Abstract
Atypical manifestation of skin rejection observed in a series of hand transplant recipients suggests that appearance and mechanisms of rejection may vary and depend on skin type and location. We herein characterize skin rejection and inflammation at 3 defined areas of a rat hind-limb allograft. Allograft skin was harvested at the thigh, dorsum and planta pedis on postoperative day 5 and analyzed for histopathology, T-cell composition and expression of cytokines associated with skin inflammation. Isografts and naïve animals served as controls. Histology of the allograft skin collected on day 5 showed a diffuse cell infiltrate in the dermis and at the dermal-epidermal interface in the thigh and dorsum, whereas in the planta pedis it was mainly located perivascularly in the dermis. At all areas, epidermal involvement such as apoptotic keratinocytes and infiltrating lymphocytes were observed. Isograft skin showed only very mild or no signs of inflammation at all 3 locations. No significant difference was observed in the proportion of skin infiltrating CD45+CD3+CD4+ T-cells and CD45+CD3+CD8+ T-cells between the 3 sampling areas. Significant differences in cytokine protein expression were observed between hair bearing (thigh, dorsum) and hairless (planta pedis) skin, but not between the thigh and the dorsum (both hair bearing). MCP-1, IL-4, and GRO-KC exhibited the greatest individual expression at both locations thigh and dorsum in comparison with planta pedis. Differences in histopathologic appearance and cytokine expression in skin suggest area-specific mechanisms of rejection in a limb allograft, which should be considered for assessment and treatment of rejection.
Abbreviations:
- VCA, vascularized composite allotransplantation
- POD, postoperative day
- H&E, haematoxylin-eosin
- IL, interleukin
- MCP, monocyte chemotactic protein
- LEW, Lewis
- BN, Brown Norway
- IFN, interferon
- TNF, tumor necrosis factor
- GM-CSF, granulocyte-macrophage colony stimulating factor
- ANOVA, analysis of variance
- RM-ANOVA, repeated measure analysis of variance
- FDR, false discovery rate
- TH, thigh
- PP, planta pedis
- DO, dorsum
Introduction
Skin has been shown to be a highly immunogenic tissue and hence the primary target of rejection in a vascularized composite allograft (VCA).Citation1,2 Macroscopic skin changes and histopathologic characteristics of skin rejection have been well described.Citation3–6 As for hand allografts, skin rejection most often affects the dorsal and volar aspects of the forearm and wrist and/or the dorsum of the hand. Clinical macroscopic manifestations thereby can range from mild pink discoloration or erythema to lichenoid papules. Skin lesions may be limited to a small area of the skin or can spread over large parts of the transplant. However, a limited number of hand transplant patients have developed a specific and novel type of skin rejection with regard to appearance, localization and microscopic changes.Citation7 The lesions observed in these patients were characterized by an erythematous rash of the palm and/or the fingers, together with swelling of the fingers. Dryness, scaling and thickness of the palmar skin were combined with nail changes, resembling psoriatic lesions. Interestingly, the histological appearance of this type of rejection differed form conventional rejection with regard to e.g. prominence of parakeratosis and spongiosis.
Inspired by the observation that skin rejection can manifest in different regions of a hand allograft representing different skin types (palm vs thin skin and hair bearing vs hairless skin) we aimed to investigate the characteristics and patterns of skin rejection and inflammation at various locations representing various skin types of a rat hind-limb allograft. Therefore, rejecting skin collected from the thigh (hair bearing skin), the dorsum of the foot (thin, hair bearing skin) and the planta pedis (thick, hairless skin) was evaluated for macroscopic and microscopic skin changes, distribution of the cell infiltrate, T-cell composition and cytokine profiles on protein level. We were able to show that the distribution of the immune cell infiltrate is characteristic for the skin type. Furthermore, significant differences in cytokine protein expression were found between hair bearing (thigh, dorsum) and hairless (planta pedis) skin.
Understanding localization- and skin type- specific rejection helps to develop targeted, skin type- and area- specific treatment and should be taken into consideration in the course of skin biopsy sampling in humans and VCA animal trials.
