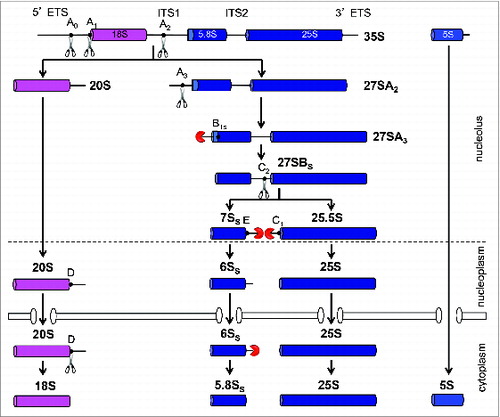
Figure 2. Pre-rRNA processing pathway in the yeast, Saccharomyces cerevisiae. Mature 18S, 5.8S, and 25S rRNAs are contained in the 35S pre-rRNA that is transcribed by RNA Polymerase I. The precursor for 5S rRNA resides on the same rDNA locus but is independently transcribed by RNA polymerase III. Sequential exo- and endonucleolytic events at the indicated processing sites remove the spacer sequences to generate mature rRNAs. The pathway for the maturation of large ribosomal subunit rRNA was simplified by excluding the alternative minor pathway that generates the longer 5.8S rRNA species.Citation24 Note that the A0, A1, and A2 sites are primarily cleaved co-transcriptionally in rapidly dividing cells.Citation82,113
Constructing ribosomes is the single most expensive metabolic process for cells and is essential to maintain cellular homeostasis.Citation27 A rapidly growing bacterial cell requires 20,000 ribosomes Citation28 and a dividing mammalian cell requires as many as 10 million.Citation29 It is therefore paramount that ribosome biogenesis is highly efficient and stringently regulated to produce accurately functioning ribosomes, given its tight linkage with cell growth and proliferation. In metazoans, haploinsufficiency or partial loss-of-function mutations in r-protein genes result in developmental abnormalities, hypoproliferative diseases collectively called ribosomopathies.Citation30-33 Mammalian r-protein genes regulate and are regulated by the Myc oncogene and the P53 tumor suppressor. Thus it is not surprising that a number of these mutations also lead to increased cancer predisposition.Citation34 One well-documented ribosome disorder is Diamond-Blackfan anemia, characterized by bone marrow failure.Citation35 The majority of mutations linked with Diamond-Blackfan anemia map to r-proteins, which often are associated with defects in the formation of mature ribosomal subunits. The longstanding conundrum of how early onset ribosomopathies are manifested by cellular hypoproliferation, but evolve into cancerous hyperproliferation has recently been addressed.Citation36 Mutations that suppress ribosome biogenesis defects nevertheless compromise the fidelity of aberrant ribosomes that escape into the translation pool, resulting in an abnormal gene expression profile, eventually leading to cancer.
This review aims to summarize the current general mechanisms by which ribosomes assemble. With the advent of high-resolution ribosome structures as well as the emergence of robust structural, biophysical, and biochemical techniques to survey assembly intermediates,Citation37-42 the field has seen a renaissance in the investigation of how binding of r-proteins with rRNA drives the construction of ribosomal subunits. The intricate structural data that have emerged in the past 15 y provide a strategic foundation for more comprehensive investigations of how r-proteins shape the assembly of ribosomal subunits. Lately, there has been a surge in research with respect to the functions of r-proteins in the formation of ribosomes in vivo. We will therefore discuss the principles that emerged from studies that focused on r-proteins and their interaction with rRNAs. The field has certainly taken significant strides to better understand how these individual parts are assembled together to create nature's complex molecular machine that is the ribosome.
First things first: Hierarchy and cooperativity in ribosome assembly
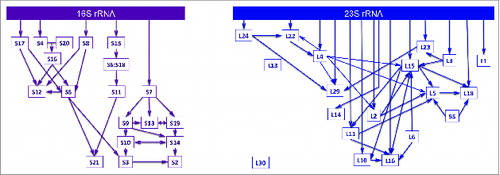
The elegance of being able to reassemble bacterial ribosomal subunits in vitro was reinforced when individual r-proteins were systematically excluded during sequential reconstruction experiments. By examining the interdependencies of r-protein binding with mature rRNA at equilibrium, assembly maps for the individual ribosomal subunits were generated.Citation18,21 The classic Nomura and Nierhaus assembly maps for the 30S and 50S subunits, respectively, remain largely unchanged almost 50 y after their initial discovery (). In general, r-proteins can be grouped into 3 classes based on their order of binding: primary binding r-proteins bind independently and directly to rRNA, secondary binding r-proteins require prior association of primary binders, and tertiary binding r-proteins require the presence of primary and secondary binders. This thermodynamic hierarchy of r-protein binding results in cooperativity during ribosome assembly.Citation43 The ability to resolve rRNA structures by equilibrium chemical probing and hydroxyl radical footprinting has been a powerful tool to investigate how binding of r-proteins stimulates assembly in vitro.Citation44-47 These techniques enable one to assay rRNA conformations in the presence or absence of r-proteins. Early in ribosome assembly, autonomous folding of rRNA creates a platform for binding of primary r-proteins. R-protein-induced conformational changes in rRNA provide the binding site for secondary binders, which then rearrange the RNP particle to accommodate the tertiary binding r-proteins. Hence, an alternating cascade of rRNA folding and r-protein binding events is initiated in a cooperative manner. Recently, a stable isotope labeling approach combined with mass spectrometry to monitor the incorporation of r-proteins with assembling bacterial ribosomes has demonstrated that the hierarchical assembly maps in vitro are fundamentally similar to those in vivo.Citation48
Figure 3. Hierarchy in ribosome assembly. In vitro assembly maps for the small (left) and large (right) ribosomal subunits in E. coli. The Nomura small subunit map has been modified based on the recent in vivo work by Williamson and colleagues.Citation48 The simplified Nierhaus large subunit assembly map was adapted from FoxCitation114 to show only universally conserved ribosomal proteins.
In the ribosome, there are significantly fewer interactions among r-proteins than between r-proteins and rRNA. Thus, r-protein-induced changes in rRNA structure play an important role in the cooperative nature of ribosome assembly. rRNAs can be divided into different secondary structure domains, which fold up into tertiary structures observed in mature ribosomal subunits (, middle and bottom panel). The small subunit rRNA contains 4 major domains that largely correspond to its structural landmarks: the 5′ rRNA domain is involved in the formation of the body of the small subunit, the central domain corresponds to the platform and a fraction of the body, the 3′ major domain comprises the head region, and the 3′ minor domain forms part of the small subunit body structure. The large subunit rRNA, on the other hand, is composed of 6 secondary structure domains (domains I-VI, from 5′ to 3′), which fold into a more monolithic 3-dimensional structure where the domains are not as distinctly segregated into the known structural landmarks as they are in the small subunit.
