Abstract
Epidemiological data suggest that prenatal exposure to either phthalates or flame retardants can affect mental and motor development, and can provoke internalizing behavior and attention deficit. We hypothesize that simultaneous exposure in utero to several environmental endocrine disruptors such as phthalates and flame retardants at low doses impairs brain development and leads to behavioral traits similar to those observed in Autism Spectrum Disorders (ASD). To characterize behavior relevant to ASD and common co-morbidities such as ADHD (Attention Deficit Hyperactivity Disorder) rat offspring were exposed perinatally to a mixture of phthalates and flame retardants (DEHP, DBP, DiNP, BDE-47, BDE-99) at low doses. Pregnant Lewis rats were divided into 3 groups: negative control; exposed to the mixture of endocrine disruptors; and a positive control for ASD, valproic acid, an antiepileptic drug known to cause autism in humans. Following perinatal exposure by daily gavage, behavioral tests were administered to offspring: nest-seeking behavior, auditory startle reflex, open field, elevated plus maze, and a test of social interactions. Offspring exposed to the mixture of phthalates and PBDEs showed hyperactivity, and the males had lower maternal bonding and reduced social interactions. In addition, both males and females from the exposure group showed a remarkable escaping behavior, not present in the other groups. Exposure to low doses of phthalates and flame retardants provokes behavioral alterations consistent with certain autistic- and ADHD-like traits.
Abbreviations
| ADHD | = | Attention Deficit/Hyperactivity Disorder |
| AGD | = | ano-genital distance |
| ASD | = | Autism Spectrum Disorders |
| ATSDR | = | Agency for Toxic Substances & Disease Registry |
| BDE-47 | = | 2,2´,4,4´-tetrabromodiphenyl ether |
| BDE-99 | = | 2,2´,4,4,5´-pentabromodiphenyl ether |
| CDC | = | Center for Disease Control and Prevention |
| CI | = | confidence interval |
| DBP | = | Dibutyl Phthalate |
| DEHP | = | di(2-ethylhexyl)phthalate |
| DiNP | = | Diisononyl Phthalate |
| E6 | = | Embryonic day 6 or Gestational Day 6 |
| MRL | = | Minimum Risk Level |
| NOAEL | = | No Observable Adverse Effect Level |
| P4 | = | Post-natal day 4 |
| PBDE | = | Poly Bromo Diphenyl Ether |
| PVC | = | PolyVinyl Chloride |
| SD | = | standard deviation |
| VPA | = | valproic acid |
Introduction
Autism Spectrum Disorders (ASD), are characterized by 3 core symptoms: altered communications, social difficulties, and stereotyped and restricted behaviors and interests. An explosion in the prevalence of ASD the last 50 years has been well documented and the prevalence has now reached 1/68 children in North America.Citation1 In California, a 600% increase in ASD over 20 years has been reported.Citation2 The increase in ASD cases is only partly attributable to better diagnosis and therefore suggests an underlying interaction of genetic susceptibility with unknown environmental factors,Citation3 since genetics alone clearly cannot explain the exponential increase. Considering that ASD is 5-fold more prevalent in boys, which corresponds to a prevalence of 1/42,Citation1 we hypothesize that exposure to ubiquitous environmental endocrine disruptors during a critical window of brain development can cause ASD. The use of endocrine disrupting chemicals has increased exponentially over the last several decades, in parallel with the dramatic increase in the prevalence of ASD.
Phtalates and flame retardants such as PBDEs (Polybrominated Diphenyl Ethers) are 2 groups of chemical compounds well-known for acting as endocrine disruptors. Phthalates are present in virtually all plastic objects, in PVC (PolyVinyl Chloride), and also in cosmetics.Citation4 PBDEs, which regroup 209 congeners, are used as flame retardants in plastic objects, foams, and textiles.Citation5 Several studies have established that the entire population living in industrialized countries is exposed daily to these endocrine disruptors at different doses depending on occupation and lifestyle.Citation`6-9
Several human epidemiological studies suggest that perinatal exposure to phthalates and flame retardants is associated with behavioral alterations in infants and children toward autistic traits. Higher gestational metabolite concentrations of several phthalates have been associated with: lower mental and physical developmental scores among infantsCitation10; reduced physical development and more internalizing behavior in 3-year-oldsCitation11; symptoms of ADHD (Attention Deficit Hyperactivity Disorder), aggressive behaviors, depression and altered emotional control among 4–7-year-oldsCitation12,13; and autistic traits among 7–9-year-olds.Citation14 Gestational PBDE exposure has been associated with less sustained attention in 5–6-years-oldsCitation15 and lactational PBDE exposure with increased risk of hyperactivity/impulsivity behaviors, in toddlers.Citation16 An epidemiological study has associated post natal PBDE exposure with attention deficit and poor social competence.Citation17
The toxicity data given in the following paragraph are directly extracted from either the website of the Agency for Toxic Substances & Disease Registry (ATSDR), Atlanta, GA, or the website of Health Canada, Ottawa, ON. The estimated Minimal Risk Level (MRL) of human exposure for several congeners of PBDEs including BDE-47 and BDE-99, based on animal studies, is 0.03 mg/kg/day for acute exposure (14 days or less) according to ATDSR. This is calculated with the NOAEL (No Observable Adverse Effect Level) estimated at 1 mg/kg in rats divided by an uncertainty factor of 30: a factor of 10 for the translation of rats to humans (toxicokinetics and metabolic differences) and a factor of 3 for human variability; the usual factor of 10 for human variability is not used specifically here because the NOAEL is already based on effects observed in a sensitive subgroup, otherwise all other MRLs presented here used a factor 10. The same computation has been made for chronic exposure and the MRL is 0.007 mg/kg/day. The Canada Health agency calculated that newborns/infants in Canada have a mean exposure to PBDEs of approximately 0.002 mg/kg/day by taking into account of PBDE levels in human milk, in ambient air and in water. Concerning phthalates, data from the ATDSR are available mainly for DEHP for which the estimated MRL for acute exposure is 0.1 mg/kg/day and for chronic exposure is 0.06 mg/kg/day. Health Canada had estimated the mean exposure of DEHP for newborns/infants to be between 0.01 to 0.02 mg/kg/day.