Results
Histopathologic differences of skin rejection between defined anatomical locations-the thigh, dorsum and planta pedis-of a rat limb allograft
First, we assessed whether the rejection severity according to the histopathologic Banff classification and the immune cell distribution pattern differed between the 3 sampling locations. On postoperative day (POD) 5 all allotransplants macroscopically revealed a grade II rejection, representing as erythema and edema. Both, swelling and erythema were most prominent on the forefoot, but also included the thigh. Isografts did not show any macroscopic alterations. In haematoxylin-eosin (H&E) stained skin samples a Banff grade II rejection was observed in all biopsies of the allograft limbs collected at the 3 defined sampling locations, with the exception of one biopsy taken at the dorsum, which showed a grade I rejection on POD 5. However, a different pattern of the distribution of the cell infiltrate was observed between sampling sites. In the thigh the cell infiltrate was found diffusely distributed in the dermal layer (). Furthermore, an interface reaction and mild epidermal involvement with lymphocytes infiltrating the epidermis were observed. These attributes were also found in skin of the dorsum but with a tendency toward less overall cell infiltration (). Compared to the thigh, the interface reaction and epidermal involvement were restricted to certain areas of the dermal-epidermal interface in the dorsum. In the planta pedis the cell infiltrate was mainly located perivascularly in the dermis, while only a very mild diffuse infiltrate was observed in the surrounding dermis (). However, immune cells were observed at the dermal-epidermal interface and infiltrating the epidermis thereby qualifying for a grade II rejection.
Figure 1. Histopathologic characteristics and immune cell distribution in the skin of the thigh, dorsum and planta pedis of limb allografts and isografts on POD 5. (A) (thigh), (B) (dorsum), and (C) (planta pedis) depict a Banff grade II rejection in allograft skin on POD 5, however, the extent of the cell infiltrate and cell distribution varied between the sampling spots. In general, the diffuse dermal cell infiltrate was more prominent in the thigh (A), compared to the dorsum (B). An interface reaction and dermal involvement with migrated immune cells was found at both sampling spots, but with a focal restriction in the dorsum (B). In the planta pedis the cell infiltrate was very prominent in the perivascular areas, but epidermal involvement with epidermal immune cell migration was found as well, which is per definition a grade II rejection. In isograft skin only a mild dermal cell infiltrate (D) (thigh) or no signs of inflammation (E) (dorsum) and (F) (planta pedis) were found on POD 5.
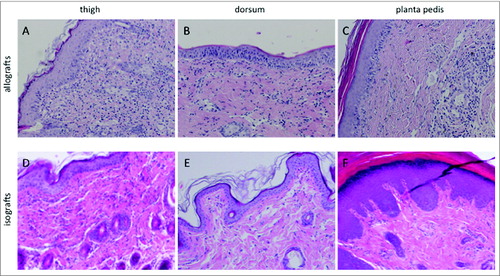
In isograft skin, a mild cell infiltrate was observed in 3 out of 5 samples taken at the thigh and the dorsum on POD 5, but only in one sample collected at the planta pedis (-). Control skin samples from untreated animals did not show cell infiltration or signs of inflammation (grade 0 per rejection definition) at any sampling location.
Characterization of the infiltrating T cells in skin of the thigh, dorsum and planta pedis during allograft rejection on POD 5
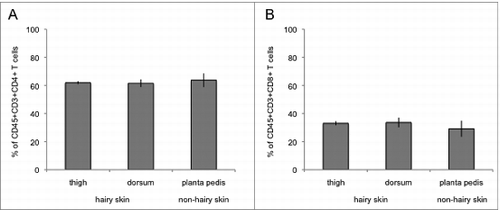
We addressed the question whether there is a difference in the proportion of infiltrating CD45+CD3+CD4+ T cells and CD45+CD3+CD8+ T cells between different skin types (hair bearing skin at the thigh and the dorsum vs hairless skin at the planta pedis) and sampling areas (thigh vs dorsum vs planta pedis) in a rat limb allograft during moderate rejection by flow cytometry analysis. As displayed in and B, the proportion of skin infiltrating CD45+CD3+CD4+ T cells and CD45+CD3+CD8+ T cells among all CD45+CD3+ T cells was nearly equal in hair bearing (thigh and dorsum) vs hairless (planta pedis) skin (P > 0.05). Our findings further indicate that there is no significant difference of infiltrating CD45+CD3+CD4+ and CD45+CD3+CD8+ T cells between the 3 sampling locations (CD4+: thigh 61.98%+/−1.04 vs dorsum 61.58%+/−2.68 vs planta pedis 63.74%+/−4.90, P > 0.05; CD8: thigh 33.03%+/−1.47 vs dorsum 33.61%+/−3.45 vs planta pedis 29.13%+/−5.73, P > 0.05).