Using time-resolved RNA structure probing to assay the kinetics of RNA folding upon r-protein binding, the laboratories of Woodson and Hill showed how a pre-organized rRNA tertiary structure allows binding of an r-protein, which in turn reconfigures RNA architecture.Citation49,50 It is remarkable that naked 16S rRNA or its individual domains can fold independently to near-native structure in the absence of r-proteins.Citation51-53 This has also been recently shown for 23S rRNA.Citation54 However, these rRNA structures are unstable and some tertiary interactions are Mg2+-dependent, requiring higher than physiological concentrations to achieve a stable final form. The addition of r-proteins facilitates folding at lower Mg2+ concentrations; thus, r-proteins are required to stabilize RNA structure. Upon contact with an r-protein, the rRNA undergoes a structural change that stabilizes the association of that r-protein and creates a binding pocket for another r-protein – a so-called mutually induced fit mechanism.Citation49,50,55 Binding of an r-protein can have both proximal and allosteric effects on the structure and stability of RNA-RNA and RNA-protein interactions.Citation56-59 For example, the stabilizing effect of primary-binding S4 and S17 on rRNA tertiary structure is not only localized, but also extends throughout the entire 16S rRNA 5′ domain.Citation60 Formation of the 5′ domain promotes the assembly of the secondary binder S16, whose binding then drives an allosteric conformational switch that stabilizes pseudoknot interactions at the decoding site.Citation56 Similarly, binding of S15 to the central domain of 16S rRNA imposes long-range conformational changes in the 5′ and 3′ 16S rRNA domains.Citation57 Furthermore, the multiphasic assembly kinetics of protein-rRNA interactions indicated that labile RNA-protein encounter complexes are rapidly formed, but slowly transition to more stable interactions that resemble the final complexes.Citation49,55
Many roads, one destination: Multiple parallel pathways of ribosome assembly
Kinetic assays of RNA footprinting demonstrated that different domains of 16S rRNA can independently and simultaneously form into a heterogenous population of intermediates, seeding the assembly of r-proteins.Citation49,52 By monitoring the binding kinetics ofCitation15 N-labeled r-proteins, Williamson and colleagues showed that there is no single rate-limiting bottleneck in 30S assembly,Citation61 supporting the relative malleability in the order of addition of r-proteins.Citation62 More recently, the same group followed up this finding by employing time-resolved single particle electron microscopy to visualize assembling 30S subunits over time.Citation42 Together, these studies unequivocally showed that ribosome assembly proceeds via multiple parallel pathways in vitro. As in electrical circuits, parallel pathways of ribosome assembly likely ensure ribosome production even in conditions that disfavor one specific route.
Importantly, however, the studies above also indicated that not all pathways are productive. Consistent with the parallel nature of assembly, RNAs can fold more into multiple alternative structures at equilibrium, which may represent either online intermediates or kinetic traps. The large rRNAs range from ∼1500 to several thousand nucleotides in length, and thus are susceptible to fold into quasi-stable structures that can stall the assembly pathway. Indeed, although 16S rRNA can fold independently of r-proteins, a significant fraction of these folded rRNAs represent kinetically-trapped intermediates that possess misfolded segments or mispaired interactions.Citation52 Binding of r-proteins to rRNA favors the stabilization of native architecture over antagonistic misfolded conformers.Citation56,60 rRNA conformations poised to form the final tertiary interactions are preferred, augmenting the rate of folding to native state. This depopulation of non-native intermediates occurs cooperatively as more r-proteins join the RNP complex, synergistically rerouting the assembly pathway toward the formation of native structures. Therefore, assembly of r-proteins not only guides the formation of stable interactions, but also biases against metastable abortive intermediates and streamlines the route to subunit maturation. These are key aspects that propel the cooperative nature of ribosome assembly. In vivo, r-proteins bind as the pre-rRNA is transcribed, hence, only a subset of rRNA structural motifs can be populated at any given time. This reduces the likelihood of forming dead-end intermediates, contributing to the increased cooperativity of ribosome biogenesis.Citation42,49 Nevertheless, parallel and heterogeneous ribosome assembly pathways remain to be explored in vivo.
Strong interactions are built over time: Transitioning from a labile encounter complex to a stable final complex
In eukaryotic cells, almost all r-proteins assemble with nascent pre-rRNA in the nucleolus.Citation63,64 Only a handful of r-proteins associate much later in the cytoplasm. However, in general, yeast r-proteins initially bind weakly to pre-rRNA in early assembly intermediates in vivo, then become more stably associated as assembly proceeds. The association of r-proteins with early pre-rRNAs is more sensitive to increasing salt concentrations relative to their interaction with late precursors.Citation37,65 This reinforces the model of encounter complexes initially proposed for bacterial ribosome assembly in vitro, wherein labile initial interaction of r-proteins with 16S rRNA transitions to a more robust binding as more protein-RNA interactions are established.Citation49,55 Hence, strengthening of r-protein association with rRNA leads to increasing RNP particle stability as maturation progresses. Consistent with this, misassembled yeast pre-ribosomes blocked at early stages of assembly in vivo are more rapidly turned over than particles blocked at later assembly steps.Citation66-69
Constructing ribosomal subunits in vivo, one neighborhood at a time
The majority of genes encoding r-proteins are essential.Citation66,70-74 Even so, how the binding of r-proteins coincides with biogenesis of ribosomal subunits in the cellular context has largely been understudied until recently. Ribosome assembly in vivo has been most extensively studied in yeast, and this has set the stage to investigate more complex systems in metazoans. Investigations of the functions of r-proteins in eukaryotic ribosome biogenesis have primarily focused on monitoring the production of ribosomal subunits by sucrose gradient fractionation, detecting nuclear export via fluorescent reporters, and assaying individual pre-rRNA processing intermediates by primer extension, northern blotting, and pulse-chase analysis. More recently, the ability to isolate assembling ribosomes combined with proteomic tools to characterize pre-ribosome constituents have allowed systematic global surveys of the roles of r-proteins in the formation of 40S and 60S subunits.
Knockdown or repression of small subunit r-protein genes in mammalian and yeast cells led to a bipartite classification of 40S r-protein functions. By assaying for defects in pre-rRNA processing, small subunit r-proteins can be grouped into those required to initiate early pre-18S rRNA processing steps, and those necessary for late nucleocytoplasmic maturation and export.Citation71,74 Interestingly, bacterial homologues of the yeast 40S r-proteins belonging to the early group are mostly primary binders, whereas homologues of the late group are mostly tertiary binders. The topological distribution of these 2 subgroups also correlates with the major structural domains of the small subunit (). 40S assembly is initiated by the formation of the body domain followed by the construction of the head domain. Indeed, by examining the assembly of r-proteins after in vivo depletion of representative primary or secondary r-proteins in the body and head domains, respectively, Milkereit and colleagues demonstrated that the formation of a stable intermediate constituting the body of the small subunit is required for the formation of head substructure, but not vice versa.Citation37
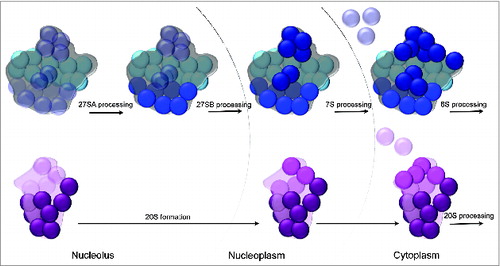
Figure 4. Clustering of ribosomal protein functional classes in the 40S and 60S structures. (Top) Large subunit ribosomal proteins required for 27SA pre-rRNA processing (early-acting, light blue) are localized on the convex solvent-exposed side, those necessary for 27SB pre-rRNA processing (middle-acting, royal blue) cluster around the polypeptide exit tunnel, and finally, ribosomal proteins important for processing of 7S/6S pre-RNAs and nuclear export (late-acting, dark blue) are present on the subunit interface as well as around the central protuberance. (Bottom) Small subunit ribosomal proteins required for early processing steps (dark purple) are primarily localized on the body domain while ribosomal proteins necessary for late nucleocytoplasmic maturation and export (magenta) are clustered on the 40S head domain. Structural landmarks of the small and large subunits are labeled as in .