In experimental studies, perinatal exposure of rodents to DBP (Dibutyl Phthalate), or DiNP (Diisononyl Phthalate), or DEHP (di(2-ethylhexyl)phthalate), 3 of the most prevalent phthalates in our environment, causes altered sexual development and impaired spatial learning at very high exposures (500 to 900 mg/kg of body weight per day).Citation18-20 Exposure to DBP at 10 μg/kg/day caused decreased grooming behavior.Citation21 Despite the abundant epidemiological evidence, no other experimental studies have focused on the impact of developmental exposure to phthalates on behavior. Concerning PBDEs, there are several studies showing behavioral alterations in developing rodents exposed to high doses (1 to 20 mg/kg/day)Citation22-24; however, few data are available on PBDE exposure at low doses. Our previous experimental data have linked perinatal exposure to BDE-47 to locomotor hyperactivity at very low doses (2 to 200 μg/kg every 5 days).Citation25 In addition, exposure to BDE-99 (60 μg/kg/day) has been associated with locomotor hyperactivity after a single exposure in utero at gestational day 6 (E6).Citation26
However, there is no animal study simulating real-life human exposure to phthalates and flame retardants (i.e. simultaneous chronic low dose exposure) to detect autistic- and ADHD-like traits in perinatally exposed animals. Our goal is to characterize behavior relevant to autistic features in rats perinatally exposed to a mixture of phthalates and flame retardants at low doses. In order to have a positive control group for autistic-like traits, we used valproic acid, known to induce ASD in humans and commonly used to induce this phenotype in rats.
Results
Development
No change in the timing of developmental landmarks was found in rats exposed to endocrine disruptors vs. controls (see ): litter size, ear canal opening, incisor eruption, fur growth, eye opening, body weight, and reflex acquisition. One female from the negative control group and 2 females from the positive control were not pregnant when we assigned them, which explains the unequal number of litters per group in the results (6 negative controls, 7 exposed to the mixture, 5 VPA-exposed with 3 pregnancy losses).
Table 1. Summary of developmental indicators
Exposure to 600 mg/kg of VPA had effects on gestation and the pups’ development. For the 5 dams exposed to VPA, only 2 delivered, and the 3 remaining had embryo implantation spots revealing embryo resorption following the exposure. In the 2 surviving litters, we observed a decreased body weight at birth (mean ± SD weight in controls: 6.76 g ± 0.09 vs VPA: 5.98 g ± 0.12; P < 0.0001) which persisted until the last weighing at P40 (mean ± SD weight in controls: 132.59 g ± 2.24 vs VPA: 111.23 g ± 2.36; P < 0.0001). Pups from these 2 litters presented a general developmental delay: a one to 2 day delay in all developmental landmarks. At P42, the brains of VPA-exposed animals were significantly lighter compared to controls (mean ± SD weight in controls: 1.269 g ±0.009 vs VPA: 1.096 g ± 0.009; P < 0.0001), while there were no differences in brain weight between offspring exposed to endocrine disruptors (mean ± SD 1.253 g ± 0.008; p = 0.16) and controls.
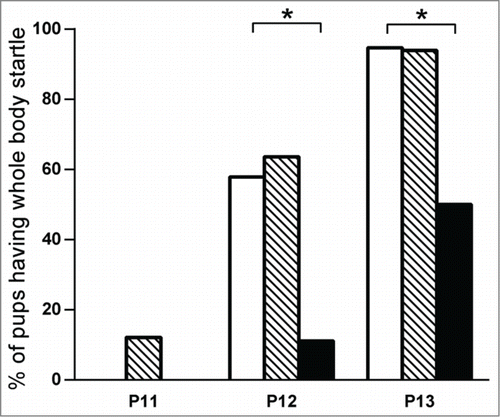
The auditory startle reflex was acquired between P12 and P13 in pups from the control group as well as the endocrine disruptors group. We observed a one day delay for the acquisition of this reflex in pups exposed to VPA, confirming the general developmental delay (see ). When pups were retested at P14, all had acquired the reflex (not shown).
Behavioral tests
Nest Seeking Behavior: No difference was found in the percentage of pups exhibiting nest seeking behavior (discrimination of maternal olfactory cues) between the control group and the group exposed to endocrine disruptors in both trials and in both sexes. A drastic effect was observed in pups exposed to VPA with very few pups showing nest seeking behavior, specifically in males in both trials (Controls: 65% and 76% vs VPA: 10% and 30%; p = 0.006 and p = 0.018) (see ). For pups which were able to find the maternal bedding, the time needed to reach the maternal area was recorded in 2 trials. Control pups were faster to reach the maternal area in the second trial for both males (mean ± SD time: 21.9 s ± 1.9 vs. 14.8 ± 1.4; p = 0.004) and females (mean ± SD time: 23.3 s ± 2.3 vs. 16.8 s ± 2.3; p = 0.07). This decrease in the time to locate the maternal area in the second trial was not observed in males exposed to endocrine disruptors (mean ± SD time: 21.8 s ± 2.0 vs. 20.9 s ± 1.5; p = 0.72) but was observed for females (mean ± SD time: 24.3 s ± 1.4 vs. 17.3 s ± 1.4; p = 0.003). In pups exposed to VPA, there was no difference between the first and second trials in time spent to reach the maternal area in both males (mean ± SD time: 23.2 s ± 6.1 vs. 19.7 s ± 3.1; p = 0.59) and females (mean ± SD time: 25.1 s ± 3.7 vs. 21.9 s ± 3.7; p = 0.54), but this analysis was performed on a small number since very few pups were successful in this task (n = 4/10 males and 4/8 females) (see ).
Figure 2. Nest-seeking behavior test performed at PND8. * P < 0.01; # P < 0.1. (A) Percentage of pups crossing the line toward maternal area with the head and the forepaws in males (left) and females (right): negative control group (in white) n = 30; exposed to endocrine disruptors group (striped) n = 44; positive control (VPA) group for autistic features (in black) n = 18. (B) Time spent to reach maternal area for successful pups: males (left) and females (right): negative control group (in white) n = 24; exposed to endocrine disruptors group (striped) n = 32; positive control (VPA) group for autistic features (in black) n = 8.
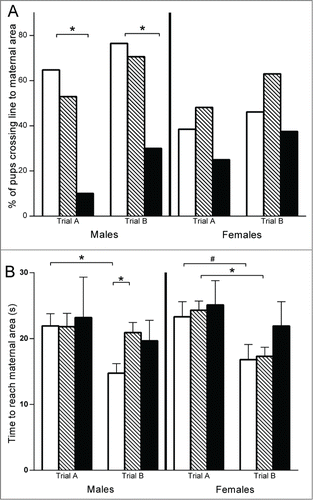
Figure 3. Open Field (A) and Elevated Plus Maze (B). Negative control group (in white) n = 20; exposed to endocrine disruptors group (striped) n = 28; positive control (VPA) group for autistic features (in black) n = 18. * P < 0.01; # P < 0.1. (A) Results expressed as a percentage of control for distance traveled and time of mobility in open field test performed at P20. All statistical tests were performed on raw data, and data were transformed to percent of controls for the figures. (B) Results expressed as a percentage of control for distance traveled (overall, in open arms, and in closed arms) in elevated plus maze test performed at P25.