Figure 2. Characterization of allograft skin T cells at the 3 sampling areas on POD 5 using flow cytometry analysis. Skin T cells were isolated ex vivo and stained with antibodies against CD45, CD3, CD4 and CD8. Among the CD45+CD3+ T cell population around 60% were characterized as CD45+CD3+CD4+ T cells with no significant difference between the 3 sampling spots (thigh, planta pedis, dorsum) and the skin types (hair bearing vs hairless skin) (A). Around 30% of CD45+CD3+ T cells stained positive for CD8, again with no significant difference between the 3 sampling locations and the skin types (B).
Cytokine levels are elevated in hair bearing (thigh and dorsum) vs hairless (planta pedis) skin during allograft rejection
To investigate the molecular background during skin rejection at 3 defined sampling locations, we assessed skin cytokine/chemokine protein levels using the LuminexTM assay technique. Tissue protein concentrations of 14 analytes in the thigh, planta pedis and dorsum are shown in log-scale in . Baseline cytokine levels in naïve, non-transplanted control skin are depicted in . Bonferroni corrected p-values from pair-wise analyses showed a significant differential expression between the thigh and the planta pedis with higher abundance in the thigh for 7 out of 14 studied mediators (). Significant differences were also observed for the expression of 5 cytokines between the planta pedis and the dorsum (). Again, protein levels were higher in the skin of the dorsum compared to planta pedis. The protein level of 4 cytokines [interleukin (IL)-4, IL-1ß, IL-2 and IL-12p70] were significantly different in both the skin of the thigh and dorsum (hair bearing skin) vs the skin of the planta pedis (hairless skin). No significant differences of cytokine protein levels were found between the skin of the thigh and the dorsum (). The heatmap in displays the individual fold changes (log2 fold-changes) of the cytokines/chemokines between the 3 different locations within the allografts. Monocyte chemotactic protein (MCP)-1, IL-4, and GRO-KC exhibited the greatest individual expression (at both locations thigh and dorsum in comparison with planta pedis) and show similar profiles over all 5 allografts. High individual fold-changes and similar profiles were also observed for IL-1ß and IL-2 as evident by hierarchical clustering and the heatmap in . In contrast, no significant difference of baseline cytokine protein levels in naïve, non-transplanted control skin was observed between the different sampling locations and skin types.
Table 1. Mean cytokine/chemokine levels and their significant changes in the three different skin areas of limb allografts on POD 5. Mean levels in pg/mg total protein from samples at the three different skin locations [thigh (TH), planta pedis (PP), dorsum (DO)] are presented. Adjusted p-values from Repeated Measure ANOVA and Bonferroni corrected p-values from pair-wise analyses are shown. Significant differences between the respective sites are indicated bold (p<0.05).
Figure 3. Comparison of the different cytokine levels in samples from different skin areas in limb allografts on POD 5 and control skin. Levels in pg/mg tissue protein are shown in log-scale at the different skin locations (thigh, planta pedis and dorsum). Lines indicate the paired design as samples at the different skin areas were taken from the same allograft. In general, tissue cytokine levels were found to be higher in the thigh and dorsum of allograft skin during moderate grade II rejection, compared to the planta pedis (A). This phenomenon was not true for baseline cytokine protein levels observed in naïve, non-transplanted control skin collected at the 3 defined sampling sites (B).
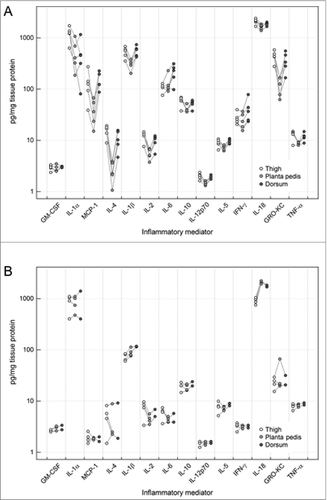
Figure 4. Heatmap visualizing log2-fold changes of cytokine levels between different locations [thigh (TH) versus planta pedis (PP) and dorsum (DO) vs. planta pedis] in each of the allografts on POD 5. Relative higher levels of inflammatory mediators (log2-fold changes>0 ) are indicated in red and lower levels (log2-fold changes<0 ) are indicated in blue according to the legend. Similar profiles of mediators across all allografts are grouped by average linkage hierarchical clustering as indicated by the dendogram (tree) at the left.
