Abstract
Five patients received a bilateral hand (n = 3), a bilateral forearm (n = 1) and a unilateral hand transplant (n = 1) between 03/2000 and 03/2014. We herein describe the long-term outcome with emphasis on function, immunosuppression (IS), histomorphology and graft vascular changes.
Induction therapy with antithymocyte globulin or alemtuzumab was followed by tacrolimus, prednisolone ± mycophenolate mofetil (MMF) or tacrolimus and MMF maintenance IS. Later, an mTOR-Inhibitor was added under simultaneous withdrawal or dose reduction of tacrolimus or MMF. Steroids were avoided in one and withdrawn in 2 patients.
Range of motion reached up to 70% of normal with a grip strength up to 10kg. Hand function correlated with time after transplantation and amputation level and remained stable after year 5 in all cases. Intrinsic hand muscle function recovery, discriminative sensation and temperature sensation were observed after hand transplantation. Three, 7, 6, 3 and one rejection episodes were successfully treated with steroids, anti-CD25, anti-CD52 and anti-CD20 antibodies and/or intensified maintenance IS. Repetitive events of skin rejection/inflammation late after transplantation were observed in one case. Skin histology at current shows no or mild perivascular lymphocytic infiltrates without signs of progression. Vessels are patent without signs for luminal narrowing or intimal proliferation. The overall functional outcome and patient satisfaction are highly encouraging. All patients are now free of rejection with moderate levels of IS.
Abbreviations:
- ADL, activities of daily living
- ATG, antithymocyte globulin
- CNI, calcineurin inhibitor
- CMAP, compound motor action potentials
- DSA, donor specific antibodies
- HTSS, hand transplantation score system
- iRT-PSP, Innsbruck Psychological Screening Program for Reconstructive Transplantation
- IS, immunosuppression
- MMF, mycophenolate mofetil
- RHT, reconstructive hand transplantation
- RTI, Reconstructive Transplantation Innsbruck
Introduction
Human hand and face transplantation have become valid therapeutic options after the loss of a hand or a forearm. Over 100 single and double hand transplantations have been performed worldwide between 1998 and 2014 and outcomes have been satisfactory in the majority of cases. Immunosuppressive protocols similar to those used in solid organ transplantation were effective in preventing early graft loss as reflected by a 1-year survival of 95%. Long term graft survival and immunologic challenges late after transplantation, however, remains unknown.Citation1-4
Seven hand/forearm transplantations have been performed in Innsbruck. Between March 2000 and May 2006, 3 patients underwent bilateral hand and forearm transplantation.Citation5 After careful consideration unilateral hand amputation has been accepted as an indication for transplantation and the first unilateral hand transplant was performed in July 2009. In March 2014 another bilateral hand transplantation was performed and the early course of this case is included in the data analysis.
Patients have been monitored closely by regular inspection of skin, evaluation of hand function, histological and immunohistological assessment of skin biopsies, monitoring of de novo donor specific antibodies (DSA) by LUMINEX (Luminex® 200™) and ELISA, X-ray, ultrasound, CT scan with CT-angiography, conventional angiography, nerve conduction studies and somatosensory evoked potentials performed at regular intervals. We herein summarize the clinical courses and important events during a 14-year follow up. We focus in this manuscript on findings considered particularly relevant and on novel observations not published previously.
Patient and Methods
Over a course of 14 years, 7 hands have been transplanted in 5 patients at the Innsbruck Medical University. The first hand transplantation in Innsbruck was performed on March 2000. In the second bilateral transplant performed in 2003, the level of amputation was just below the elbow and transplantation included transfer of all forearm muscles.Citation6 A third bilateral transplant at the level of the mid forearm was performed in 2006.Citation5 After reviewing the functional outcomes of the first 3 cases and a reflection on the outcome of unilateral hand transplantation, our working group decided to accept unilateral amputees for transplantation. The first unilateral hand transplant was eventually performed in July 2009. In March 2014 patient #5 received a bilateral hand transplant. The level of amputation was the metacarpal area (left) and the wrist (right).
Preformed panel reactive HLA antibodies were only present in patient 1 (5%). Donor/Recipient HLA match was 0/6, 2/6, 3/6, 0/6 and 0/6 in patients 1, 2, 3, 4 and 5 respectively. The pre-transplant lymphocytotoxic crossmatch was negative in all 5 patients. Patient characteristics are shown in . Mean waiting time was 10.03 ± 4.7 months (mean ± SD). The cold ischemia time was 169.8 ± 34.1min (mean ± SD).
Table 1. Patient characteristics at the time of transplantation
Surgery
Detailed descriptions of patient and donor selection, surgical procedure, hand therapy and postoperative follow up for the first 2 patients have been published earlier.Citation6,7 Patient 3 had lost both hands and one eye after a bomb explosion. The surgical technique was equal in sequence and principals as in the previous cases.Citation5 The mean warm ischemia time was 190.8 ± 41.8 minutes (mean ± SD).
In July 2009, a unilateral hand transplantation at the very distal forearm was performed applying the same principles as in the previous, bilateral cases: (i) donor and recipient match for blood group, gender, size, ethnicity, skin color and appearance, CMV match (ii) completion of donor and recipient operation in adjacent theatres to keep ischemia time short (iii) sequence of reconstruction–bone, vessels, tendons, nerves, skin (iv) early passive mobilization (v) fitting of a cosmetic, skin tone matched prosthesis to the donor. Inclusion criteria for unilateral hand transplantation included a high degree of psychological impairment and significant loss of quality of life attested by a 2-step psychological evaluation. The patient was a healthy, 54 y old male suffering from traumatic right hand amputation in 2004. He was fitted but unsatisfied with myoelectric prosthesis.