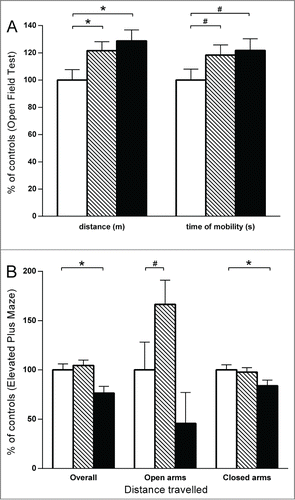
Open Field and Elevated Plus Maze: Pups from litters exposed to endocrine disruptors and those exposed to VPA showed the same increased level of locomotor behavior in the open field test with increased distance traveled (mean ± SD distance in controls: 4.49 m ± 0.35 vs. endocrine disruptors: 5.46 m ± 0.29 p = 0.038; vs. VPA: 5.78 m ± 0.40 p = 0.017) and time of mobility (mean ± SD time in controls: 79.0 s ± 6.4 vs. endocrine disruptors: 93.5 s ± 5.4 p = 0.08; vs. VPA: 96.2 s ± 6.8 p = 0.06) compared to the negative control group (+ 20% to 30%) with no differences between males and females (see ). Males from control and endocrine disruptors group had more distance traveled in the central area of the open field compared to their respective females (mean ± SD distance in central area for male controls: 0.82 m ± 0.12 vs. female controls: 0.51 m ± 0.12 p = 0.041; males endocrine disruptors: 1.00 m ± 0.1 vs. females endocrine disruptors: 0.61 m ± 0.1 p = 0.003) but there was no differences between the sexes in the VPA group (males VPA: 0.73 m ± 0.14 vs. females VPA: 0.72 m ± 0.15 p = 0.97). There were also no differences between the sexes in the control and VPA groups for the time of mobility in the central area (mean ± SD time in center for males controls: 17.7 s ± 5.1 vs. females controls: 15.5 s ± 3.1 p = 0.685; males VPA: 17.9 s ± 6.6 vs. females VPA: 14.09 s ± 6.9 p = 0.510); but males exposed to endocrine disruptors had more time of mobility in the central area (male, endocrine disruptors: 29.5 s ± 4.3 vs. female, endocrine disruptors: 14.0 s ± 4.3 p = 0.0015)
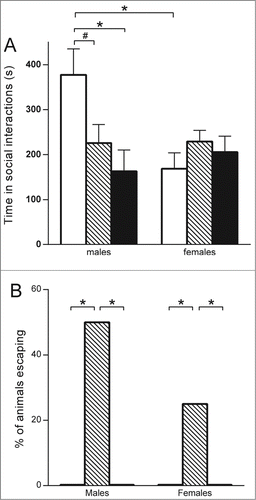
Figure 4. Social interaction test performed at P40. * P < 0.01; # P < 0.1. (A) Mean time spent in social interactions (active playing, pursuing, and sniffing and grooming each other), in males (left) (control n = 4; exposed to endocrine disruptors n = 8; positive control (VPA) group n = 6) and in females (right) (control n = 4; exposed to endocrine disruptors n = 8; positive control (VPA) group n = 6). (B) Escaping behavior specific to rats exposed to endocrine disruptors (striped, n = 8 males and 8 females), no rats in the negative control group (in white, n = 4 males and 4 females) nor in the positive control (VPA) group (n = 6 males and 6 females) demonstrated this behavior.
The increased locomotor activity of pups exposed to endocrine disruptors was confirmed in the elevated plus maze (Fig. 3B) with more distance traveled in the open arms of the maze (+60% compared to controls) with no difference between males and females (mean ± SD distance in controls: 0.59 m ± 0.17 vs. endocrine disruptors: 0.99 m ± 0.15; p = 0.08). Pups exposed to VPA, but not those from the group exposed to endocrine disruptors, exhibited an anxious behavior profile in the elevated plus maze with less distance traveled overall (mean ± SD distance in controls: 9.87 m ± 0.61 vs. VPA: 7.55 m ± 0.69; p = 0.008), in both open arms (-50% compared to control; p = 0.21) and closed arms (-20% compared to controls; p = 0.04) (see ).
Social Interactions: Total mean time spent in social interactions was decreased specifically in male pups exposed to endocrine disruptors and those exposed to VPA, compared to controls (mean ± SD time in interaction for controls: 377.4 s ± 58.0 vs. endocrine disruptors: 225.8 s ± 41.0 p = 0.06; and vs. VPA: 162.9 ± 47.4 p = 0.02) (see ). The total number of social interactions was reduced only in males exposed to VPA compared to controls, but not in males exposed to endocrine disruptors (mean ± SD number of interaction in controls: 44.0 ± 6.8 vs. VPA: 23 ± 5.5 p = 0.05; and vs. endocrine disruptors 39.8 ± 4.8; p = 0.6). As expected, females from the control group spent less time in social interactions compared to males (mean ± SD time in interaction in males: 377.4 s ± 58.0 vs. females: 168.3 s ± 35.4; p = 0.01). However, this naturally occurring gender effect in social behavior was absent in rats exposed to endocrine disruptors (mean ± SD time in interaction in males: 225.8 s ± 41.0 vs. females: 229.1 s ± 25.0; p = 0.95) and in those exposed to VPA (mean ± SD time in interaction in males: 163.0 s ± 41.4 vs. females: 205.6 ± 35.4; p = 0.48). Males from these 2 groups showed 40% fewer social interactions compared to males from the control group. Moreover, we observed an unexpected escaping behavior during this test specifically in rats exposed to endocrine disruptors, in both males and females. Fifty percent of males and 25% of females (exclusively in the group exposed to endocrine disruptors) tried, at least once, to escape by climbing out of the cage during the test (see ). Some animals exhibited this behavior over the entire session of the test, both males and females, with up to 10 escaping episodes per session.
Discussion
Our study is the first to examine autistic-like traits in rodents exposed to phthalates and PBDEs. Our data show that perinatal exposure to low doses of a mixture of endocrine disruptors substantially alters the behavior of the offspring, with gender specificity. Male offspring showed altered olfactory discrimination of maternal odour in the nest-seeking behavior test and reduced social interactions. In addition, both male and female offspring exposed to this mixture show increased locomotor activity and a remarkable escaping behavior.
Exposure of rats to 600 mg/kg of VPA at E12 is a model first characterized by Schneider and Przewlocki, 2005Citation27 for its relevance to autistic behavior. It has since been used as a model of autism in numerous animal studies, given that VPA is known to increase the risk of ASD/autism 5-fold in children exposed in utero to this antiepileptic drug,Citation28 and many altered behaviors relevant to ASD features in humans have already been described for this model (see Citation29 for an exhaustive review). Using this model in our present study allows us to compare the behavior of pups exposed to endocrine disruptors with those of pups exposed to VPA, in order to validate the relevance to autistic behaviors ().