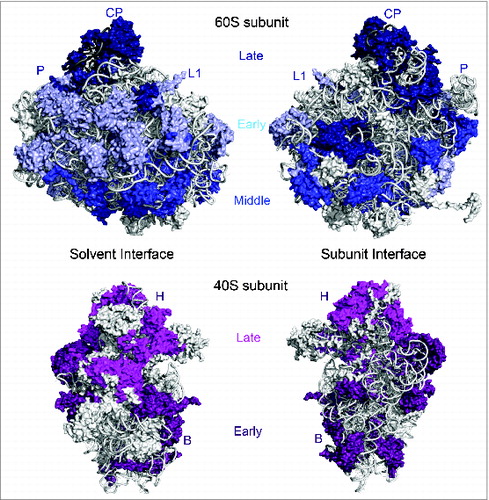
Although the large subunit has prominent landmarks such as the central protuberance, L1 stalk, and acidic stalk, the segregation of the 60S subunit into distinct structural domains is not as apparent as for the 40S subunit, because 25S rRNA domains are more intertwined.Citation22,75 Hence, contrary to conventional scientific wisdom and expectations, it was striking to find that the absence of yeast large subunit r-proteins affects specific steps in processing of 25S/5.8S rRNA precursors rather than having more global processing defects. These observations led to the phenotypic classification of yeast 60S r-proteins,Citation66 based on their function in the processing of early 27SA pre-rRNAs, middle 27SB pre-rRNA, and finally, late 7S/6S pre-RNAs and nuclear export. In the yeast large subunit structure,Citation6 r-proteins required for early processing steps are localized on the convex solvent-exposed interface, middle-acting r-proteins are clustered around the rim of the polypeptide exit tunnel, and late-acting r-proteins are present on the intersubunit surface as well as the central protuberance (). This remarkable clustering of r-proteins belonging to different phenotypic groups in the structure of the 60S subunit argues for a sequential order of stable assembly of various RNP neighborhoods of eukaryotic large subunits, akin to small subunits.Citation66 In support of this model, depletion of an early-acting r-protein affects the stable association of other early-acting r-proteins, as well as members of the middle- and late-acting groups. On the other hand, depleting a middle-acting r-protein around the exit tunnel only affects the stable binding of middle- and late-acting r-proteins. Lastly, the absence of late-acting r-proteins has very minor effects on the assembly of neighboring late-acting r-proteins and does not affect the early or middle r-protein group.Citation65-67 Thus, assembly of eukaryotic large subunits appears to proceed in a hierarchical fashion, beginning with the convex solvent-side, followed by the polypeptide exit tunnel, and finally the inter-subunit side as well as the central protuberance. It is also important to note that the sequential order of stabilized binding of each r-protein group coincides with stepwise pre-rRNA processing events from the nucleolus to the cytoplasm ().
Figure 5. Models for hierarchical assembly of yeast ribosomal subunits. Cartoon representations of the subunit interfaces of the large (top) and small (bottom) ribosomal subunits are shown. The models show the sequential stabilization of binding of ribosomal proteins around discrete structural neighborhoods of the small and large subunits. Ribosomal proteins are represented by spheres and the groups are colored according to . Ribosomal proteins that are loosely associated with pre-ribosomes are faint and transparent while those that are already tightly bound to assembling ribosomes are bright and solid. Hierarchical stabilization of ribonucleoprotein neighborhoods coincides with the step-wise steps of pre-rRNA processing.
In bacteria, pre-rRNA processing is much simpler and not compartmentalized. It is therefore striking to find that largely similar assembly hierarchies for E. coli ribosomal subunits exist in vivo,Citation48 where pulse-labeled populations of 30S and 50S assembly intermediates were monitored by quantitative mass spectrometry. These observations argue for general conservation of mechanisms that govern the biogenesis of these RNP complexes. Importantly, in vivo studies of small subunit assembly in bacteria and in eukaryotes corroborate the extensive in vitro work on reconstituted bacterial 30S subunits and their domain derivatives.
Taken together, the hierarchy revealed by investigating the roles of r-proteins in the assembly of eukaryotic ribosomal subunits demonstrates a stringent spatio-temporal regulation of ribosome biogenesis. The specific order of properly constructing different structural features of the 40S and 60S subunits serves as a checkpoint for each of the irreversible pre-rRNA maturation steps, and importantly, the export of nearly mature subunits to the cytoplasm (, also see below). Curiously, all 3 rRNA pseudoknots in the small subunit, which are important for its decoding function, are formed late.Citation45,52 Similarly, the last stages in large subunit assembly occur around the functionally important domains including the central protuberance that mediates intersubunit joining and the peptidyltransferase center.Citation66,76,77 Hence, the final steps of ribosomal subunit biogenesis evolved to focus on the sites involved in ribosome function, preventing premature translation initiation by earlier assembly intermediates.
Finding direction: 5′ to 3′ polarity of subunit assembly
In the small subunit, most E. coli primary binding r-proteins and eukaryotic r-proteins that function in early pre-rRNA processing events are bound to 5′ rRNA sequences, which together form the body substructure. 30S subunit tertiary binding r-proteins and 40S subunit r-proteins required for nucleocytoplasmic export and maturation are bound to 3′ rRNA sequences forming the head substructure.Citation37,48,71,74 In the large subunit, early-acting proteins have more interactions with the 5′ half of 25S rRNA, while late-acting r-proteins have more interactions with the 3′ half of 25S rRNA.Citation66 This correlation between the timing of binding or function of RPLs and their primary binding sites suggests that the 5′ to 3′ directionality of r-protein binding facilitates stepwise RNP maturation events. Indeed, assembly is tightly coupled with transcription of pre-rRNA in vivo ().
Figure 6. Co-transcriptional assembly of ribosomal proteins. In cells, pre-rRNAs are transcribed from the rDNA locus by RNA Pol I. Folding of nascent transcripts enables the co-transcriptional binding of ribosomal proteins (blue). Individual secondary structure domains of mature rRNA sequences are represented by different shades of gray.
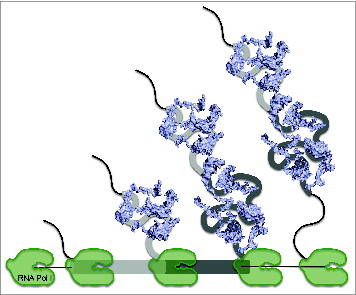
Although kinetic footprinting studies demonstrated that the different domains of 16S rRNA can simultaneously and independently fold in vitro,Citation49,52 there is strong evidence that 5′ to 3′ polarity is favored,Citation51,61,78 mirroring the co-transcriptional model of assembly in vivo. rRNA conformational changes are localized to the 5′ domain during early stages of assembly, whereas late folding events are concentrated in the 3′ domain.Citation51,78 Measurements by isotope pulse-chase and mass spectrometry techniques showed that r-proteins that bind the 5′ body domain rapidly associate with 16S rRNA, whereas those that bind the 3′ head domain are incorporated more slowly.Citation61 More direct evidence by visualization of these reconstituted intermediates by single particle EM demonstrated that early particles depict a stably formed 5′ body domain followed by later complexes that finally bear the 3′ head domain.Citation42 Taken together, in vitro and in vivo studies point to a general 5′ to 3′ direction of assembly, which underscores the relevance of cooperativity during ribosome assembly.
Making ends meet: Importance of 5′ and 3′ ends during ribosome assembly
Although the observations above suggest 5′ to 3′ assembly, there is evidence that rRNA sequences at the 3′ end are also important to initiate pre-rRNA processing at sites near the 5′ end. Maturation of bacterial 16S and 23S rRNAs in vivo requires that the spacer sequences flanking both the 5′ and the 3′ ends of mature rRNAs are base paired together before liberation from the nascent rRNA transcript by RNAse III.Citation23 A theme that emerged from systematic examination of mammalian and yeast r-protein assembly is that the 2 ends of pre-18S and pre-5.8S/25S rRNAs are also likely brought together early during assembly. Co-positioning of both the 5′ and 3′ spacers of 18S rRNA is a requirement for initiating early pre-rRNA processing steps in assembly of 40S subunits.Citation74 Yeast L3, which is required for the first step of pre-25S processing,Citation79 is positioned close to both the 5′ end of 5.8S rRNA and the 3′ end of 25S rRNA.Citation6 Thus, together with previous observations from mutations in the 3′ spacer sequence,Citation80,81 proper formation of pre-rRNA sequences at the 3′ end and possibly bringing it close to the 5′ end are important to signal early pre-rRNA maturation steps.Citation68 This likely facilitates the formation of a compact intermediate during 60S assembly. Consistent with these models, pre-18S rRNA is packaged into a compact structure immediately after the entire 18S is transcribed ().Citation82 Moreover, the 5′ and 3′ processing events of pre-25S rRNA are coordinated to facilitate the compaction of pre-ribosomes.Citation83 This may reflect a general principle underlying assembly of large RNPs; the formation of a compact intermediate that exhibits native tertiary interactions such as correctly oriented RNA helices is a crucial step in the cooperative folding of large rRNAs.Citation84-86 Furthermore, these observations suggest that while binding of r-proteins with rRNA occurs co-transcriptionally, further processing of pre-18S and pre-5.8S/25S rRNAs occurs post-transcriptionally. Such a mechanism enables quality check at the 3′ end to ensure that entire pre-rRNAs are correctly transcribed and structured before committing to invest in more energy-consuming downstream maturation processes.