Proximal extrinsic musculature was preserved. Surgical modifications included augmentation of the extensor pollicis longus tendon by transposing and attaching the proximal aspect of the tendon of the brachioradialis muscle at the radiodorsal site.
As in previous cases, cold ischemia of the transplant has been kept short to minimize damage of the intrinsic musculature and other tissues. Time interval between crossclamping and reperfusion of the transplant was 200 minutes. Transplant perfusion was performed using 500 ml HTK preservation solution. To prevent thromboembolic events, the patient was given heparin intravenously starting at 500 IE/h and adjusted to reach a partial thromboplastin time of 40 seconds. The fifth case, a bilateral hand transplantation in a 55 y old male patient, was performed in March 2014. The recipient lost both hands in a car accident in 2009. Transplantation of the left hand at the level of the wrist was technically similar to previous cases. On the right side, osteosynthesis was performed at the metacarpal bones. Vascular and nerve reconstruction were completed at the wrist level
Immunosuppression
Antithymocyte globulin (ATG; ATG-Fresenius S ®, Fresenius Biotech) was used for induction therapy in the first 2 patients and alemtuzumab, an anti-CD52 antibody, (Campath®, Genzyme Corporation, MA, USA) in patients 3 and 4. For maintenance IS, patients received tacrolimus, mycophenolate mofetil (MMF) and prednisolone in the first 3 cases. A steroid free regimen (tacrolimus and MMF) was used in the fourth patient. Reduction of tacrolimus trough levels and steroid withdrawal was cautiously performed in all patients. Steroids were tapered between year 3 and 5 after transplant. Conversion from tacrolimus to an mTOR (sirolimus) inhibitor was attempted at 2 y and tacrolimus was withdrawn at 5 y in patient #1. Tacrolimus is currently being weaned over a 4-year course in the second patient since therapy with everolimus was initiated. In patient #3, treatment with sirolimus and weaning of tacrolimus was started 6 months ago. Our fourth patient is currently being treated with tacrolimus and MMF, after an attempt to treat with belatacept (Nulojix®, Bristol-Myers Squibb) initiated in response to increasing serum creatinine and mTOR-inhibitor induced proteinuria, had failed after 7 months. In all patients, adjustment of immunosuppressive therapy is performed under closer surveillance of skin appearance and skin histology. Patient #5 received induction therapy with 30mg alemtuzumab followed by triple-drug maintenance immunosuppressive therapy based on tacrolimus. MMF was switched to MPA 2 months after the transplantation due to diarrhea. Steroids were withdrawn on postoperative day 21. On day 100 after transplantation belatacept (Nulojix®, Bristol-Myers Squibb) was started because of renal insufficiency according to the calcineurin inhibitor- (CNI) conversion protocol published by Grinyo et al.Citation8
Rehabilitation program
Immediate therapy aims included pain control, swelling control, joint stiffness prophylaxis, soft tissue adhesions prevention, tendon glide maintenance, motor re-education, sensory re-education, and independence. The respective treatment goals and therapy methods were adapted individually and with time according to the amputation level, additional interventions and patient´s daily activities and avocations. The long-term rehabilitation protocol consisted of early protective motion (EPM), cognitive exercise training according to Perfetti, electro-stimulation, occupational therapy, activities of daily living (ADL), sensibility training, EMG-biofeedback training, lymphatic drainage and use of positioning splints.Citation9
Histology and immunohistochemistry
Skin biopsies were taken every month during the first 3 months, every third month until one year after transplantation and annually thereafter.Citation10 Additional biopsies were taken when rejection was suspected. Four-mm punch biopsies were fixed in 4% buffered paraformaldehyde, embedded in paraffin and stained with haematoxylin and eosin (H&E). Rejection was graded according to the Banff 2007 guidelines for skin containing VCAs.Citation11 The characterization of the cellular infiltrate was done using antibodies for CD3 (Daco, Vienna, Austria, dilution 1:50), CD4 (Menarini Diagnostics, Vienna, Austria, dilution 1:10), CD8, CD20, CD68 (all Daco, dilution 1:50, 1:700 and 1:100) and Foxp3 (Biocare Medical, Concord CA, dilution 1:50). C4d staining (Biomedica, Vienna, Austria, dilution 1:40) was performed to detect antibody-mediated rejection.
Imaging modalities
Helical CT examinations were performed on multi-detector row CT scanners (LightSpeed QX/i; LightSpeed 16; VCT; General Electric Medical Systems, Milwaukee, WI) with a 0.35-0.8 second gantry rotation period. CT-angiography was acquired, in a proximal-to-distal direction by using 1.25 mm (LightSpeed QX/i) or 0.625 mm (LightSpeed 16; VCT) collimation, a 0.626 - 1.25 mm reconstructed slice thickness, and the standard reconstruction kernel. According to the anatomical region of interest, the pitch and the reconstruction interval were adjusted. CT data sets were transferred to a picture archiving and communicating system (PACS; J-Vision, TIANI, Austria) as well as to a 3-dimensional rendering workstation (Ultra 60; Sun Microsystems, Mountain View, CA) running the Advantage Windows software (Advantage Windows 4.0; General Electric Medical Systems, Milwaukee, WI), both of which are equipment of the emergency CT suite. Multiplanar 2D (MPR) and Volume Rendering 3D (VR) reconstructions were performed.