Table 2. Summary of behavioral testing results: Comparison between groups exposed to endocrine disruptors and valproic acid
The dose of VPA we used is that which is currently used in the literature to induce an autistic behavior in rats (600 mg/kg at E12). This dose clearly creates autistic traits in rat offspring, but it also alters their development and even induces embryo resorption. These effects are consistent with the adverse pregnancy outcomes observed with VPA exposure in pregnant women. In the absence of prophylactic supplementation of folic acid, a rate of 25% of spontaneous abortion in women under VPA monotherapy has been observed.Citation30 In addition, a rate of 16% of fetal malformations has been reported following in utero exposure to VPA compared to a 3.1% rate in pregnancies without anti-epileptic drugs or 2.4% in pregnancies with other anti-epileptic medication.Citation31 Moreover, in utero VPA exposure has already been linked to an increased risk of ASD (OR 2.9 [95% CI 1.7–4.9]) and childhood autism (OR 5.2 [95% CI 2.7–10.0]).Citation28 Finally, it is interesting to note that in a cohort of young children (3–5 years old) with ASD, it has been observed that 25% of the children also have a mild developmental delay and 44% have a moderate to severe developmental delayCitation32 consistent with our observations in rats. Thus, the autistic rat model induced by 600 mg/kg of VPA may be more representative of the severe autistic phenotype rather than the mild form. In agreement with our findings, a teratogenic effect has been reported for in utero exposure to VPA at the dose commonly used for the autistic rat model: a 50% rate of fetal resorption after exposure to 600 mg/kg and 25% after exposure to 500 mg/kg both at E12.Citation33 In order to develop a model of mild autistic phenotypes, the effects of lower doses of VPA need to be characterized. Finally, the rat model of VPA induced autism needs to be refined with better characterization of behavioral phenotypes using a range of doses and the effects on development and autistic traits need to be compared.
According to the CDC, the sex ratio for ASD is 5:1 (boys/girls),Citation1 and we therefore characterized alterations in behavior that were specific to males. Adult males from litters exposed to either VPA or endocrine disruptors showed marked alterations in social interactions. First, we observed the typical sex difference in social behavior in control rats already described in the scientific literature: males from the control group spent more time in social interactions compared to females, as expected.Citation34,35 In contrast, in our study, males exposed to endocrine disruptors or VPA exhibited fewer social interactions than control males, and their interactions declined to the same level as for females from their corresponding groups. This finding is highly relevant to ASD for which social impairment is one of the most important features and is one of the main criteria for ASD diagnosis.Citation36
In addition, we characterized one other altered sex-specific behavior. The nest seeking behavior test is based on the recognition of maternal olfactory cues as a means of communication between very young pups and their dam in order to orient themselves and to be able to find the nest.Citation37 Compared to control pups, which executed the task faster in the second trial, male pups exposed to endocrine disruptors and those exposed to VPA showed no improvement in the second trial. This lack of improvement could be due to a deficit in short term memory or alterations in the learning process. As already discussed by Favre, et al.,Citation33 this observation cannot be explained by a primary olfactory deficit in these animals. If this were the case, we would expect animals to gravitate toward the maternal area or the fresh clean bedding area in a random manner. However, approximately the same percentage of pups in the control group and the group exposed to endocrine disruptors found the nest. Furthermore, in accordance with Favre, et al.,Citation33 this observation is unlikely to be due to deficits in motor locomotion because we did not observe any spontaneous motor defects later in life (open field test and elevated plus maze). We propose that the lack of improvement in the time to reach the maternal area in the second trial, specifically in males exposed to endocrine disruptors or VPA is indicative of a defect in the communication between the dam and the pup, reflecting one specific type of maternal bonding. However, this observation can also be interpreted as a learning deficit compared to controls. We will need further studies on maternal behavior to confirm this link between these findings in the nest-seeking behavior and maternal bonding, and to perform specific tests to evaluate the learning ability of the rats exposed to the endocrine disruptors mixture.
For now, we think that this lack of seeking the maternal odour might be correlated with human autistic behavior such as lack of eye contact, lack of response to name and altered communication which are important impairments in autistic children.Citation38,39
Our hypothesis to explain why PBDEs and phthalates affect male rats more than females is that these endocrine disruptors interfere with sexual hormone homeostasis during fetal development. The development of the brain networks responsible for the behavioral differences between the sexes is highly dependent on in utero sex hormone balance (i.e., testosterone and estradiol).Citation40 The enzyme aromatase transforms testosterone to estradiol directly in both neural and glial cells,Citation41 and the brain exhibits an important sexual dimorphism regarding the expression and the activity of this enzyme in specific brain areas at specific times such as during the early period of sex differentiation.Citation42 Males undergo a major production of testosterone by the fetal testis, beginning at the sixth week of pregnancy in humans and reaching a maximum near the second trimester. This burst of testosterone production in the male rat fetus starts at E15Citation43 which is why we started our perinatal exposure at this date. This high level of testosterone is essential for the development of male typical behaviors, which is called masculinisation of the brain. Female brain development is much less dependent on the testosterone/aromatase pathway since they have a very limited production of testosterone.Citation44 PBDEs and phthalates have been reported to inhibit production of testosterone in the fetal testis.Citation45-47 Moreover, PBDEs inhibit aromatase activityCitation48 and phthalates alter aromatase activity in a non-linear dose-response manner: they inhibit aromatase activity at low doses, but increase aromatase activity at high doses of exposure.Citation49 Thus, at the low dose exposures used in our study, these endocrine disruptors have the potential to reduce estrogen availability in neural and glial cells during the testosterone peaks of male brain development, and therefore to provoke behavioral alterations, especially in males.
We also characterized alterations in the behavior in both males and females in our experimental groups compared to the controls. The remarkable escaping behavior we observed during the social interactions test in both males and females exposed to endocrine disruptors deserves special attention. This behavior occurred only during the test session for social interactions, when there were 2 animals per cage; during the familiarization session the day preceding the test, when the animal was alone in the apparatus, none exhibited this escaping behavior. This behavior could reflect a high social avoidance and/or the display of a social anxiety phenotype heightened by the presence of the unknown congener. However, this behavior is also consistent with hyperactivity and/or impulsivity and we think it may also be related to the risk-taking/avoidance behavior which is a common trait among autistic subjects.Citation50,51 Furthermore, in our previous experiments with BDE-47,Citation25 this escaping behavior was noted in pups after weaning and housed with 3-4 rats per cage. At that time, Wistar rats exposed to BDE-47 escaped their cages on a daily basis by forcing open the tops of the cages until we took special measures (i.e. reinforcing the tops of their cages and giving them toys). At that time, the observation of this escaping behavior was only anecdotal but our findings in the present study confirm the previous observations.