![Figure 4. Heatmap visualizing log2-fold changes of cytokine levels between different locations [thigh (TH) versus planta pedis (PP) and dorsum (DO) vs. planta pedis] in each of the allografts on POD 5. Relative higher levels of inflammatory mediators (log2-fold changes>0 ) are indicated in red and lower levels (log2-fold changes<0 ) are indicated in blue according to the legend. Similar profiles of mediators across all allografts are grouped by average linkage hierarchical clustering as indicated by the dendogram (tree) at the left.](/cms/asset/ea53677d-0888-4ef8-9464-c46225300c40/kvca_a_973795_f0004_c.jpg)
These findings suggest that the cytokine patterns expressed in the skin during moderate grade II rejection at an early time-point (POD 5) are specific for the skin type – hair bearing (thigh and dorsum) vs hairless (planta pedis) skin in a rat limb allograft.
Discussion
Rejection is a common immunological complication after hand and face transplantation,Citation8 which mainly manifests as skin rejection. The macroscopic pattern of skin rejection of a hand/forearm allograft is quite heterogenous. Most often, an erythemateous rash of various extent and intensity affects the dorsum and volar aspects of the forearm and the dorsum of the hand.Citation5,9–11 In a limited number of patients, so-called atypical rejection episodes have been recorded, which mainly manifested at the palm, including the fingers.Citation7 These observations suggest that skin rejection may affect all parts of a hand/forearm allograft, including hair bearing and hairless skin – and that different skin parts may not be affected simultaneously. However, there is no evidence whether histopathologic rejection severity and molecular rejection patterns are the same at different locations and in different skin types of a hand allograft. Utilizing an experimental rat hind-limb allotransplant model we showed for the first time that immune cell distribution varied between 3 defined skin areas during allograft rejection. Furthermore, a pattern of cytokines was found differentially expressed in hair bearing vs hairless skin. However, we did not find differences in the proportion of skin infiltrating CD4+ and CD8+ T cells.
In general, one single skin biopsy is collected for histopathologic evaluation of rejection at a proximal, cosmetically advantageous site of a human hand allograft.Citation12 This might be sufficient for diagnosis of rejection, though, it does not clarify the dissemination and extent of inflammation within the allograft. The investigation in a rat animal VCA model offers the advantage to map and characterize rejection at several areas of a VCA. An interesting observation in the course of macroscopic assessment of rat allograft limbs upon moderate rejection on POD 5 was that skin inflammation appeared more prominent at the forefoot (dorsum and planta pedis), compared to the thigh. However, this tendency was not confirmed by histopathologic analysis of H&E stained skin sections collected at the 3 defined sampling spots thigh, dorsum and planta pedis. The most pronounced diffuse inflammatory skin infiltrate was found in the samples taken from the thigh, whereas it was somewhat less in the dorsum. In samples collected from the sole of the rat hind-limb the changes were restricted to the perivascular areas in the dermis and the dermal-epidermal interface. This phenomenon might be attributed to the specialized properties and functions of the skin on the planta pedis and palm of the hand. The skin in this location is relatively thick with a prominent stratum corneum. The epidermal layer and the tissue of the superficial dermis appear very compact. Due to its exposed position on feet and hands this skin type is challenged with mechanical stress and chemical irritation to a greater extent. Hence, it is speculated that this type of skin innately is less vulnerable to inflammation and rejection and/or cell migration within the relatively compact and thick tissue of the planta pedis is hindered. Moreover, the folliculo-sebaceous-apocrine units of hair-bearing skin (thigh and dorsum) are known to represent areas of immune cell accumulation during skin rejection.Citation5
Overall, inflammation on POD 5 as a result of the transplantation procedure and limb ischemia alone (isografts) was much less severe when compared to allografts. Comparable to allografts, isograft skin biopsies taken from the planta pedis were also affected least by inflammation, compared to the thigh and the dorsum of a rat isograft limb.
When characterizing the phenotype of skin infiltrating CD45+CD3+CD4+ and CD45+CD3+CD8+ T cells during allograft rejection in rat hind-limbs on POD 5, no significant difference between the 3 sampling locations was observed. In our rat skin samples about 2 thirds (61 to 63%) of T cells were positive for CD4 and one third (29 to 33%) was positive for CD8 during grade II rejection. In a previous study characterizing skin rejection in skin biopsies of human hand allografts 47% of the CD3+ T cells were found positive for CD4, whereas 43% were characterized as CD8+.Citation13 In contrast to our findings obtained in rat skin samples, where only grade II samples were assessed for T cell populations, all rejection grades (0 – IV) were included in the human study. Another study showed that even a higher number of CD8+ T cells was found in mildly rejecting (grade I) skin samples of human hand allografts.Citation14 To allow for an exact comparison of CD4+/CD8+ T cell populations between rat and human skin samples, data on grade II human skin samples would be necessary but are not available. Moreover, 2 different methods of analysis were used - cell isolation and flow cytometry for analysis of rat skin samples and immunohistochemical stains for evaluation of human skin samples – which makes a valid comparison even more difficult. According to our results obtained from the rat skin samples collected at distinct locations of a VCA, the proportions of T cell populations seem to be stable and do not vary within the allograft during moderate rejection. However, our results do not provide information on the absolute amounts of CD4+ and CD8+ T cells. This would be very interesting in the context of rejection severity and T cell infiltration.