All angiography procedures were performed in an angiographic suite (Integris BV 3000; Philips, Eindhoven, The Netherlands). In all patients, transarterial access was obtained via a right femoral approach. In all of them, the exact position of the catheter within the artery and that of its tip were identified by diagnostic angiography with a 4 or 5-F catheter (Cordis, Roden, The Netherlands).
Nerve conduction studies
Motor and sensory nerve conduction velocity studies were performed according to standard procedures using a Nicolet Viking IV (Nicolet Biomedical, Madison, WI), Oxford Medelec Synergy (Witney, Oxfordshire, UK) or Dantec Keypoint.Net (Skavlunde, Denmark). Disposable surface electrodes were used to record compound motor action potentials (CMAP) form the abductor pollicis brevis and abductor digiti minimi muscles after stimulating the median and ulnar nerve just proximal the site of hand or forearm transplantation. Compound sensory action potentials were recorded from the index and fifth finger using band electrodes.
Psychological assessment
Innsbruck Psychological Screening Program for Reconstructive Transplantation (iRT-PSP):
Reconstructive Hand Transplantation (RHT) needs not only standardization in surgical techniques and immunological procedures but also psychosocial evaluation and treatment. The small number of transplanted patients makes the study RHT specific psychosocial components leading to successful outcome difficult. Psychological and social factors are keys to long-term success. A multi-staged multidisciplinary psychosocial protocol including an comprehensive assessment of the candidates’ past history, current stressors, family support, phantom limb pain, utilization of prosthesis, understanding of RHT and motivation for RHT was implemented. In addition to the identification of psychological contraindications, the thorough interdisciplinary screening thought to identify the opportunity for supportive psychological treatments in order to enhance the candidates’ quality of life (QOL) and adherence.Citation12,13Considering the lessons learned from different psychosocial evaluation programs and based on our clinical experience with hand transplant patients in the past 14 years, we have developed the iRT-PSP as a standardized multi-staged psychosocial evaluation protocol. The evaluation of key psychosocial domains in RHT is provided by a semi-structured interview addressing motivational aspects, body image, QOL etc., and a standardized battery of psychometric instruments (a comprehensive description of the iRT-PSP is given by Kumnig et al.).Citation14 Furthermore, the iRT-PSP provides a standardized protocol not only for pre-transplant evaluation but also for pre- and postoperative follow-up ratings, so that patients requiring supportive psychological and/or psychiatric treatment can be identified.Citation14
Results
Surgery
Two episodes of immediate postoperative swelling due to hematoma required surgical revision in 2 patients (#3 and 5) and coverage of a defect on the proximal forearms using split thickness skin grafts in one case (patient #3).
Postoperative swelling of soft tissue at the level of skin suture on day 3 in patient # 4 did not require further surgical treatment. Patency of vascular anastomoses was achieved in all patients as monitored using ultrasound. Bone healing of the forearms as monitored with plain radiographs and ultrasound was within normal range.
Secondary surgical procedures since 2006 included a surgical scar correction in the face done simultaneously with scar and skin graft resection on both forearms at one year after transplantation in patient #3. Despite repeated ophthalmological interventions retinal damage in the second eye of this patient (consequence of the bomb blast) progressed and ultimately resulted in blindness. Insufficient palmar abduction and thumb opposition required an opponensplasty by transposing the superficial flexor tendon of the patient's ring finger at 2 y. At 4 y, a scar correction at forearm level was performed by skin z-plasty upon the patients’ request. All secondary surgeries and tissue healing were uneventful.
Immunologic complications and side effects
Overall a total of 3, 7, 6, 3 and one rejection episodes were observed during the 14-, 11-, 8- and 5-year and 3 mo follow-up (). Time to first rejection was 55, 9, 51, and 14 d in patients 1, 2, 3 and 4, respectively. More detailed descriptions of the immunological courses and side effects for patients 1–3, including a bullous pemphigoid of our first patient, have been published previously.Citation15 In summary, all rejections have been detected by visual inspection of skin lesions. The appearance of skin lesions differed significantly between rejection episodes, individual patient and skin area. In each patient, an individual but characteristic pattern of rejection with regard to appearance and localization was observed. Rarely did skin rejection appear on the entire allograft, but was rather restricted to a specific region. All rejections except for one appeared on the forearm or the dorsum of the hand. In most cases, lesions were scattered and non-confluent. Only rarely was skin rejection associated with swelling of the allograft. Edema of the graft did also occur without skin lesions. At this point it remains unclear if edema has to be counted as a sign for rejection. Patients 2 and 4 presented with edematous hands and forearms and described a sensation of tension without any exanthema typical