Finally, offspring exposed to endocrine disruptors display hyperactive behavior in the open field test at the same level as our autistic VPA-exposure model. This hyperactivity was confirmed in the elevated plus maze test for pups exposed to endocrine disruptors (both sexes) but not for the VPA model, for which anxious behavior was present. Moreover we observe a sexually dimorphic behavior concerning the distance traveled and the time of mobility in the center of the box during the open field test. This difference was present in both groups: control and exposed to endocrine disruptors, and can be explained as mentioned before, that is, female rats are more anxious than male rats. But the males from the endocrine disruptors group were more active in this zone that the other males. This is consistent with the hyperactive behavior of the males exposed to endocrine disruptors, and also correlates with the above mentioned escaping behavior. Hyperactive behavior is relevant to autistic phenotypes in humans considering the high rate of co-morbidity between ASD and ADHD (41 to 78% of children with ASD also meet the criteria for ADHD).Citation32,52
Methods
Animals and treatment
We obtained 21 timed pregnant Wistar rats (180–240 g) from Charles River Laboratories (St. Constant, Québec, Canada) on E11–13. They were housed individually in plastic cages with bedding and under regulated temperature (21 ± 2°C) and humidity (50 ± 10%), and a 12 h light/dark cycle (6 h-18 h). Food (Rodent Chow 5075; Charles River Laboratories) and water were provided ad libidum. All animals received care in compliance with the Guide to the Care and Use of Experimental Animals from the Canadian Council of Animal Care and the protocol was approved by our institutional animal research ethics committee. As soon as we received the pregnant dams, we randomly assigned them to 3 groups of 7 dams each: control (exposed to vehicle peanut oil), exposed to the mixture of endocrine disruptors, or exposed to valproic acid (VPA).
At E12, dams assigned to the positive control (VPA) group for autistic features received a gavage of 600 mg/kg of VPA solution (Depakene) purchased from the pharmacy of the Center Hospitalier Universitaire de Sherbrooke (CHUS), QC, Canada. All other chemical substances were purchased from Chromatographic Specialties INC (Brockville, ON, Canada). 2,2´,4,4´-tetrabromodiphenyl ether (BDE-47), and 2,2´,4,4,5´-pentabromodiphenyl ether (BDE-99) (between 99% and 100% purity, 5 mg solid each) were dissolved with methanol in one solution of 10 mL (1 mg/mL). Di(2-ethylhexyl)phthalate (DEHP), diisononyl phthalate (DiNP), and di-n-butyl phthalate (DBP) (between 99% and 100% purity, 100 mg liquid each) were mixed into one solution of 10 mL with peanut oil as the vehicle (10 mg/mL). Solutions were stored at 4°C during the experiment. Every morning from E15 to Post-natal day 4 (P4) dams were weighed and the appropriate gavage mixture, diluted in peanut oil (vehicle), was prepared daily. The gavage took place every morning from E15 to P4 except for the 3 days around the delivery (namely G22, GD23, P1) in order to prevent stress to the dam around delivery, giving a total of 10 days of exposure. Dams from the control group received 500 μL of vehicle by gavage daily. Dams from the exposed group received 500 μL of gavage solution containing 14 μg/kg of BDE-47 and 99, and 4.5 mg/kg of DEHP, DBP, and DiNP.
Our doses of exposure were based on our previous work and on NOAEL (No Observable Adverse Effect Level) in rats estimated by ATSDR (Agency for Toxic Substances & Disease Registry, Atlanta, GA) in order to be well below toxic doses and to simulate daily human exposure. All data listed below are from the ATSDR website (http://www.atsdr.cdc.gov). Concerning the phthalates in the mixture: (i) for DEHP, only NOAEL for intermediate-duration oral exposure (15–364 days) is available: 14 mg/kg/day; (ii) for DBP, NOAEL for acute-duration oral exposure (14 days or less) is 50 mg/kg/day; (iii) no NOAEL for DiNP is available. Concerning PBDEs, NOAEL for individual congeners are not available but for penta-BDE, a commercial mixture containing both BDE-47 and BDE-99, is 1 mg/kg/day for acute-duration oral exposure (14 days or less). Our previous study had shown that perinatal exposure to BDE-47 at doses ranging from 2 μg to 200 μg/kg of BDE-47 every 5 days results in an exposure within the range reported for human exposure in North America.Citation25 In continuity with this work, we choose to administer an equivalent dose by daily gavage.
Dams were allowed to deliver and the litters were not reduced. All pups were identified at P1 and weighed at P1, P3, P5 and then every 5 days until euthanasia at P42. Developmental landmarks were evaluated in all pups daily at appropriate developmental windows for each, such as: ear canal opening (checked from P2 to P4), eruption of incisors (P6 to P9), hair appearance and fur development (P5 to P14), and eye opening (P14 to P17). Dams and pups were kept in the same cage until weaning at P30. At PND15, 2 males and 2 females from each litter were randomly selected for further behavioral testing. At least one male and one female from each litter went through all the behavioral tests. All other pups were euthanized at P15. All pups from litters exposed to VPA were tested to increase the number of subjects after the loss of 3 litters (see first section of results). After weaning, males and females were separated, housed 3 to 4 per cage, and dams were euthanized.
Behavioral testing
All behavioral tests were video recorded for further observations and analysis, and performed between 9 a.m. and 4 p.m. by the same person.
Nest-seeking behavior: This test was performed as previously described elsewhere.Citation37 At P8, the pup was placed at the center of an experimental cage (26 cm x 17 cm x 14.5 cm) divided into 3 equal compartments: (i) left: covered with home cage bedding; (ii) center: no bedding; (iii) right: fresh clean bedding. Each pup had 2 trials of 30 seconds with an interval of 1 min with orientation of the head counterbalanced between trials. When the pup walked into the zone filled with home cage bedding and the head and the front paws crossed the line of this area, it was scored as a success. The time spent choosing the home bedding zone was recorded. At the time of testing, pups still had closed eyelids, so the test is based solely on the recognition of maternal olfactive cues in order to locate the nest.