Figure 7. Chromatin spread of actively transcribed rDNA locus in yeast. Electron microscopy is used to visualize rDNA transcription and initial stages in rRNP assembly, which resemble “Christmas trees.” Newly synthesized rRNA “branches” emanate from the rDNA “trunk.” Large “knobs” at the end of each branch illustrate the compaction of pre-18S rRNA to form the small subunit processome. This yeast chromatin spread is adapted from Osheim et al.Citation82

Unity in diversity: Concerted efforts of ribosomal proteins and assembly factors
Ribosome assembly in vivo also involves the orchestrated binding and release of trans-acting biogenesis factors. These include snoRNP components, RNA binding proteins, RNA and protein chaperones, scaffolding proteins, methyltransferases, energy-consuming enzymes, and nucleases.Citation23-26 While folding of rRNA and binding of r-proteins are the primary drivers of assembly, assembly factors increase the efficiency and accuracy of ribosome biogenesis. Accumulating evidence indicates that binding of r-proteins acts in concert with assembly factors in order to form stable intermediates that are capable of undergoing pre-rRNA processing. In yeast, for example, a group of assembly factors referred to as the “A3 cluster proteins” must stably bind to assembling pre-60S complexes in order for accurate processing of the 27SA3 pre-rRNA to take place.Citation87 60S r-proteins bound to domains I and II of 25S rRNA are required for stable association of the A3 factors into pre-ribosomes.Citation66,67 The A3 cluster factors are necessary to recruit r-proteins surrounding the polypeptide exit tunnel,Citation87 and these tunnel r-proteins in turn enable stable binding of assembly factors required for 27SB pre-rRNA processing (“B factors”) and pre-ribosome export (“export factors”).Citation66,68 On the other hand, depletion of r-proteins around the central protuberance prevents the release of a subset of 60S assembly factors,Citation65 which is likely important for major repositioning of the 5S RNP and binding of export factors.Citation88,89 There are examples of cytoplasmic assembling r-proteins that are required for the release and subsequent recycling of assembly and export factors.Citation90-92 Moreover, some assembly factors serve as placeholders whose release allows subsequent binding of r-proteins.Citation89,93 From the known location of some of these factors on pre-ribosomes,Citation88,94-98 it can be inferred that the influence of r-proteins on the association and dissociation of many assembly factors, and vice versa, is proximal. Taken together, the coordinated dynamics of r-proteins and assembly factors ensure that formation of ribosome assembly intermediates proceeds in an efficient manner.
Practice makes perfect: test-driving and proof-reading of functional regions
Ribosome biogenesis is under rigorous quality control exerted in almost every step of the assembly pathway.Citation99 The checkpoint mechanisms of structural proofreading and functional test-driving in eukaryotes were first revealed by genetic studies of r-protein L10.Citation100 Mutations in the loop of L10 that extends toward the P-site of the peptidyltransferase center disrupt the GTPase-mediated release of the subunit anti-association factor Tif6. The conformational dynamics of such a GTPase during assembly is akin to the mechanism involved in the GTPase-dependent tRNA translocation during protein synthesis. These observations reinforce a model where an assembly factor interrogates the structural and functional integrity of the P-site before release of Tif6, ensuring that only 60S subunits with properly assembled functional centers engage in translation. Test-driving results are likely also communicated between ribosomal subunits. It was recently shown that final maturation of pre-40S complexes involves GTPase-mediated functional proofreading in a translation-like complex.Citation101,102 Interestingly, this quality control process is abolished by a mutation in the 60S r-protein L3 that alters the structure of the GTPase-associated center.Citation103 Hence, assessment of the bona fide structure of the 60S subunit is also required for 40S subunit assembly.
What lies ahead: Marching from ribosome assembly toward ribosome biogenesis
There is no doubt that studies on in vitro reconstitution of bacterial ribosomal subunits have immensely informed the field about the general principles of ribosome assembly. Nonetheless, the holy grail in understanding ribosome assembly is to learn what happens inside the cell, especially in metazoans. We envision the next frontier to be a push toward more studies in vivo, while achieving as much, if not more, detail as previous in vitro studies.
The power of genetics will continue to inform us about the mechanisms of ribosome biogenesis. In addition, the next steps will also involve state-of-the-art technologies, many of them already at hand. For example, it would be valuable to comprehensively analyze the spatio-temporal dynamics of eukaryotic ribosome biogenesis by pulse-chase using isotope- or epitope-labeling combined with mass spectrometry.Citation48,104 This will provide a better resolution of when individual r-proteins assemble with pre-ribosomes, as well as the coordinated association and dissociation of assembly factors. Since assembling ribosomes are heterogeneous, better methods to isolate narrowly defined sets of intermediates will facilitate analysis of protein composition and rRNA structure at each step of the biogenesis pathway.
Time-resolved monitoring of protein-induced rRNA conformational changes in homogeneous pre-ribosome intermediates can give information on the in vivo kinetics of ribosome assembly. Furthermore, this can reveal conformational changes that may be pre-requisites to the formation of different domains or the processing of pre-rRNA. High-throughput techniques to assay RNA structure and pre-ribosomal protein components in vivo are now available.Citation105-107 Moreover, since intrinsic disorder is a common feature in many r-proteins, especially in eukaryote-specific regions,Citation108 another facet to explore in order to understand protein-induced RNA folding is the role of these disordered domains in chaperoning rRNA. It will also be important to further investigate post-translational modifications in r-proteins as they may regulate remodeling of RNP interactions within assembling subunits.Citation109,110
Another informative approach is to visualize purified assembling ribosomal subunits by biophysical techniques such as cryo-EM, and correlate the observations with biochemical characterization of composition and structure. In eukaryotes, for instance, taking 3-dimensional “snapshots” throughout the life of maturing subunits, starting from the 90S pre-ribosome to the individual intermediates in the pre-60S pathway, will be very valuable. Structural analysis of pre-ribosomes may also shed light on the multiple parallel pathways of assembly occurring in vivo. In view of emerging questions about heterogenous specialized ribosomes,Citation111,112 single-particle monitoring of biogenesis intermediates will be an asset. Together, these approaches will facilitate teasing apart the interwoven dynamics among folding of rRNA, binding of r-proteins, processing of pre-rRNA, as well as the assembly and release of biogenesis factors. Absolutely, the field is looking forward to another exciting era of enlightenment.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors thank Jelena Jakovljevic and Jason Talkish for critical feedback on this manuscript.
Funding
Ribosomal protein-related research in the Woolford laboratory was supported by grants to J.L.W. from the National Science Foundation (grant MCB0818534) and the David Scaife Family Charitable Foundation as well as the de Vries Fellowship to M.G.