for rejection at 9 and 3 y after bilateral forearm and unilateral hand transplantation. Biopsies revealed rejection grade Banff II-III in both patients. Immunhistochemical analysis identified large aggregates of lymphocytes with an architecture resembling lymph nodes. CD20 staining identified the center of the aggregates almost entirely consisted of B-lymphocytes. DSA (Luminex® 200™) were found at high levels in both patients for the first time since transplantation. Based on the predominance of B-cells and DSA with lack of response to conventional treatment with steroids and tacrolimus dose increase, rituximab, an anti-CD20 antibody (MabThera®, F. Hoffmann La-Roche AG), was given at 375 mg/m2 BSA. In response, clinical symptoms disappeared and biopsies showed normal skin with absence of B-cells. DSA were negative at 3 months after rituximab.Citation16
Table 2. Rejection episodes
In most cases, rejection responded to topical treatment with tacrolimus and steroid ointments. In the early stage, skin rejection was treated more aggressively and 500 mg methylprednisolone was used routinely for treatment. Also, tacrolimus dose was transiently increased in all cases. In one patient who was off tacrolimus (patient 1), tacrolimus was restarted and withdrawn again 8 months later. While most rejections responded promptly to this treatment, more aggressive rejections observed in patients 2 and 3 did not change or even progressed further and prompted therapy with basiliximab, an anti-CD25 antibody (Simulect®, Novartis Pharmaceuticals Corporation), thymoglobuline and/or alemtuzumab. Alemtuzumab had shown a rapid effect in one very severe rejection in patient 2. In total, 3 rejections resistant to i.v. steroids + tacrolimus dose increase + tacrolimus and steroid ointments were eventually treated with alemtuzumab. While this had a prompt and sustained effect on skin rejections, repeated administration of alemtuzumab resulted in nausea, fever, headache and edema requiring hospitalization and monitoring of hemodynamic parameters. The significant overall amounts of immunosuppression given in patients 2 and 3 resulted in repetitive CMV infections, polyoma virus-associated skin warts, invasive fungal infection with Alternaria Alternata, hypertension, headache, transient creatinine increase, hyperlipidemia and myocarditis, each requiring specific treatment ().
Table 3. Complications and side effects
In the first patient all acute rejections were histologically grade I or II and responded promptly to i.v. steroid bolus or topical treatment with tracrolimus and steroid ointments. Calcineurin-based triple-drug IS was gradually reduced and ultimately switched to low-dose sirolimus monotherapy (trough level 4-6 ng/mL).
Since the 8-year report of this program, 3 additional (fourth, fifth and sixth) mild rejection episodes (Grade I-II) occurred between 2.5 and 6 y after transplantation in patient 3 and were treated with topical tacrolimus and steroid ointments and a transient increase of tacrolimus dose (trough levels 10 ng/ml → 15 ng/ml).
After a single, early rejection episode on day 14 treated with an increase in maintenance tacrolimus, MMF and topical ointments only, patient #4 developed a second acute rejection episode 18 months after transplantation. A mechanical irritation from the patient's work in Christmas tree business seems to have triggered a Banff II skin rejection on the mechanically stimulated areas of the skin. Rejection was treated successfully with topical steroids and intensified maintenance IS. No intravenous or oral steroids were used in this patient and the overall amount of immunosuppression is significantly less when compared to cases 1–3. Subsequent to non-adherence and overdosing of tacrolimus combined with a creatinine increase and after consultation with the local ethic committee, patient #4 was switched to belatacept (Nulojix®, Bristol-Meyers Squibb) in February 2013. Seven months after the start with belatacept according to the CNI-conversion protocol (8), the patient developed a deterioration of hand function, painful tension and limited mobility, without histology signs of rejection.
The first rejection episode in patient 5 occurred on the 112th postoperative day with the occurrence of a light red exanthema on the dorsum of the hands. The skin punch biopsy revealed acute rejection episode Banff grade I to II. The rejection could be treated successfully with 3 × 500 mg steroids intravenously.
Patients are now free of rejection for 10, 2, 3, 1.5 y and 22 d. All most recent protocol biopsies taken annually show normal skin histology without any histopathologic evidence of rejection. Neither systemic nor intragraft chimerism were observed in any patient.
Current immunosuppression
The current immunosuppressive regimen in our patients consists of sirolimus with targeted trough levels of 4 to 6 ng/mL (patient #1), tacrolimus (trough levels 3-4 ng/ml and 9–10 ng/ml) in combination with everolimus/sirolimus (trough levels 5-6 ng/ml and 7-8 ng/ml) (patient #2 and #3 respectively), tacrolimus (trough levels 4-5 ng/ml) and MMF (2 g/d) (patient 4) and belatacept (Nulojix®, Bristol-Meyers Squibb), tacrolimus (in a weaning dosage) and MMF (2g/day) (patient #5). Overall, IS was gradually and continuously tapered to moderate levels in all 4 patients without triggering severe rejection or graft loss. Close monitoring of the vasculature was performed to rule out potential luminal narrowing as a consequence of myointimal proliferation.