Auditory startle: Every day for P11 to P13, the pup was placed on a flat surface and the ability to show a whole-body startle response to a clicker sound at approximately 10 cm from the ears was measured and scored, as previously described elsewhere.Citation37
Open Field: At P20, the rat was placed in the apparatus, a box with a 40 cm2 base with video camera placed above connected to a computer running ANY-Maze® software (Stoelting CO, USA). Each rat was initially placed in the same orientation and all trajectories were analyzed during a 5 min session, as we described previously.Citation25
Elevated Plus-Maze: At P25, the rat was placed at the center of the apparatus composed of an elevated cross (50 cm above the floor) with 2 open arms (16 lux) and 2 enclosed arms (8 lux). A video camera was placed above the apparatus and was connected to a computer running ANY-Maze® software (Stoelting CO, USA). Each rat was initially placed in the same orientation and all trajectories were analyzed during a 5 min session, as previously described.Citation53
Social Interactions: At P40, 2 rats from the same group, same sex, and approximately the same weight, but who had never met before, were placed in the same cage (26 cm × 17 cm × 14.5 cm). A Plexiglas separation in the center of the cage created 2 distinct areas, which the rats are able to reach through a passage allowing the passage of one rat at a time, as described previously.Citation54 The day before the test, all animals had a 5 min session alone in the apparatus to become familiarized with the cage. The 10 min session of testing was recorded with a camera placed above the apparatus and was manually analyzed by a person blinded to the exposure group of each pup. Behavior recorded as social interactions was: playing, pursuing, sniffing and grooming each other.
Statistical analysis
All statistical analyses were performed using SAS/STAT software (SAS Institute, version 9.2). Mixed modeling with random statement to control within-litter effect was applied for analysis of the time variable in the nest-seeking behavior test (with repeated measures statement for the 2 trials), the open field test, and the elevated plus maze test. Individual weights on the day of testing and litter size were introduced in each model as fixed effects, but these variables were not significantly related to test performances and were removed from the final models. ANOVA was applied for analyses of: (i) the social interaction test since we used only one pup of each sex per litter, (ii) body weight and brain weight monitoring data, both normally distributed. Sex was introduced in each model as fixed effect, and when there was a significant interaction of this factor, secondary analyses were performed for each sex separately and compared to each other in all groups. Chi squared test, or Fisher exact test when needed, was performed for analysis of scores in nest-seeking behavior and auditory startle tests.
Conclusion
Perinatal exposure to a mixture of well-known endocrine disruptors, phthalates and PBDEs, at low doses alters the behavior of rat offspring toward autistic traits with more alterations observed in males, consistent with the observed sex ratio for ASD in humans (5:1 male:female). In addition, we observed less maternal bonding, hyperactivity, decreased social interactions, and an unusual escaping behavior. More studies are needed in order to understand how these endocrine disruptors provoke neurobehavioral alterations, as well as the role of the brain aromatase pathway as a potential target of these endocrine disruptors.
Disclosure of Potential Conflicts of Interest
There are no potential conflicts of interest.
Acknowledgment
We wish to thank Julie Bergeron, Marie-Elsa Brochu, and Jessica Deslauriers for their advice concerning the protocol development. These results were presented at the Gordon Research Conference: Environmental Endocrine Disruptors (May 11–16, 2014, Lucca, Italy).
Funding
This research was funded by the Foundation of Stars and the Centre d’Excellence Mère-Enfant de l’Université de Sherbooke.
References
- Developmental Disabilities Monitoring Network Surveillance Year 2010 Principal Investigators. Prevalence of autism spectrum disorder among children aged 8 years - autism and developmental disabilities monitoring network, 11 sites, United States, 2010. MMWR Surveill Summ 2014; 63 Suppl 2:1-21
- Hertz-Picciotto I, Delwiche L. The rise in autism and the role of age at diagnosis. Epidemiology 2009; 20(1):84-90; PMID:19234401; http://dx.doi.org/10.1097/EDE.0b013e3181902d15
- Weintraub K. The prevalence puzzle: Autism counts. Nature 2011; 479:22-24; PMID:22051656; http://dx.doi.org/10.1038/479022a
- Berge A, Cladiere M, Gasperi J, Coursimault A, Tassin B, Moilleron R. Meta-analysis of environmental contamination by phthalates. Environ Sci Pollut Res Int 2013; 20(11):8057-8076; PMID:23917738; http://dx.doi.org/10.1007/s11356-013-1982-5
- Williams AL, DeSesso JM. The potential of selected brominated flame retardants to affect neurological development. J Toxicol Environ Health B Crit Rev 2010; 13(5):411-448; PMID:20582854; http://dx.doi.org/10.1080/10937401003751630
- Abdelouahab N, Langlois MF, Lavoie L, Corbin F, Pasquier JC, Takser L. Maternal and cord-blood thyroid hormone levels and exposure to polybrominated diphenyl ethers and polychlorinated biphenyls during early pregnancy. Am J Epidemiol 2013; 178(5):701-713; PMID:23924579; http://dx.doi.org/10.1093/aje/kwt141
- Lee J, Lee JH, Kim CK, Thomsen M. Childhood exposure to DEHP, DBP and BBP under existing chemical management systems: A comparative study of sources of childhood exposure in korea and in denmark. Environ Int 2014; 63:77-91; PMID:24270398; http://dx.doi.org/10.1016/j.envint.2013.10.020
- Lenters V, Thomsen C, Smit LA, Jönsson BA, Pedersen HS, Ludwicki JK, Zviezdai V, Piersma AH, Toft G, Bonde JP, et al. Serum concentrations of polybrominated diphenyl ethers (PBDEs) and a polybrominated biphenyl (PBB) in men from greenland, poland and ukraine. Environ Int 2013; 61:8-16; PMID:24091254; http://dx.doi.org/10.1016/j.envint.2013.09.001