References
- Ban N, Nissen P, Hansen J, Moore PB, Steitz TA. The complete atomic structure of the large ribosomal subunit at 2.4 A resolution. Science 2000; 289:905-20; PMID:10937989; http://dx.doi.org/10.1126/science.289.5481.905
- Nissen P, Hansen J, Ban N, Moore PB, Steitz TA. The structural basis of ribosome activity in peptide bond synthesis. Science 2000; 289:920-30; PMID:10937990; http://dx.doi.org/10.1126/science.289.5481.920
- Wimberly BT, Brodersen DE, Clemons WM, Jr., Morgan-Warren RJ, Carter AP, Vonrhein C, Hartsch T, Ramakrishnan V. Structure of the 30S ribosomal subunit. Nature 2000; 407:327-39; PMID:11014182; http://dx.doi.org/10.1038/35030006
- Yusupov MM, Yusupova GZ, Baucom A, Lieberman K, Earnest TN, Cate JH, Noller HF. Crystal structure of the ribosome at 5.5 A resolution. Science 2001; 292:883-96; PMID:11283358; http://dx.doi.org/10.1126/science.1060089
- Schuwirth BS, Borovinskaya MA, Hau CW, Zhang W, Vila-Sanjurjo A, Holton JM, Cate JH. Structures of the bacterial ribosome at 3.5 A resolution. Science 2005; 310:827-34; PMID:16272117; http://dx.doi.org/10.1126/science.1117230
- Ben-Shem A, Garreau de Loubresse N, Melnikov S, Jenner L, Yusupova G, Yusupov M. The structure of the eukaryotic ribosome at 3.0 A resolution. Science 2011; 334:1524-9; PMID:22096102; http://dx.doi.org/10.1126/science.1212642
- Armache JP, Jarasch A, Anger AM, Villa E, Becker T, Bhushan S, Jossinet F, Habeck M, Dindar G, Franckenberg S, et al. Cryo-EM structure and rRNA model of a translating eukaryotic 80S ribosome at 5.5-A resolution. Proc Natl Acad Sci U S A 2010; 107:19748-53; PMID:20980660; http://dx.doi.org/10.1073/pnas.1009999107
- Klinge S, Voigts-Hoffmann F, Leibundgut M, Arpagaus S, Ban N. Crystal structure of the eukaryotic 60S ribosomal subunit in complex with initiation factor 6. Science 2011; 334:941-8; PMID:22052974; http://dx.doi.org/10.1126/science.1211204
- Hashem Y, des Georges A, Dhote V, Langlois R, Liao HY, Grassucci RA, Hellen CU, Pestova TV, Frank J. Structure of the mammalian ribosomal 43S preinitiation complex bound to the scanning factor DHX29. Cell 2013; 153:1108-19; PMID:23706745; http://dx.doi.org/10.1016/j.cell.2013.04.036
- Hashem Y, des Georges A, Fu J, Buss SN, Jossinet F, Jobe A, Zhang Q, Liao HY, Grassucci RA, Bajaj C, et al. High-resolution cryo-electron microscopy structure of the Trypanosoma brucei ribosome. Nature 2013; 494:385-9; PMID:23395961; http://dx.doi.org/10.1038/nature11872
- Melnikov S, Ben-Shem A, Garreau de Loubresse N, Jenner L, Yusupova G, Yusupov M. One core, two shells: bacterial and eukaryotic ribosomes. Nat Struct Mol Biol 2012; 19:560-7; PMID:22664983; http://dx.doi.org/10.1038/nsmb.2313
- Ban N, Beckmann R, Cate JH, Dinman JD, Dragon F, Ellis SR, Lafontaine DL, Lindahl L, Liljas A, Lipton JM, et al. A new system for naming ribosomal proteins. Curr Opin Struct Biol 2014; 24:165-9; PMID:24524803; http://dx.doi.org/10.1016/j.sbi.2014.01.002
- Steitz TA. A structural understanding of the dynamic ribosome machine. Nat Rev Mol Cell Biol 2008; 9:242-53; PMID:18292779; http://dx.doi.org/10.1038/nrm2352
- Voorhees RM, Ramakrishnan V. Structural basis of the translational elongation cycle. Annu Rev Biochem 2013; 82:203-36; PMID:23746255; http://dx.doi.org/10.1146/annurev-biochem-113009-092313
- Schmeing TM, Ramakrishnan V. What recent ribosome structures have revealed about the mechanism of translation. Nature 2009; 461:1234-42; PMID:19838167; http://dx.doi.org/10.1038/nature08403
- Traub P, Nomura M. Structure and function of E. coli ribosomes. V. Reconstitution of functionally active 30S ribosomal particles from RNA and proteins. Proc Natl Acad Sci U S A 1968; 59:777-84; PMID:4868216; http://dx.doi.org/10.1073/pnas.59.3.777
- Nomura M, Traub P. Structure and function of Escherichia coli ribosomes. 3. Stoichiometry and rate of the reconstitution of ribosomes from subribosomal particles and split proteins. J Mol Biol 1968; 34:609-19; PMID:4938560; http://dx.doi.org/10.1016/0022-2836(68)90184-8
- Mizushima S, Nomura M. Assembly mapping of 30S ribosomal proteins from E. coli. Nature 1970; 226:1214; PMID:4912319; http://dx.doi.org/10.1038/2261214a0
- Held WA, Ballou B, Mizushima S, Nomura M. Assembly mapping of 30 S ribosomal proteins from Escherichia coli. Further studies. J Biol Chem 1974; 249:3103-11; PMID:4598121
- Nierhaus KH, Dohme F. Total reconstitution of functionally active 50S ribosomal subunits from Escherichia coli. Proc Natl Acad Sci U S A 1974; 71:4713-7; PMID:4612527; http://dx.doi.org/10.1073/pnas.71.12.4713
- Rohl R, Nierhaus KH. Assembly map of the large subunit (50S) of Escherichia coli ribosomes. Proc Natl Acad Sci U S A 1982; 79:729-33; PMID:7038683; http://dx.doi.org/10.1073/pnas.79.3.729
- Holbrook SR. Structural principles from large RNAs. Annu Rev Biophys 2008; 37:445-64; PMID:18573090; http://dx.doi.org/10.1146/annurev.biophys.36.040306.132755
- Shajani Z, Sykes MT, Williamson JR. Assembly of bacterial ribosomes. Annu Rev Biochem 2011; 80:501-26; PMID:21529161; http://dx.doi.org/10.1146/annurev-biochem-062608-160432
- Woolford JL Jr, Baserga SJ. Ribosome biogenesis in the yeast Saccharomyces cerevisiae. Genetics 2013; 195:643-81; PMID:24190922; http://dx.doi.org/10.1534/genetics.113.153197
- Karbstein K. Inside the 40S ribosome assembly machinery. Curr Opin Chem Biol 2011; 15:657-63; PMID:21862385; http://dx.doi.org/10.1016/j.cbpa.2011.07.023
- Kressler D, Hurt E, Bergler H, Bassler J. The power of AAA-ATPases on the road of pre-60S ribosome maturation–molecular machines that strip pre-ribosomal particles. Biochim Biophys Acta 2012; 1823:92-100; PMID:21763358; http://dx.doi.org/10.1016/j.bbamcr.2011.06.017
- Raska I, Koberna K, Malinsky J, Fidlerova H, Masata M. The nucleolus and transcription of ribosomal genes. Biol Cell 2004; 96:579-94; PMID:15519693; http://dx.doi.org/10.1016/j.biolcel.2004.04.015
- Berg JM, Tymoczko JL, Stryer L. Biochemistry. New York: WH Freeman, 2002
- Cooper GM. The Cell: A Molecular Approach. Sunderland (MA): Sinauer Associates, 2000
- Trainor PA, Merrill AE. Ribosome biogenesis in skeletal development and the pathogenesis of skeletal disorders. Biochim Biophys Acta 2014; 1842:769-78; PMID:24252615; http://dx.doi.org/10.1016/j.bbadis.2013.11.010