Histology and immunohistochemistry
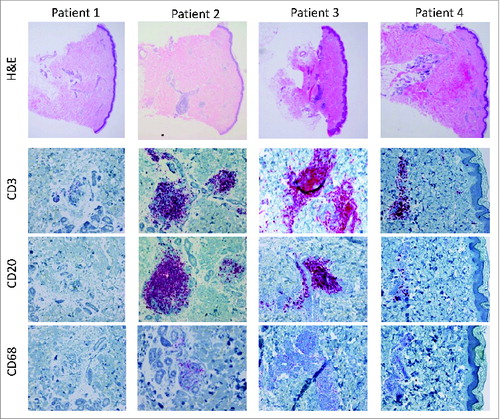
In all patients, histologically proven acute rejections were documented during the postoperative course. Patient 1 experienced 3 histologically mild to moderate (grade I-II) rejection episodes on day 55, 4 y and 9.5 y after transplantation, respectively. The majority of the infiltrating cells were CD3+ T lymphocytes (CD4:CD8 T cells 3:1). Sparse macrophages and no infiltrating B-cells were detected. During the most recent rejection episode, 5–10% Foxp3+ infiltrating T cells were observed. At 11 y after transplantation the patient developed a bullous pemphigoid.Citation15 Histology of the recipient's own skin and the skin of the allografts revealed a partial separation along the dermal-epidermal junction. A mild perivascular cellular infiltrate consisted mainly of lymphocytes, whereas a small number of eosinophils and granulocytes were located interstitially and toward the epidermis. The histology of the protocol biopsy taken at 12 y after transplantation showed normal morphology of the epidermis, dermis and subcutis without histopathologic alterations and skin atrophy (). Cutaneous nerves and skin appendages such as sebaceous glands, perspiratory glands and hair follicles displayed normal structure. No signs of vasculopathy or intimal hyperplasia were encountered in vessels of the dermis and subcutis. Only single CD3+ T cells were observed perivascularly without evidence for rejection.
The clinical course of the forearm transplant patient (patient 2) was complicated by repeated acute rejection episodes during the first year post transplant, 2 of the rejections were more severe than in the first patient (grade III). Immunohistochemical analysis revealed an increase in infiltrating Foxp3+ T lymphocytes upon rejections at later time points. At 9 y post-transplant, he experienced an antibody-mediated rejection episode.Citation16 Lymphocytic accumulations resembling lymph nodes were found in the deep dermis and consisted mainly of B cells, CD20+ () accompanied by peripheral node addressin (PNAd)+ vessels. C4d staining showed an intense pattern in most vessels. Moreover, the patient was positive for HLA class I and II antibodies.
In patient 3, a first severe (grade III-IV) acute rejection was recorded on day 51. He further experienced mild to moderate rejections (grade I-II) between years 1-and 4. Immunohistochemical analysis revealed an increased number of infiltrating macrophages during these rejection episodes of patient 3, which comprised approximately 30-50% of the cellular infiltrate. A mild, diffuse CD68+ infiltrate was also found during absence of rejection. Five% Foxp3+ T cells were detected during rejection after one year post transplant. The protocol biopsy at 6 y after transplantation showed grade II rejection (). A prominent cell infiltrate mainly located perivascularly and around the adnexae in the dermis was comprised of CD3+ T lymphocytes (CD4:CD8 T cells 2:1) and CD20+ B lymphocytes (). Macrophages comprised only 5% of the infiltrating cells ().
Patient 4 experienced a mild grade I rejection episode on day 15. At 18 months a severe rejection (grade III) was diagnosed after exposure to mechanical stress. The localization was atypical and manifested on the skin of the thumb, the index finger and the fingertips. The diffuse cell infiltrate in the superficial dermis and epidermis was dominated by CD3+ T cells, whereas a lymph-node-like cell accumulation in the deep dermis mainly consisted of CD20+ B cells in the center and few CD3+ T cells in the periphery. The patient was DSA+ at this time. A grade II rejection was observed at 2 y post-transplant, which was similar to the previous episode in respect to the distribution and characterization of the cell infiltrate. The protocol biopsy at 3 y revealed only a mild perivascular cell infiltrate in the dermis and subcutis (grade I), which comprised of 85% CD3+ T lymphocytes (CD4:CD8 T cells 2:1), 10% CD20+ B lymphocytes and 5% CD68+ macrophages ().
Imaging
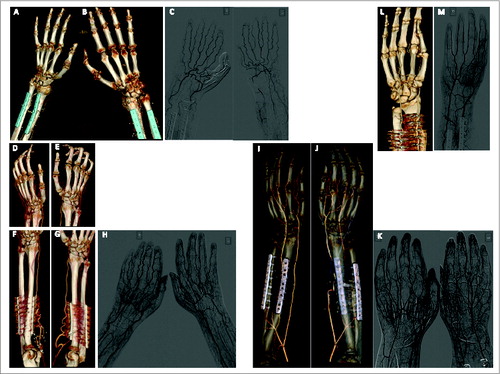
X-ray, CT-angiography, conventional angiography and ultrasound were performed at regular intervals according to an internal protocol in all patients. Bone healing, vascular patency, muscle texture and nerve regeneration were monitored and documented over a 14-year period. Some of the findings for individual patients have been published previously. In summary, the most relevant findings observed in all or the majority of patients included: (i) Elongation and kinking of ulnar und radial arteries. This resulted from the excess length of vessels available in the transplant setting. This is different to replantation where vessels are at original length or too short for reconstruction (requiring interposition). Never has the kinking resulted in malperfusion and the condition of the vasculature remained largely unchanged over the course of 12 y. (ii) Enhanced vascularization of the muscle just proximal to the level of transplantation. This was interpreted as a residual of the hyperperfusion and inflammatory response to the surgical trauma. (iii) Hyperemia of the fingers with elongation and ectasis of finger veins. shows 3D-reconstructions of CT-angiography for the first 4 patients.
Functional outcome
In summary, all 4 patients displayed improvement of hand function in the first one (hand transplantation) to 5 (forearm transplantation) years. In the majority of cases the functional parameters at large have remained unaltered. Although insignificant, reduction in range of motion, grip strength and sensitivity has been observed in individual cases. In contrast, DASH (disabilities of the arm, shoulder and hand) score as well as ARAT (action research arm test) score express the improvement of activity of daily living and patient´s independence. Some inconsistent variations related to the side, joint were observed but did not seem to be related to any specific event or immunological courses.