- Fromme H, Lahrz T, Kraft M, Dietrich S, Sievering S, Burghardt R, Schuster R, Bolte G, Völkel W. Phthalates in german daycare centers: Occurrence in air and dust and the excretion of their metabolites by children (LUPE 3). Environ Int 2013; 61:64-72; PMID:24103347; http://dx.doi.org/10.1016/j.envint.2013.09.006
- Kim Y, Ha EH, Kim EJ, Park H, Ha M, Kim JH, Hong YC, Chang N, Kim BN. Prenatal exposure to phthalates and infant development at 6 months: Prospective mothers and children's environmental health (MOCEH) study. Environ Health Perspect 2011; 119(10):1495-1500; PMID:21737372; http://dx.doi.org/10.1289/ehp.1003178
- Whyatt RM, Liu X, Rauh VA, Calafat AM, Just AC, Hoepner L, Diaz D, Quinn J, Adibi J, Perera FP, et al. Maternal prenatal urinary phthalate metabolite concentrations and child mental, psychomotor, and behavioral development at 3 years of age. Environ Health Perspect 2012; 120(2):290-295; PMID:21893441; http://dx.doi.org/10.1289/ehp.1103705
- Kim BN, Cho SC, Kim Y, Shin MS, Yoo HJ, Kim JW, Yang YH, Kim HW, Bhang SY, Hong YC. Phthalates exposure and attention-deficit/hyperactivity disorder in school-age children. Biol Psychiatry 2009; 66(10):958-963; PMID:19748073; http://dx.doi.org/10.1016/j.biopsych.2009.07.034
- Engel SM, Miodovnik A, Canfield RL, Zhu C, Silva MJ, Calafat AM, Wolff MS. Prenatal phthalate exposure is associated with childhood behavior and executive functioning. Environ Health Perspect 2010; 118(4):565-571; PMID:20106747; http://dx.doi.org/10.1289/ehp.0901470
- Miodovnik A, Engel SM, Zhu C, Ye X, Soorya LV, Silva MJ, Calafat AM, Wolff MS. Endocrine disruptors and childhood social impairment. Neurotoxicology 2011; 32(2):261-267; PMID:21182865; http://dx.doi.org/10.1016/j.neuro.2010.12.009
- Roze E, Meijer L, Bakker A, Van Braeckel KN, Sauer PJ, Bos AF. Prenatal exposure to organohalogens, including brominated flame retardants, influences motor, cognitive, and behavioral performance at school age. Environ Health Perspect 2009; 117(12):1953-1958; PMID:20049217; http://dx.doi.org/10.1289/ehp.0901015
- Hoffman K, Adgent M, Goldman BD, Sjodin A, Daniels JL. Lactational exposure to polybrominated diphenyl ethers and its relation to social and emotional development among toddlers. Environ Health Perspect 2012; 120(10):1438-1442; PMID:22814209; http://dx.doi.org/10.1289/ehp.1205100
- Gascon M, Vrijheid M, Martinez D, Forns J, Grimalt JO, Torrent M, Sunyer J. Effects of pre and postnatal exposure to low levels of polybromodiphenyl ethers on neurodevelopment and thyroid hormone levels at 4 years of age. Environ Int 2011; 37(3):605-611; PMID:21237513; http://dx.doi.org/10.1016/j.envint.2010.12.005
- Li XJ, Jiang L, Chen L, Chen HS, Li X. Neurotoxicity of dibutyl phthalate in brain development following perinatal exposure: A study in rats. Environ Toxicol Pharmacol 2013; 36(2):392-402; PMID:23736097; http://dx.doi.org/10.1016/j.etap.2013.05.001
- Boberg J, Christiansen S, Axelstad M, Kledal TS, Vinggaard AM, Dalgaard M, Nellemann C, Hass U. Reproductive and behavioral effects of diisononyl phthalate (DINP) in perinatally exposed rats. Reprod Toxicol 2011; 31(2):200-209; PMID:21075200; http://dx.doi.org/10.1016/j.reprotox.2010.11.001
- Lyche JL, Gutleb AC, Bergman A, Eriksen GS, Murk AJ, Ropstad E, Saunders M, Skaare JU. Reproductive and developmental toxicity of phthalates. J Toxicol Environ Health B Crit Rev 2009; 12(4):225-249; PMID:20183522; http://dx.doi.org/10.1080/10937400903094091
- Hoshi H, Ohtsuka T. Adult rats exposed to low-doses of di-n-butyl phthalate during gestation exhibit decreased grooming behavior. Bull Environ Contam Toxicol 2009; 83(1):62-66; PMID:19399352; http://dx.doi.org/10.1007/s00128-009-9729-1
- Blanco J, Mulero M, Heredia L, Pujol A, Domingo JL, Sanchez DJ. Perinatal exposure to BDE-99 causes learning disorders and decreases serum thyroid hormone levels and BDNF gene expression in hippocampus in rat offspring. Toxicology 2013; 308:122-128; PMID:23578391; http://dx.doi.org/10.1016/j.tox.2013.03.010
- Williams AL, DeSesso JM. The potential of selected brominated flame retardants to affect neurological development. J Toxicol Environ Health B Crit Rev 2010; 13(5):411-448; PMID:20582854; http://dx.doi.org/10.1080/10937401003751630
- Driscoll LL, Gibson AM, Hieb A. Chronic postnatal DE-71 exposure: Effects on learning, attention and thyroxine levels. Neurotoxicol Teratol 2009; 31(2):76-84; PMID:19068229; http://dx.doi.org/10.1016/j.ntt.2008.11.003
- Suvorov A, Girard S, Lachapelle S, Abdelouahab N, Sebire G, Takser L. Perinatal exposure to low-dose BDE-47, an emergent environmental contaminant, causes hyperactivity in rat offspring. Neonatology 2009; 95(3):203-209; PMID:18799892; http://dx.doi.org/10.1159/000155651
- Kuriyama SN, Talsness CE, Grote K, Chahoud I. Developmental exposure to low dose PBDE 99: Effects on male fertility and neurobehavior in rat offspring. Environ Health Perspect 2005; 113(2):149-154; PMID:15687051; http://dx.doi.org/10.1289/ehp.7421
- Schneider T, Przewlocki R. Behavioral alterations in rats prenatally exposed to valproic acid: Animal model of autism. Neuropsychopharmacology 2005; 30(1):80-89; PMID:15238991; http://dx.doi.org/10.1038/sj.npp.1300518
- Christensen J, Gronborg TK, Sorensen MJ, Schendel D, Parner ET, Pedersen LH, Vestergaard M. Prenatal valproate exposure and risk of autism spectrum disorders and childhood autism. JAMA 2013; 309(16):1696-1703; PMID:23613074; http://dx.doi.org/10.1001/jama.2013.2270
- Roullet FI, Lai JK, Foster JA. In utero exposure to valproic acid and autism–a current review of clinical and animal studies. Neurotoxicol Teratol 2013; 36:47-56; PMID:23395807; http://dx.doi.org/10.1016/j.ntt.2013.01.004
- Pittschieler S, Brezinka C, Jahn B, Trinka E, Unterberger I, Dobesberger J, Walser G, Auckenthaler A, Embacher N, Bauer G, et al. Spontaneous abortion and the prophylactic effect of folic acid supplementation in epileptic women undergoing antiepileptic therapy. J Neurol 2008; 255(12):1926-1931; PMID:18677647; http://dx.doi.org/10.1007/s00415-008-0029-1