Upregulation of proinflammatory cytokines and chemokines is a typical feature of inflammation. The goal of a previous study performed by our group was to identify a set of proinflammatory cytokines (among the same 14 analytes that were chosen for the present study) that are specific for skin rejection.Citation15 We addressed our research question in the experimental rat hind-limb allotransplant model and used advanced statistical methods in search of novel biomarkers of skin rejection. IL-1α, IL-18, IL-1β, and IL-4 were identified as principal drivers of transplant rejection upon a moderate grade of rejection in a rat VCA. Furthermore, we were able to show that the patterns of inflammatory mediator expression in skin were fundamentally different in isograft and allograft skin of transplanted rat hind-limbs on POD 5 as well as some days earlier and later. In the present study we focused on the differences of cytokine protein expression between the 3 defined skin sampling areas (thigh, dorsum and planta pedis) in a rat limb allograft on POD 5. In a pair-wise study design 8 out of 14 analytes were significantly differentially expressed in hair bearing (thigh, dorsum) vs hairless (planta pedis) skin with MCP-1, IL-4, and GRO-KC exhibiting the greatest individual effects between both skin types. This observation suggests a skin-type specific inflammatory milieu during moderate rejection in our rat VCA model. Interestingly, cytokine levels in pg/mg protein were found to be lower in skin of the planta pedis (hairless skin), compared to the thigh and the dorsum (hair bearing skin), which again supports our histopathologic findings discussed above and the theory that the immune reaction might be somewhat suppressed in the skin of the planta pedis (thick, hairless, robust skin). As this phenomenon was not found in skin samples collected from naïve rat hind-limbs we rule out that it is related to difficulties in protein isolation of rat planta pedis skin.
From a clinical point of view it has to be mentioned that skin rejection episodes have been recorded in human hand transplant recipients which almost only affected the skin of the palmCitation7 (and personal unpublished data). Interestingly, the review of the patients’ history and behavior revealed mechanical stress to the palm prior to a so-called atypical rejection episode (intense physical hand therapy, intense manual work). It is hypothesized that this specific type of rejection might be initiated by an external stimulus.
Together with these observations our findings obtained in rat allograft skin of the thigh, dorsum and planta pedis during moderate rejection suggest that different parts of a VCA including different skin types respond differently to allograft rejection and inflammation. Underlying mechanisms of skin rejection may differ between the various locations, especially between the hair bearing skin and hairless, thick, robust skin of the palms/foot soles. This should be taken into consideration when choosing the ´ideal´ area for skin biopsy sampling in human hand transplant recipients and animal models of VCA. Moreover, our findings may be of relevance when designing localized, targeted skin rejection therapy in the field of VCA.
Material and Methods
Animals
Eight- to ten-week-old male rats weighing between 200–250 g were used for all experiments (Charles River). Lewis (LEW) rats served as recipients and Brown-Norway (BN) rats were chosen as donors. This strain combination enables transplantation across a full major-histocompatibility-complex mismatch. Animals were housed under standard conditions with access to food and water ad libitum before and after transplantation. Rats received care in compliance with the Principles of Laboratory Animal Care formulated by the National Society for Medical Research and the Guide for the Care and Use of Laboratory animals prepared by the National Academy of Science and published by the National Institutes of Health (NIH Publication No. 86–23, revised 1985). Experimental protocols were approved by the Austrian Federal Ministry of Science and Research.
Rat hind-limb transplant model
Anesthesia was performed using a combination of xylazin (Xylasol ®, Dr. E. Graueb, Bern, Switzerland, 5 mg/kg) and ketamin (Ketasol ®, Dr. E. Graueb, Bern, Switzerland, 100 mg/kg), administered intramuscularly. The rat hind-limb transplantation model was carried out as described previously.Citation16 In summary, the BN donor femoral vessels were dissected and transected at the level of the inguinal ligament and the graft was flushed through the femoral artery with cold saline. Thereafter, the limb was amputated at the level of the distal femur. The LEW recipient hind-limb was prepared similarly. After bone fixation, repair of the ventral and dorsal muscle groups was performed. After anastomosis of the femoral artery and vein using 10–0 interrupted nylon suture the skin was closed with 4–0 Vicryl suture. Analgesia included caprofen (Rimadyl®, 4 mg/kg) twice a day until POD 5.