Patient 1: The patient had rapid return of good motor function and sensation and little changes had occurred after the first year. However, annual variations in grip strength, key pinch, 2-point discrimination, DASH score, ARAT score and Ipsen (modified version of the Tamai classification; treatment of amputated hand and fingers) score were observed. Over the course of 14 years, a subtle but continuous decline in grip strength and key pinch were seen. This may correlate with the level of exercise, as it has not been accompanied by changes in sensitivity and range of motion. Key pinch showed a positive development for the first 6 y but started to decrease after that. Grip strength was best at 2 y after transplantation. At 14 years, grip strength is 6.7 kg on the right side and 5.4 kg on the left. Key pinch is 0.9 kg on the right side and 1.4 kg on the left side. At the patient`s 14-year annual assessment the ARAT score was 50 (out of possible 57) for the right hand and 47 for the left hand, DASH measures 20.8, and HTSS (hand transplantation score system) was 91.5 on the right side and 91 on the left, respectively. This patient is able to perform all activities of daily living, independently. Sensory changes are still being observed, currently static 2-point discrimination scores of 3–8, depending on the digit.
Patient 2: The forearm transplant recipient displayed continuous improvement in grip strength, key pinch and DASH score while ROM (range of motion) and sensitivity remained overall unaltered up to 9 y post-transplant. At the 9-year assessment sudden worsening of the overall function was observed. Afterwards total active ROM improved slightly without achieving the former status, in generally. In contrast, the grip strength and the key pinch recovered considerably. Grip strength in position 3 measured 9.2 kg on the right side and 6.5 kg on the left at 11-years after transplantation. At the same time this patient showed good result for the key pinch: 3.2 kg on the right and 1.3 kg on the left side. This patient has no 2-point discrimination. He is able to detect the stimulus and to discriminate it. The adequate localization of the stimulus is not always possible. The patient`s ARAT score is 29 on the right side and 32 on the left. The 3 jaw function is hindered. DASH measures 62.5% at this point. The course in this patient illustrates the significant time span for functional recovery after forearm transplantation. Despite an immunologically challenging course, no stiffening or progressive atrophy of forearm and hand muscles was observed.
Patient 3: Motor as well as sensory function improved continuously over the course of the first 5 y post-transplant in this patient. While grip strength in the left hand is less when compared to the right hand, 2 point discrimination and key pinch are particularly satisfactory in this case. The loss of vision in this patient complicated the rehabilitation, but does not seem to have negatively impacted on the outcome. At 7 y after transplantation, the functional outcome evaluation was performed under difficult circumstances with respect to the skin alterations. Despite this fact the moving 2-point discrimination scored 3–6. The static 2-point discrimination was not measurable on the right side, scoring 5–10 on the left side, at the same time. Neither grip strength nor key pinch was measurable on the left side. Grip strength was 5.9 kg and the key pinch 1.1 kg on the right side. The ARAT score was 33 on the right side and 30 on the left. DASH score was 52%.
Patient 4: At 4 y after unilateral hand transplantation, motor and sensory function continued to improve at a similar range as after bilateral hand transplantation (Patient 1). It is unclear why the Kapandji opposition score decreased abruptly, from 5 (at the 3-year assessment) to one (at the 4-year evaluation). Semmes-Weinstein monofilament testing showed a loss of protective sensation. The patient is very satisfied with his capability to perform all activities of daily living. The DASH score was 5%, and the ARAT score 53. While grip strength is still inferior to the contralateral non-transplanted hand, we have not observed significant differences between unilateral and bilateral hand transplantation with regard to the patient's motivation during rehabilitation, the functional outcome as well as the patient satisfaction.
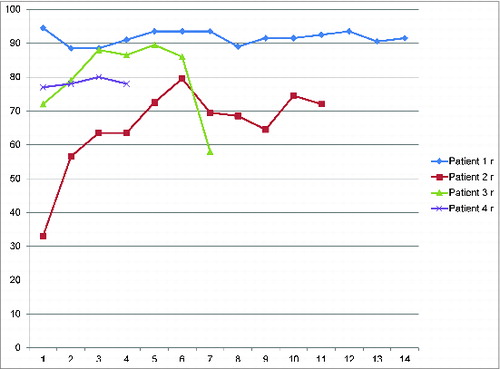
The results of the international registry for each patient (HTSS, hand transplantation score system) are shown in . Recovery of function takes longer than initially expected and continues to improve up to 7 y after transplantation. While reinnervation and reorganization of the sensorimotor cortex are present after 6–18 months, it is believed that improved innervation as well as continuous cortical rearrangement further enhances reintegration of the transplanted limbs late after transplantation. Improvements in nerve conduction studies indicates continuous regeneration of peripheral nerves while enhanced cortical reintegration remains a speculation based on functional outcome assessment.
Figure 3. Hand Tansplantation Score System (HTSS) The total score for patient #1: 90.5 for the right and 90 for the left hand; for patient #2: 74 for the right and 71.5 for the left forearm; for patient #3: 86 for the right and 85.5 for the left hand; for patient #4: 80 for the right hand. The hand transplantation score system of the international registry includes appearance (15 points), sensibility (20 points), movement (20 points), psychological and social acceptance (15 points), daily activities and work status (15 points), patient satisfaction and general well being (15 points). 0–30 points &Equals; poor, 31–60 points &Equals; fair, 61–80 points &Equals; good, 81–100 points &Equals; excellent.