- Vajda FJ, O’brien TJ, Hitchcock A, Graham J, Cook M, Lander C, Eadie MJ. Critical relationship between sodium valproate dose and human teratogenicity: Results of the australian register of anti-epileptic drugs in pregnancy. J Clin Neurosci 2004; 11(8):854-858; PMID:15519862; http://dx.doi.org/10.1016/j.jocn.2004.05.003
- Close HA, Lee LC, Kaufmann CN, Zimmerman AW. Co-occurring conditions and change in diagnosis in autism spectrum disorders. Pediatrics 2012; 129(2):e305-16; PMID:22271695; http://dx.doi.org/10.1542/peds.2011-1717
- Favre MR, Barkat TR, Lamendola D, Khazen G, Markram H, Markram K. General developmental health in the VPA-rat model of autism. Front Behav Neurosci 2013; 7:88; PMID:23898245; http://dx.doi.org/10.3389/fnbeh.2013.00088
- Suckow MA, Weisbroth SH, Franklin CL. The laboratory rat. Vol 2. Academic Press ed. American College of Laboratory Animal Medicine; 2005:928
- Johnston AL, File SE. Sex differences in animal tests of anxiety. Physiol Behav 1991; 49(2):245-250; PMID:2062894; http://dx.doi.org/10.1016/0031-9384(91)90039-Q
- Brentani H, Paula CS, Bordini D, Rolim D, Sato F, Portolese J, Pacifico MC, McCracken JT. Autism spectrum disorders: An overview on diagnosis and treatment. Rev Bras Psiquiatr 2013; 35 Suppl 1:S62-72; PMID:24142129; http://dx.doi.org/10.1590/1516-4446-2013-S104
- Baharnoori M, Bhardwaj SK, Srivastava LK. Neonatal behavioral changes in rats with gestational exposure to lipopolysaccharide: A prenatal infection model for developmental neuropsychiatric disorders. Schizophr Bull 2012; 38(3):444-456; PMID:20805287; http://dx.doi.org/10.1093/schbul/sbq098
- Mastrogiuseppe M, Capirci O, Cuva S, Venuti P. Gestural communication in children with autism spectrum disorders during mother-child interaction. Autism 2014; PMID:24699229
- Barbaro J, Dissanayake C. Early markers of autism spectrum disorders in infants and toddlers prospectively identified in the social attention and communication study. Autism 2013; 17(1):64-86; PMID:22735682; http://dx.doi.org/10.1177/1362361312442597
- Peper JS, Koolschijn PC. Sex steroids and the organization of the human brain. J Neurosci 2012; 32(20):6745-6746; PMID:22593044; http://dx.doi.org/10.1523/JNEUROSCI.1012-12.2012
- Montelli S, Peruffo A, Zambenedetti P, Rossipal E, Giacomello M, Zatta P, Cozzi B. Expression of aromatase P450(AROM) in the human fetal and early postnatal cerebral cortex. Brain Res 2012; 1475:11-18; PMID:22902617; http://dx.doi.org/10.1016/j.brainres.2012.08.010
- Vizziano-Cantonnet D, Anglade I, Pellegrini E, Gueguen MM, Fostier A, Guiguen Y, Kah O. Sexual dimorphism in the brain aromatase expression and activity, and in the central expression of other steroidogenic enzymes during the period of sex differentiation in monosex rainbow trout populations. Gen Comp Endocrinol 2011; 170(2):346-355; PMID:20955710; http://dx.doi.org/10.1016/j.ygcen.2010.10.009
- Kalat J. Biological psychology. 10th ed. Great Britain: Wadsworth Publishing Company; 2009:549
- Schwarz JM, McCarthy MM. Steroid-induced sexual differentiation of the developing brain: Multiple pathways, one goal. J Neurochem 2008; 105(5):1561-1572; PMID:18384643; http://dx.doi.org/10.1111/j.1471-4159.2008.05384.x
- Parks LG, Ostby JS, Lambright CR, Abbott BD, Klinefelter GR, Barlow NJ, Gray LE Jr. The plasticizer diethylhexyl phthalate induces malformations by decreasing fetal testosterone synthesis during sexual differentiation in the male rat. Toxicol Sci 2000; 58(2):339-349; PMID:11099646; http://dx.doi.org/10.1093/toxsci/58.2.339
- Fisher JS, Macpherson S, Marchetti N, Sharpe RM. Human ‘testicular dysgenesis syndrome’: A possible model using in-utero exposure of the rat to dibutyl phthalate. Hum Reprod 2003; 18(7):1383-1394; PMID:12832361; http://dx.doi.org/10.1093/humrep/deg273
- Zimmer KE, Montano M, Olsaker I, Dahl E, Berg V, Karlsson C, Murk AJ, Skaare JU, Ropstad E, Verhaegen S. In vitro steroidogenic effects of mixtures of persistent organic pollutants (POPs) extracted from burbot (lota lota) caught in two norwegian lakes. Sci Total Environ 2011; 409(11):2040-2048; PMID:21420147; http://dx.doi.org/10.1016/j.scitotenv.2011.01.055
- Canton RF, Scholten DE, Marsh G, de Jong PC, van den Berg M. Inhibition of human placental aromatase activity by hydroxylated polybrominated diphenyl ethers (OH-PBDEs). Toxicol Appl Pharmacol 2008; 227(1):68-75; PMID:18022659; http://dx.doi.org/10.1016/j.taap.2007.09.025
- Grande SW, Andrade AJ, Talsness CE, Grote K, Golombiewski A, Sterner-Kock A, Chahoud I. A dose-response study following in utero and lactational exposure to di-(2-ethylhexyl) phthalate (DEHP): Reproductive effects on adult female offspring rats. Toxicology 2007; 229(1-2):114-122; PMID:17098345; http://dx.doi.org/10.1016/j.tox.2006.10.005
- Matson JL, Mahan S, Fodstad JC, Worley JA, Neal D, Sipes M. Effects of symptoms of co-morbid psychopathology on challenging behaviours among infants and toddlers with autistic disorder and PDD-NOS as assessed with the baby and infant screen for children with aUtIsm traits (BISCUIT). Dev Neurorehabil 2011; 14(3):129-139; PMID:21548853; http://dx.doi.org/10.3109/17518423.2011.557029
- Zurcher NR, Rogier O, Boshyan J, Hippolyte L, Russo B, Gillberg N, Helles A, Ruest T, Lemonnier E, Gillberg C, et al. Perception of social cues of danger in autism spectrum disorders. PLoS One 2013; 8(12):e81206; PMID:24324679; http://dx.doi.org/10.1371/journal.pone.0081206
- Matson JL, Rieske RD, Williams LW. The relationship between autism spectrum disorders and attention-deficit/hyperactivity disorder: An overview. Res Dev Disabil 2013; 34(9):2475-2484; PMID:23751293; http://dx.doi.org/10.1016/j.ridd.2013.05.021
- Bergeron JD, Deslauriers J, Grignon S, Fortier LC, Lepage M, Stroh T, Poyart C, Sébire G. White matter injury and autistic-like behavior predominantly affecting male rat offspring exposed to group B streptococcal maternal inflammation. Dev Neurosci 2013; 35(6):504-515; PMID:24246964
- Varlinskaya EI, Spear LP. Social interactions in adolescent and adult sprague-dawley rats: Impact of social deprivation and test context familiarity. Behav Brain Res 2008; 188(2):398-405; PMID:18242726; http://dx.doi.org/10.1016/j.bbr.2007.11.024