Experimental design and tissue sampling
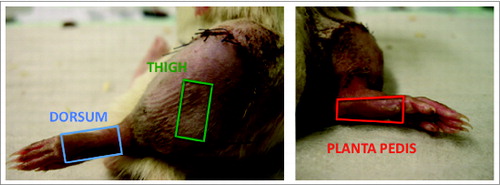
Skin biopsies from allogenic rat hind-limbs (n = 5) were obtained at the endpoint of the study on POD 5, as a moderate grade of rejection without epidermolysis and gross tissue damage was expected by then. The macroscopic skin changes were evaluated using a classification including 5grades of rejection severity (grade 0: no skin changes, grade I: erythema, grade II: erythema and edema, grade III: epidermolysis, grade IV: necrosis and mummification). Animals were sacrificed and skin biopsies (size 1.5 × 1.0 cm) were collected at 3 defined anatomical sites of each transplanted rat limb: 1) skin obtained from the lateral thigh representing hair bearing skin; 2) skin collected at the dorsum of the foot representing hair bearing, thin skin; and 3) skin collected at the planta pedis typifying hairless, thick skin (). Each skin sample was divided into 3 pieces for histopathologic evaluation, cell isolation/flow cytometry analysis and protein isolation/Luminex assay. Skin biopsies collected from isogenic rat hind-limbs on POD 5 (n = 5) and non-transplanted, naive limbs (n = 5) served as controls.
Figure 5. Areas of skin biopsy sampling at the 3 defined spots of a rat limb allograft. Three defined areas representing 2 skin types (hair bearing and hairless skin) were chosen to assess for skin rejection characteristics and severity in a rat vascularized composite allograft. Samples were taken from the lateral aspect of the thigh (green rectangle) with some distance to the coaptation site of the allograft and recipient skin, the dorsum excluding the toes (blue rectangle) and the planta pedis, again excluding the toes (red rectangle). The size of a skin sample was approximately 1.5 × 1.0 cm. The 3 different skin samples were collected from the same limb allograft on POD 5.
Histology
Skin samples were fixed in 4% paraformaldehyde and embedded in paraffin. Four μm sections were stained with H&E and evaluated by a blinded pathologist for inflammation, localization of the cell infiltrate and tissue damage. Histopathologic changes were described in detail and additionally scored according to the Banff 2007 guidelines.Citation17 Photos were taken using a PL-A642 camera (Pixelink) mounted on a BX50F4 microscope (Olympus).
Cell isolation and flow cytometry analysis
To assess for the phenotype of the infiltrating T cells (CD45+CD3+CD4+ T cells and CD45+CD3+CD8+ T cells) at the 3 different anatomical sites including different skin types of a limb allograft, T cells were isolated of the allograft skin and flow cytometry analysis was performed. First, skin samples were stored in dispase (1 mg/ml, Roche, cat. no.: 10269638001) overnight. After washing with complete medium (10% FCS and 1% pen/strep in RPMI 1640, Lonza, cat. no.: BE12–167F) skin biopsies were digested in specific collagenase D (1 mg/ml, Roche, cat. no.: 11088866001) for one hour at 37°C. Next, the cell suspension was strained through a Nylon cell strainer and washed twice. For lymphocyte isolation, the cell suspension was slowly placed on histopaque (histopaque 1083, Sigma-Aldrich, cat. no.: 10831) and centrifuged at 800 rcf for 30 minutes at 20°C without breaks. The white blood cell ring fraction was then transferred into PBS, washed 3 times and used for flow cytometry analysis. For 4-color flow cytometry cell suspensions were incubated at 4°C for 30 minutes with appropriate dilutions of directly labeled monoclonal rat antibodies against CD45 (APC, OX1, cat. no.: 17–0461–82), CD3 (PE, G4.18, cat. no.: 12–0030–83), CD4 (FITC, OX35, cat. no.: 11–0040–85), and CD8a (PE-Cy7, OX8, cat. no.: 25–0085–82); all eBioscience). Fluorescence intensity was analyzed on a FACS Calibur (BD) and data analysis was carried out using CellQuestPro software.