Nerve conduction studies
Repeated nerve conduction velocity studies demonstrated motor reinnervation in all and sensory reinnervation in 3 patients. shows the changes of CMAP amplitude over time for patients 1‐ 4. Maximum CMAPs were recorded after 2-5 y and remained stable afterwards. The reduced CMAPs seen in patient 4 after 4 y are most likely temporary due to a significant swelling of his hand.
EVP
Somatosensory evoked potentials were studied 3 y after hand and forearm transplantation in patients 1 and 2. The left and right index and fifth fingers and the left and right median and ulnar nerves at the wrist were electrically stimulated and recordings were performed over Erb's point, C5 and the somatosensory cortex according to standard procedures using a Nicolet Viking IV, Nicolet Biomedical, Madison, WI).
In both patients cortical responses were present at 3 y after hand transplantation, while no potentials could be recorded over Erb's point or C5. The latencies of the cortical responses were prolonged but the amplitudes within normal range in patient 1 and slightly reduced in patient 2. Theses findings not only confirm peripheral sensory reinnervation, but also indicate reorganization of the somatosensory cortex.
Psychological assessment
The iRT-PSP has been used for pre-transplant evaluation of potential candidates considering uni-/bilateral RHT and to monitor patients after RHT. The iRT-PSP has been only recently developed and the current data on the pre- and post-transplant psychosocial outcomes are incomplete. All candidates assessed had a different history of hand loss and showed diverse psychological reactions and reduced QOL before RHT. The overall psychosocial outcomes are very satisfying, neither sever psychosocial/psychiatric complications (apart from normal post-transplant stress reactions) nor body image distortions have been recorded. The diverse psychological reactions due to hand loss has been thoroughly investigated, but no common defense concept for RHT patients has been found.Citation12,13
Our results describe essential psychosocial similarities and also major differences between individual patients allowing for a better understanding of psychosocial conditions in patients awaiting uni-/bilateral RHT. Low-risk candidates typically have greater psychological stability and adjustment, medical compliance, are more interested in the treatment options and the course of RHT and have a healthier lifestyle than high-risk candidates. The iRT-PSP may also serve as the basis for developing or providing support toward developing coping mechanisms for patients to prepare for transplantation and cope well with the therapeutic challenges after hand transplantation.
Discussion
Fourteen years after initiation of vascularized composite allotransplantation at the Innsbruck Medical University, all 5 patients with their 9 transplanted hands/forearms are in an overall satisfactory condition. Without a doubt, the team of physicians, nurses and therapists have gone through a learning curve and a great many aspects are better understood now when compared to the initiation of the program.
Hand transplantation represents an unique medical development offering the possibility to regain sensation and the sense of body integrity, which are major limitations of current prostheses.Citation17,18 In bilateral hand amputees, the indication for transplantation builds on the overall functional limitations. For patients with unilateral hand amputation, the decision for surgery is more challenging since many functional, sensory and social capacities are presented. The patients´ functional needs and psychological impairment, however, are different between individuals and some patients suffer more from the hand loss than others. Based on surgical, immunological and psychological parameters, guidelines for donor and recipient selection for bilateral and unilateral hand transplantation have been developed. Step 1: basic hand, surgical, immunological, psychological and general health test in conformity with the screening test for potential solid organ transplant recipients. Step 2: advanced psychological evaluation. Step 3: advanced hand surgical/immunological diagnostics, Step 4: interdisciplinary consensus.
The main issues of the transplant candidates’ psychological strain deal with the improvement of quality of life, in the sense of improving a disturbed body image due to the loss of one hand.
The psychological assessment addresses the status of a transplant candidate as well as psychological resources and copying strategies for the postoperative period.Citation12-14
Outcome assessment is an essential tool for evaluating, monitoring and reporting the results of interventions with patients, particularly because many different modalities of rehabilitation are being used. It is important to assess a patients’ ability to actually use his hands. Not all tests applied to assess the outcome were deemed suitable. We applied the ARAT, DASH, WEST Monofilament test and ROM test for the evaluation of function after hand and forearm transplantation.
Restoration of sensation after hand transplantation progresses rapidly and nerve endings reach the fingertips at 6-9 months depending on the anatomical level of transplantation.Citation1,19 Protective and discriminative sensation returned in all of the patients. In hand transplantation, the patient´s forearm muscles enable finger movement early after transplantation. In many cases, the return of intrinsic hand muscle function can also be observed. Transplantation at more proximal levels requires prolonged rehabilitation, is overall less consistent and remains inferior when compared with hand transplantation. The reinnervation and reactivation of intrinsic hand muscles remains unlikely.Citation5,20
Despite the good functional outcome in our patients, the postoperative courses have been complicated by skin rejection in all patients. Skin rejections were heterogeneous in appearance and location. While lesions were confluent with progression of rejection, the location of first appearance was the dorsum of hand and forearm in the majority of cases. When rejection appeared on the palm, mechanical stress was timely associated and considered a triggering agent. A systematic analysis of such an association is currently ongoing.