- McGowan KA, Li JZ, Park CY, Beaudry V, Tabor HK, Sabnis AJ, Zhang W, Fuchs H, de Angelis MH, Myers RM, et al. Ribosomal mutations cause p53-mediated dark skin and pleiotropic effects. Nat Genet 2008; 40:963-70; PMID:18641651; http://dx.doi.org/10.1038/ng.188
- Raiser DM, Narla A, Ebert BL. The emerging importance of ribosomal dysfunction in the pathogenesis of hematologic disorders. Leuk Lymphoma 2013; 55:491-500; PMID:23863123; http://dx.doi.org/10.3109/10428194.2013.812786
- Bolze A, Mahlaoui N, Byun M, Turner B, Trede N, Ellis SR, Abhyankar A, Itan Y, Patin E, Brebner S, et al. Ribosomal protein SA haploinsufficiency in humans with isolated congenital asplenia. Science 2013; 340:976-8; PMID:23579497; http://dx.doi.org/10.1126/science.1234864
- Freed EF, Bleichert F, Dutca LM, Baserga SJ. When ribosomes go bad: diseases of ribosome biogenesis. Mol Biosyst 2010; 6:481-93; PMID:20174677; http://dx.doi.org/10.1039/b919670f
- Ellis SR. Nucleolar stress in Diamond Blackfan anemia pathophysiology. Biochim Biophys Acta 2014; 1842:765-8; PMID:24412987; http://dx.doi.org/10.1016/j.bbadis.2013.12.013
- Sulima SO, Patchett S, Advani VM, De Keersmaecker K, Johnson AW, Dinman JD. Bypass of the pre-60S ribosomal quality control as a pathway to oncogenesis. Proc Natl Acad Sci U S A 2014; 111:5640-5; PMID:24706786; http://dx.doi.org/10.1073/pnas.1400247111
- Ferreira-Cerca S, Poll G, Kuhn H, Neueder A, Jakob S, Tschochner H, Milkereit P. Analysis of the in vivo assembly pathway of eukaryotic 40S ribosomal proteins. Mol Cell 2007; 28:446-57; PMID:17996708; http://dx.doi.org/10.1016/j.molcel.2007.09.029
- Chen SS, Sperling E, Silverman JM, Davis JH, Williamson JR. Measuring the dynamics of E. coli ribosome biogenesis using pulse-labeling and quantitative mass spectrometry. Mol Biosyst 2012; 8:3325-34; PMID:23090316; http://dx.doi.org/10.1039/c2mb25310k
- Woodson SA. RNA folding pathways and the self-assembly of ribosomes. Acc Chem Res 2011; 44:1312-9; PMID:21714483; http://dx.doi.org/10.1021/ar2000474
- Williamson JR. Biophysical studies of bacterial ribosome assembly. Curr Opin Struct Biol 2008; 18:299-304; PMID:18541423
- Harnpicharnchai P, Jakovljevic J, Horsey E, Miles T, Roman J, Rout M, Meagher D, Imai B, Guo Y, Brame CJ, et al. Composition and functional characterization of yeast 66S ribosome assembly intermediates. Mol Cell 2001; 8:505-15; PMID:11583614; http://dx.doi.org/10.1016/S1097-2765(01)00344-6
- Mulder AM, Yoshioka C, Beck AH, Bunner AE, Milligan RA, Potter CS, Carragher B, Williamson JR. Visualizing ribosome biogenesis: parallel assembly pathways for the 30S subunit. Science 2010; 330:673-7; PMID:21030658; http://dx.doi.org/10.1126/science.1193220
- Stern S, Powers T, Changchien LM, Noller HF. RNA-protein interactions in 30S ribosomal subunits: folding and function of 16S rRNA. Science 1989; 244:783-90; PMID:2658053; http://dx.doi.org/10.1126/science.2658053
- Jagannathan I, Culver GM. Assembly of the central domain of the 30S ribosomal subunit: roles for the primary binding ribosomal proteins S15 and S8. J Mol Biol 2003; 330:373-83; PMID:12823975; http://dx.doi.org/10.1016/S0022-2836(03)00586-2
- Holmes KL, Culver GM. Mapping structural differences between 30S ribosomal subunit assembly intermediates. Nat Struct Mol Biol 2004; 11:179-86; PMID:14730351; http://dx.doi.org/10.1038/nsmb719
- Powers T, Noller HF. Hydroxyl radical footprinting of ribosomal proteins on 16S rRNA. RNA 1995; 1:194-209; PMID:7585249
- Powers T, Noller HF. A temperature-dependent conformational rearrangement in the ribosomal protein S4.16 S rRNA complex. J Biol Chem 1995; 270:1238-42; PMID:7836385; http://dx.doi.org/10.1074/jbc.270.3.1238
- Chen SS, Williamson JR. Characterization of the ribosome biogenesis landscape in E. coli using quantitative mass spectrometry. J Mol Biol 2013; 425:767-79; PMID:23228329; http://dx.doi.org/10.1016/j.jmb.2012.11.040
- Adilakshmi T, Bellur DL, Woodson SA. Concurrent nucleation of 16S folding and induced fit in 30S ribosome assembly. Nature 2008; 455:1268-72; PMID:18784650; http://dx.doi.org/10.1038/nature07298
- Woolstenhulme CJ, Hill WE. The genesis of ribosome structure: how a protein generates RNA structure in real time. J Mol Biol 2009; 392:645-56; PMID:19563812; http://dx.doi.org/10.1016/j.jmb.2009.06.056
- Powers T, Daubresse G, Noller HF. Dynamics of in vitro assembly of 16 S rRNA into 30 S ribosomal subunits. J Mol Biol 1993; 232:362-74; PMID:8345517; http://dx.doi.org/10.1006/jmbi.1993.1396
- Adilakshmi T, Ramaswamy P, Woodson SA. Protein-independent folding pathway of the 16S rRNA 5′ domain. J Mol Biol 2005; 351:508-19; PMID:16023137; http://dx.doi.org/10.1016/j.jmb.2005.06.020
- Ramakrishnan V. Distribution of protein and RNA in the 30S ribosomal subunit. Science 1986; 231:1562-4; PMID:3513310; http://dx.doi.org/10.1126/science.3513310
- Athavale SS, Gossett JJ, Hsiao C, Bowman JC, O'Neill E, Hershkovitz E, Preeprem T, Hud NV, Wartell RM, Harvey SC, et al. Domain III of the T. thermophilus 23S rRNA folds independently to a near-native state. RNA 2012; 18:752-8; PMID:22334759; http://dx.doi.org/10.1261/rna.030692.111
- Bunner AE, Beck AH, Williamson JR. Kinetic cooperativity in Escherichia coli 30S ribosomal subunit reconstitution reveals additional complexity in the assembly landscape. Proc Natl Acad Sci U S A 2010; 107:5417-22; PMID:20207951; http://dx.doi.org/10.1073/pnas.0912007107
- Ramaswamy P, Woodson SA. S16 throws a conformational switch during assembly of 30S 5′ domain. Nat Struct Mol Biol 2009; 16:438-45; PMID:19343072; http://dx.doi.org/10.1038/nsmb.1585
- Jagannathan I, Culver GM. Ribosomal protein-dependent orientation of the 16 S rRNA environment of S15. J Mol Biol 2004; 335:1173-85; PMID:14729335; http://dx.doi.org/10.1016/j.jmb.2003.11.031
- Ha T, Zhuang X, Kim HD, Orr JW, Williamson JR, Chu S. Ligand-induced conformational changes observed in single RNA molecules. Proc Natl Acad Sci U S A 1999; 96:9077-82; PMID:10430898; http://dx.doi.org/10.1073/pnas.96.16.9077
- Agalarov SC, Sridhar Prasad G, Funke PM, Stout CD, Williamson JR. Structure of the S15,S6,S18-rRNA complex: assembly of the 30S ribosome central domain. Science 2000; 288:107-13; PMID:10753109; http://dx.doi.org/10.1126/science.288.5463.107