Protein isolation and luminex
To further characterize the rejection process and to assess for cytokine patterns specific for different rejection sites and skin types proteins from skin biopsies were isolated on ice, using a disperser (T10, basic ULTRA-TURRAX, IKA, cat. no.: 0003737000) with 1 ml 1 x Cell Lysis Buffer (Cell Signaling, cat. no.: 9803) per sample. Protein quantification for 14 analytes was performed after homogenization applying the BCA Protein Assay Kit (Pierce Biotechnology, cat. no.: 23225) according to the manufacturer's protocol. The LuminexTM inflammatory mediator bead set (CYTO-80K-PMX-14-plex Milliplex Map Kit from Millipore) which included interferon (IFN)-γ, IL-1α, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12p70, IL-18, MCP-1, GRO/KC, tumor necrosis factor (TNF)-α, and granulocyte-macrophage colony stimulating factor (GM-CSF) was used to measure cytokine/chemokine expression at the protein levels in a Luminex™ 100 IS (Luminex Corporation) and analyzed by xPonent 3.1 Rev.2 Software (Luminex Corporation). Results for each of the 14 analytes were read in pg/ml and subsequently normalized to total mass of sample protein (pg inflammatory mediator/mg protein) by multiplying with 0.025 ml standard sample volume and dividing with 0.1 mg added total protein for each sample. All samples were measured in duplicate and respective results were averaged. Any analyte indicating a concentration above 20,000 pg/μl were excluded from analysis (NA) since those concentrations were outside the linear range of the Luminex™ assay.
Statistical analysis
Flow cytometry analysis data is expressed as mean +/− standard deviation (SD). Analysis of Variance (ANOVA) was utilized to assess for differences between the 3 skin sampling sites. The Bonferroni post hoc test was used for correction of multiple comparisons. A p-value < 0 .05 was considered as statistically significant.
Repeated Measure Analysis of Variance (RM-ANOVA) were performed to assess differences in the cytokine levels (normalized to mg tissue protein) at the different sampled skin locations of the allografts. Resulting p-values were adjusted for multiple hypothesis testing based on the false discovery rate (FDR).Citation18 Significant differences between the 3 sites (thigh, dorsum, planta pedis) are indicated by adjusted p < 0 .05. Students paired t-test with Bonferroni correction were used as post hoc test to indentify significant changes of the mediator levels between the pair-wise comparisons of sites (thigh vs. planta pedis, planta pedis vs. dorsum, dorsum vs. thigh). Normal distribution of the pair-wise differences were tested using the Shapiro-Wilk test. Statistical analyses were performed using IBM® SPSS® and the statistical software environment R. Average linkage hierarchical clustering were performed and heatmap on log2-fold changes for individual allografts of cytokine levels in thigh or dorsum compared to planta pedis were generated using Genesis.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Hettiaratchy S, Melendy E, Randolph MA, Coburn RC, Neville DM, Jr., Sachs DH, Huang CA, Lee WP. Tolerance to composite tissue allografts across a major histocompatibility barrier in miniature swine. Transplantation 2004; 77:514-21; PMID:15084927; http://dx.doi.org/10.1097/01.TP.0000113806.52063.42.
- Mathes DW, Randolph MA, Solari MG, Nazzal JA, Nielsen GP, Arn JS, Sachs DH, Lee WP. Split tolerance to a composite tissue allograft in a swine model. Transplantation 2003; 75:25-31; PMID:12544866; http://dx.doi.org/10.1097/00007890-200301150-00005.
- Kanitakis J, Jullien D, Petruzzo P, Hakim N, Claudy A, Revillard JP, Owen E, Dubernard JM. Clinicopathologic features of graft rejection of the first human hand allograft. Transplantation 2003; 76:688-93; PMID:12973110; http://dx.doi.org/10.1097/01.TP.0000079458.81970.9A.
- Schneeberger S, Kreczy A, Brandacher G, Steurer W, Margreiter R. Steroid- and ATG-resistant rejection after double forearm transplantation responds to Campath-1H. Am J Transplant 2004; 4:1372-4; PMID:15268743; http://dx.doi.org/10.1111/j.1600-6143.2004.00518.x.
- Cendales LC, Kirk AD, Moresi JM, Ruiz P, Kleiner DE. Composite tissue allotransplantation: classification of clinical acute skin rejection. Transplantation 2006; 81:418-22; PMID:16477229; http://dx.doi.org/10.1097/01.tp.0000185304.49987.d8.
- Kanitakis J, Petruzzo P, Jullien D, Badet L, Dezza MC, Claudy A, Lanzetta M, Hakim N, Owen E, Dubernard JM. Pathological score for the evaluation of allograft rejection in human hand (composite tissue) allotransplantation. Eur J Dermatol 2005; 15:235-8; PMID:16048749.
- Schneeberger S, Gorantla VS, van Riet RP, Lanzetta M, Vereecken P, van Holder C, Rorive S, Remmelink M, Le Moine A, Abramowicz D. Atypical acute rejection after hand transplantation. Am J Transplant 2008; 8:688-96; PMID:18261182; http://dx.doi.org/10.1111/j.1600-6143.2007.02105.x.
- Petruzzo P, Lanzetta M, Dubernard JM, Landin L, Cavadas P, Margreiter R, Schneeberger S, Breidenbach W, Kaufman C, Jablecki J, et al. The International Registry on Hand and Composite Tissue Transplantation. Transplantation 2010; 90:1590-4; PMID:21052038; http://dx.doi.org/10.1097/TP.b013e3181ff1472.
- Schneeberger S, Ninkovic M, Piza-Katzer H, Gabl M, Hussl H, Rieger M, Loescher W, Zelger B, Brandacher G, Ninkovic M, et al. Status 5 years after bilateral hand transplantation. Am J Transplant 2006; 6:834-41; PMID:16539641; http://dx.doi.org/10.1111/j.1600-6143.2006.01266.x.
- Schneeberger S, Ninkovic M, Gabl M, Hussl H, Rieger M, Loescher W, Zelger B, Brandacher G, Bonatti H, Hautz T, et al. First forearm transplantation: outcome at 3 years. Am J Transplant 2007; 7:1753-62; PMID:17511764; http://dx.doi.org/10.1111/j.1600-6143.2007.01837.x.
- Petruzzo P, Badet L, Gazarian A, Lanzetta M, Parmentier H, Kanitakis J, Sirigu A, Martin X, Dubernard JM, et al. Bilateral hand transplantation: six years after the first case. Am J Transplant 2006; 6:1718-24; PMID:16827876; http://dx.doi.org/10.1111/j.1600-6143.2006.01369.x.
- Hautz T, Zelger BG, Weissenbacher A, Zelger B, Brandacher G, Landin L, Morelon E, Kanitakis J, Jablecki J, Lee WP, et al. Standardizing skin biopsy sampling to assess rejection in vascularized composite allotransplantation. Clin Transplant 2013; 27:E81-90; PMID:23452279; http://dx.doi.org/10.1111/ctr.12086.
- Hautz T, Zelger B, Grahammer J, Krapf C, Amberger A, Brandacher G, Landin L, Müller H, Schön MP, Cavadas P, et al. Molecular markers and targeted therapy of skin rejection in composite tissue allotransplantation. Am J Transplant 2010; 10:1200-9; PMID:20353468; http://dx.doi.org/10.1111/j.1600-6143.2010.03075.x.
- Hautz T, Zelger B, Brandacher G, Mueller H, Grahammer J, Lee AW, Cavadas P, Margreiter R, Pratschke J, Schneeberger S. Histopathologic characterization of mild rejection (grade I) in skin biopsies of human hand allografts. Transpl Int 2012; 25:56-63; PMID:21981770; http://dx.doi.org/10.1111/j.1432-2277.2011.01369.x.
- Wolfram D, Starzl R, Hackl H, Barclay D, Hautz T, Zelger B, Brandacher G, Lee WP, Eberhart N, Vodovotz Y, et al. Insights from computational modeling in inflammation and acute rejection in limb transplantation. PLoS One 2014; 9:e99926; PMID:24926998; http://dx.doi.org/10.1371/journal.pone.0099926.
- Sacks JM, Kuo YR, Horibe EK, Hautz T, Mohan K, Valerio IL, Lee WP. An optimized dual-surgeon simultaneous orthotopic hind-limb allotransplantation model in rats. J Reconstr Microsurg 2012; 28:69-75; PMID:21863543; http://dx.doi.org/10.1055/s-0031-1285822.
- Cendales LC, Kanitakis J, Schneeberger S, Burns C, Ruiz P, Landin L, Remmelink M, Hewitt CW, Landgren T, Lyons B, et al. The Banff 2007 working classification of skin-containing composite tissue allograft pathology. Am J Transplant 2008; 8:1396-400; PMID:18444912; http://dx.doi.org/10.1111/j.1600-6143.2008.02243.x.
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statist Soc B 1995; 57:289-300.