- Ramaswamy P, Woodson SA. Global stabilization of rRNA structure by ribosomal proteins S4, S17, and S20. J Mol Biol 2009; 392:666-77; PMID:19616559; http://dx.doi.org/10.1016/j.jmb.2009.07.032
- Talkington MW, Siuzdak G, Williamson JR. An assembly landscape for the 30S ribosomal subunit. Nature 2005; 438:628-32; PMID:16319883; http://dx.doi.org/10.1038/nature04261
- Nomura M. Assembly of bacterial ribosomes. Science 1973; 179:864-73; PMID:4569247; http://dx.doi.org/10.1126/science.179.4076.864
- Kruiswijk T, Planta RJ, Krop JM. The course of the assembly of ribosomal subunits in yeast. Biochim Biophys Acta 1978; 517:378-89; PMID:626744; http://dx.doi.org/10.1016/0005-2787(78)90204-6
- Hadjiolov AA. The Nucleolus and Ribosome Biogenesis. Berlin: Springer-Verlag, 1985
- Ohmayer U, Gamalinda M, Sauert M, Ossowski J, Poll G, Linnemann J, Hierlmeier T, Perez-Fernandez J, Kumcuoglu B, Leger-Silvestre I, Lin L, Woolford JL Jr. Studies on the Assembly Characteristics of Large Subunit Ribosomal Proteins in S. cerevisae. PLoS One 2013; 8:e68412; PMID:23874617; http://dx.doi.org/10.1371/journal.pone.0068412
- Gamalinda M, Ohmayer U, Jakovljevic J, Kumcuoglu B, Woolford J, Mbom B, Lin L, Woolford JL Jr. A hierarchical model for assembly of eukaryotic 60S ribosomal subunit domains. Genes Dev 2014; 28:198-210; PMID:24449272; http://dx.doi.org/10.1101/gad.228825.113
- Jakovljevic J, Ohmayer U, Gamalinda M, Talkish J, Alexander L, Linnemann J, Milkereit P, Woolford JL Jr. Ribosomal proteins L7 and L8 function in concert with six A3 assembly factors to propagate assembly of domains I and II of 25S rRNA in yeast 60S ribosomal subunits. RNA 2012; 18:1805-22; PMID:22893726; http://dx.doi.org/10.1261/rna.032540.112
- Gamalinda M, Jakovljevic J, Babiano R, Talkish J, de la Cruz J, Woolford JL, Jr. Yeast polypeptide exit tunnel ribosomal proteins L17, L35 and L37 are necessary to recruit late-assembling factors required for 27SB pre-rRNA processing. Nucleic Acids Res 2013; 41:1965-83; PMID:23268442; http://dx.doi.org/10.1093/nar/gks1272
- Babiano R, de la Cruz J. Ribosomal protein L35 is required for 27SB pre-rRNA processing in Saccharomyces cerevisiae. Nucleic Acids Res 2010; 38:5177-92; PMID:20392820; http://dx.doi.org/10.1093/nar/gkq260
- Steffen KK, McCormick MA, Pham KM, Mackay VL, Delaney JR, Murakami CJ, Kaeberlein M, Kennedy BK. Ribosome Deficiency Protects Against ER Stress in Saccharomyces cerevisiae. Genetics 2012; 191:107-18; PMID:22377630
- Ferreira-Cerca S, Poll G, Gleizes PE, Tschochner H, Milkereit P. Roles of eukaryotic ribosomal proteins in maturation and transport of pre-18S rRNA and ribosome function. Mol Cell 2005; 20:263-75; PMID:16246728; http://dx.doi.org/10.1016/j.molcel.2005.09.005
- Poll G, Braun T, Jakovljevic J, Neueder A, Jakob S, Woolford JL Jr, Tschochner H, Milkereit P. rRNA maturation in yeast cells depleted of large ribosomal subunit proteins. PLoS One 2009; 4:e8249; PMID:20011513; http://dx.doi.org/10.1371/journal.pone.0008249
- Robledo S, Idol RA, Crimmins DL, Ladenson JH, Mason PJ, Bessler M. The role of human ribosomal proteins in the maturation of rRNA and ribosome production. RNA 2008; 14:1918-29; PMID:18697920; http://dx.doi.org/10.1261/rna.1132008
- O'Donohue MF, Choesmel V, Faubladier M, Fichant G, Gleizes PE. Functional dichotomy of ribosomal proteins during the synthesis of mammalian 40S ribosomal subunits. J Cell Biol 2010; 190:853-66; PMID:20819938; http://dx.doi.org/10.1083/jcb.201005117
- Yusupova G, Yusupov M. High-resolution structure of the eukaryotic 80S ribosome. Annu Rev Biochem 2014; 83:467-86; PMID:24580643
- Jomaa A, Jain N, Davis JH, Williamson JR, Britton RA, Ortega J. Functional domains of the 50S subunit mature late in the assembly process. Nucleic Acids Res 2014; 42:3419-35; PMID:24335279; http://dx.doi.org/10.1093/nar/gkt1295
- Li N, Chen Y, Guo Q, Zhang Y, Yuan Y, Ma C, Deng H, Lei J, Gao N. Cryo-EM structures of the late-stage assembly intermediates of the bacterial 50S ribosomal subunit. Nucleic Acids Res 2013; 41:7073-83; PMID:23700310
- Holmes KL, Culver GM. Analysis of conformational changes in 16 S rRNA during the course of 30 S subunit assembly. J Mol Biol 2005; 354:340-57; PMID:16246364; http://dx.doi.org/10.1016/j.jmb.2005.09.056
- Rosado IV, Kressler D, de la Cruz J. Functional analysis of Saccharomyces cerevisiae ribosomal protein Rpl3p in ribosome synthesis. Nucleic Acids Res 2007; 35:4203-13; PMID:17569673; http://dx.doi.org/10.1093/nar/gkm388
- Allmang C, Tollervey D. The role of the 3′ external transcribed spacer in yeast pre-rRNA processing. J Mol Biol 1998; 278:67-78; PMID:9571034; http://dx.doi.org/10.1006/jmbi.1998.1693
- Hitchen J, Ivakine E, Melekhovets YF, Lalev A, Nazar RN. Structural features in the 3′ external transcribed spacer affecting intragenic processing of yeast rRNA. J Mol Biol 1997; 274:481-90; PMID:9417929; http://dx.doi.org/10.1006/jmbi.1997.1376
- Osheim YN, French SL, Keck KM, Champion EA, Spasov K, Dragon F, Baserga SJ, Beyer AL. Pre-18S ribosomal RNA is structurally compacted into the SSU processome prior to being cleaved from nascent transcripts in Saccharomyces cerevisiae. Mol Cell 2004; 16:943-54; PMID:15610737; http://dx.doi.org/10.1016/j.molcel.2004.11.031
- Lebaron S, Segerstolpe A, French SL, Dudnakova T, de Lima Alves F, Granneman S, Rappsilber J, Beyer AL, Wieslander L, Tollervey D. Rrp5 binding at multiple sites coordinates pre-rRNA processing and assembly. Mol Cell 2013; 52:707-19; PMID:24239293
- Bokinsky G, Rueda D, Misra VK, Rhodes MM, Gordus A, Babcock HP, Walter NG, Zhuang X. Single-molecule transition-state analysis of RNA folding. Proc Natl Acad Sci U S A 2003; 100:9302-7; PMID:12869691; http://dx.doi.org/10.1073/pnas.1133280100
- Buchmueller KL, Weeks KM. Near native structure in an RNA collapsed state. Biochemistry 2003; 42:13869-78; PMID:14636054; http://dx.doi.org/10.1021/bi035476k
- Behrouzi R, Roh JH, Kilburn D, Briber RM, Woodson SA. Cooperative tertiary interaction network guides RNA folding. Cell 2012; 149:348-57; PMID:22500801; http://dx.doi.org/10.1016/j.cell.2012.01.057
- Sahasranaman A, Dembowski J, Strahler J, Andrews P, Maddock J, Woolford JL Jr. Assembly of saccharomyces cerevisiae 60S ribosomal subunits: role of factors required for 27S pre-rRNA processing. EMBO J 2011; 30:4020-32; PMID:21926967; http://dx.doi.org/10.1038/emboj.2011.338
- Leidig C, Thoms M, Holdermann I, Bradatsch B, Berninghausen O, Bange G, Sinning I, Hurt E, Beckmann R. 60S ribosome biogenesis requires rotation of the 5S ribonucleoprotein particle. Nat Commun 2014; 5:3491; PMID:24662372; http://dx.doi.org/10.1038/ncomms4491
- Matsuo Y, Granneman S, Thoms M, Manikas RG, Tollervey D, Hurt E. Coupled GTPase and remodelling ATPase activities form a checkpoint for ribosome export. Nature 2014; 505:112-6; PMID:24240281; http://dx.doi.org/10.1038/nature12731
- Lo KY, Li Z, Bussiere C, Bresson S, Marcotte EM, Johnson AW. Defining the pathway of cytoplasmic maturation of the 60S ribosomal subunit. Mol Cell 2010; 39:196-208; PMID:20670889; http://dx.doi.org/10.1016/j.molcel.2010.06.018
- Fernandez-Pevida A, Rodriguez-Galan O, Diaz-Quintana A, Kressler D, de la Cruz J. Yeast ribosomal protein L40 assembles late into precursor 60 S ribosomes and is required for their cytoplasmic maturation. J Biol Chem 2012; 287:38390-407; PMID:22995916; http://dx.doi.org/10.1074/jbc.M112.400564
- Hedges J, West M, Johnson AW. Release of the export adapter, Nmd3p, from the 60S ribosomal subunit requires Rpl10p and the cytoplasmic GTPase Lsg1p. EMBO J 2005; 24:567-79; PMID:15660131; http://dx.doi.org/10.1038/sj.emboj.7600547
- Rodriguez-Mateos M, Garcia-Gomez JJ, Francisco-Velilla R, Remacha M, de la Cruz J, Ballesta JP. Role and dynamics of the ribosomal protein P0 and its related trans-acting factor Mrt4 during ribosome assembly in Saccharomyces cerevisiae. Nucleic Acids Res 2009; 37:7519-32; PMID:19789271; http://dx.doi.org/10.1093/nar/gkp806
- Ulbrich C, Diepholz M, Bassler J, Kressler D, Pertschy B, Galani K, Böttcher B, Hurt E. Mechanochemical removal of ribosome biogenesis factors from nascent 60S ribosomal subunits. Cell 2009; 138:911-22; PMID:19737519; http://dx.doi.org/10.1016/j.cell.2009.06.045
- Sengupta J, Bussiere C, Pallesen J, West M, Johnson AW, Frank J. Characterization of the nuclear export adaptor protein Nmd3 in association with the 60S ribosomal subunit. J Cell Biol 2010; 189:1079-86; PMID:20584915; http://dx.doi.org/10.1083/jcb.201001124
- Granneman S, Petfalski E, Tollervey D. A cluster of ribosome synthesis factors regulate pre-rRNA folding and 5.8S rRNA maturation by the Rat1 exonuclease. EMBO J 2011; 30:4006-19; PMID:21811236; http://dx.doi.org/10.1038/emboj.2011.256
- Greber BJ, Boehringer D, Montellese C, Ban N. Cryo-EM structures of Arx1 and maturation factors Rei1 and Jjj1 bound to the 60S ribosomal subunit. Nat Struct Mol Biol 2012; 19:1228-33; PMID:23142985; http://dx.doi.org/10.1038/nsmb.2425
- Bradatsch B, Leidig C, Granneman S, Gnadig M, Tollervey D, Bottcher B, Beckmann R, Hurt E. Structure of the pre-60S ribosomal subunit with nuclear export factor Arx1 bound at the exit tunnel. Nat Struct Mol Biol 2012; 19:1234-41; PMID:23142978; http://dx.doi.org/10.1038/nsmb.2438
- Karbstein K. Quality control mechanisms during ribosome maturation. Trends Cell Biol 2013; 23:242-50; PMID:23375955; http://dx.doi.org/10.1016/j.tcb.2013.01.004
- Bussiere C, Hashem Y, Arora S, Frank J, Johnson AW. Integrity of the P-site is probed during maturation of the 60S ribosomal subunit. J Cell Biol 2012; 197:747-59; PMID:22689654; http://dx.doi.org/10.1083/jcb.201112131
- Strunk BS, Novak MN, Young CL, Karbstein K. A translation-like cycle is a quality control checkpoint for maturing 40S ribosome subunits. Cell 2012; 150:111-21; PMID:22770215; http://dx.doi.org/10.1016/j.cell.2012.04.044
- Lebaron S, Schneider C, van Nues RW, Swiatkowska A, Walsh D, Bottcher B, Granneman S, Watkins NJ, Tollervey D. Proofreading of pre-40S ribosome maturation by a translation initiation factor and 60S subunits. Nat Struct Mol Biol 2012; 19:744-53; PMID:22751017; http://dx.doi.org/10.1038/nsmb.2308
- Garcia-Gomez JJ, Fernandez-Pevida A, Lebaron S, Rosado IV, Tollervey D, Kressler D, Kressler D, de la Cruz J. Final pre-40S maturation depends on the functional integrity of the 60S subunit ribosomal protein L3. PLoS Genet 2014; 10:e1004205; PMID:24603549; http://dx.doi.org/10.1371/journal.pgen.1004205
- Stelter P, Kunze R, Radwan M, Thomson E, Thierbach K, Thoms M, Hurt E. Monitoring spatiotemporal biogenesis of macromolecular assemblies by pulse-chase epitope labeling. Mol Cell 2012; 47:788-96; PMID:22819325; http://dx.doi.org/10.1016/j.molcel.2012.06.015
- Ding Y, Tang Y, Kwok CK, Zhang Y, Bevilacqua PC, Assmann SM. In vivo genome-wide profiling of RNA secondary structure reveals novel regulatory features. Nature 2014; 505:696-700; PMID:24270811; http://dx.doi.org/10.1038/nature12756
- Rouskin S, Zubradt M, Washietl S, Kellis M, Weissman JS. Genome-wide probing of RNA structure reveals active unfolding of mRNA structures in vivo. Nature 2014; 505:701-5; PMID:24336214; http://dx.doi.org/10.1038/nature12894
- Talkish J, May G, Lin Y, Woolford JL Jr, McManus CJ. Mod-seq: high-throughput sequencing for chemical probing of RNA structure. RNA 2014; 20:713-20; PMID:24664469; http://dx.doi.org/10.1261/rna.042218.113
- Peng Z, Oldfield CJ, Xue B, Mizianty MJ, Dunker AK, Kurgan L, Uversky VN. A creature with a hundred waggly tails: intrinsically disordered proteins in the ribosome. Cell Mol Life Sci 2014; 71:1477-504; PMID:23942625; http://dx.doi.org/10.1007/s00018-013-1446-6
- Schafer T, Maco B, Petfalski E, Tollervey D, Bottcher B, Aebi U, Hurt E. Hrr25-dependent phosphorylation state regulates organization of the pre-40S subunit. Nature 2006; 441:651-5; PMID:16738661; http://dx.doi.org/10.1038/nature04840
- Clatterbuck Soper SF, Dator RP, Limbach PA, Woodson SA. In Vivo X-Ray Footprinting of Pre-30S Ribosomes Reveals Chaperone-Dependent Remodeling of Late Assembly Intermediates. Mol Cell 2013; PMID:24207057
- Gilbert WV. Functional specialization of ribosomes? Trends Biochem Sci 2011; 36:127-32; PMID:21242088; http://dx.doi.org/10.1016/j.tibs.2010.12.002
- Xue S, Barna M. Specialized ribosomes: a new frontier in gene regulation and organismal biology. Nat Rev Mol Cell Biol 2012; 13:355-69; PMID:22617470; http://dx.doi.org/10.1038/nrm3359
- Kos M, Tollervey D. Yeast pre-rRNA processing and modification occur cotranscriptionally. Mol Cell 2010; 37:809-20; PMID:20347423; http://dx.doi.org/10.1016/j.molcel.2010.02.024
- Fox GE. Origin and evolution of the ribosome. Cold Spring Harb Perspect Biol 2010; 2:a003483; PMID:20534711