Histopathological assessment of rejection allows for rapid diagnosis of the severity of rejection and the procedure has been standardized. The histologic criteria for severity of rejection have summarized in the BANFF classification and further refined at the last BANFF meetings in Paris and Comandatuba.Citation21,22 According to our internal protocol, 8-12 serial cuttings are stained per H&E and immunostains as a routine diagnostic panel.Citation10 In addition, staining for C4d is performed whenever clinically indicated. Immunosuppression reduction over time correlated with FoxP3 expression in the skin. Immunosuppression minimization after VCA was considered important in order to reduce the risks for such a non-life-saving procedure. Efforts to develop new immunosuppressive protocols and eventually establish donor specific tolerance without the need for chronic immunosuppression remains the primary objective in this field. Some centers have investigated modified immunosuppressive protocols to reduce the burden of immunosuppressive drugs. The Pittsburgh/Johns Hopkins group indicated that a modulation of the recipient´s immune system through infusion of donor bone marrow cells may be possible.Citation23 Tolerance has been successfully established in number of patients in pilot projects in kidney transplantation.Citation24-26 It remains to be investigated if any such protocols can be successfully applied in VCA. For instance, the Massachusetts General Hospital (MGH) group could successfully establish long-term drug free graft survival through transient chimerism in 7 out of 10 patients.Citation27
It is essential to monitor VCA recipients at least annually even late after transplantation. In our experience even trivial imbalances such as a flu may influence the medical course in these patients. Further, continuous adjustment of the immunosuppressive regimen seems justifiable in order to avoid unnecessary overimmunosuppression and associated effects such as malignancies, CNI-induced renal insufficiency, metabolic and infectious disorders. In the more recent experience, increasing evidence indicating the development and the clinical relevance of donor specific antibodies has surfaced. The implementation of tolerance induction in VCA remains the center of attention. Before this can be achieved, VCA should only progress with great caution and a through risk benefit analysis in each individual case.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
Funding was provided by the TILAK Foundation, Innsbruck, Austria.
References
- Petruzzo P, Lanzetta M, Dubernard JM, Landin L, Cavadas P, Margreiter R, Schneeberger S, Breidenbach W, Kaufman C, Jablecki J, et al. The international registry on hand and composite tissue transplantation. Transplantation 2010; 90:1590-4; PMID:21052038; http://dx.doi.org/10.1097/TP.0b013e3181ff1472
- Pomahac B, Gobble RM, Schneeberger S. Facial and hand allotransplantation. Cold Spring Harb Perspect Med. 2014; 4: pii: a015651; http://dx.doi.org/10.1101/cshperspect.a015651
- Dubernard JM, Sirigu A, Seulin C, Morelon E, Petruzzo P. Fifteen years later: main lessons from composite tissue allografts. Clin Transpl 2013; 113-9; PMID:25095499
- Weissenbacher A, Hautz T, Pratschke J, Schneeberger S. Vascularized composite allografts and solid organ transplants: similarities and differences. Curr Opin Organ Transplant 2013; 18:640-4; PMID:24126807; http://dx.doi.org/10.1097/MOT.0000000000000019
- Brandacher G, Ninkovic M, Piza-Katzer H, Gabl M, Hussl H, Rieger M, Schocke M, Egger K, Loescher W, Zelger B, et al. The Innsbruck hand transplant program: update at 8 years after the first transplant. Transplant Proc 2009; 41:491-4; PMID:19328910; http://dx.doi.org/10.1016/j.transproceed.2009.01.013
- Schneeberger S, Ninkovic M, Gabl M, Ninkovic M, Hussl H, Rieger M, Loescher W, Zelger B, Brandacher G, Bonatti H, et al. First forearm transplantation: outcome at 3 years. Am J Transplant 2007; 7:1753-62; PMID:17511764; http://dx.doi.org/10.1111/j.1600-6143.2007.01837.x
- Margreiter R, Brandacher G, Ninkovic M, Steurer W. Kreczy A, Schneeberger S. A double-hand transplant can be worth the effort&Excl; Transplantation 2002; 74:85-90; PMID:12134104; http://dx.doi.org/10.1097/00007890-200207150-00015
- Grinyo J, Alberu J, Contieri FL, Manfro RC, Mondragon G, Nainan G, Rial Mdel C, Steinberg S, Vincenti F, Dong Y, et al. Improvement in renal function in kidney transplant recipients switched from cyclosporine or tacrolimus to belatacept: 2-year results from the long-term extension of a phase II study. Transpl Int 2012; 25:1059-64; PMID:22816557; http://dx.doi.org/10.1111/j.1432-2277.2012.01535.x
- Ninkovic M, Weissenbacher A, Gabl M, Pierer G, Pratschke J, Margreiter R, Brandacher G, Schneeberger S. Functional outcome after hand and forearm transplantation: what can be achieved? Hand Clin 2011; 27:455-65; PMID:22051387; http://dx.doi.org/10.1016/j.hcl.2011.08.005
- Hautz T, Zelger BG, Weissenbacher A, Zelger B, Brandacher G, Landin L, Morelon E, Kanitakis J, Jablecki J, Lee WP, et al. Standardizing skin biopsy sampling to assess rejection in vascularized composite allotransplantation. Clin Transplant 2013; 27:E81-90; PMID:23452279; http://dx.doi.org/10.1111/ctr.12086
- Cendales LC, Kanitakis J, Schneeberger S, Burns C, Ruiz P, Landin L, Remmelink M, Hewitt CW, Landgren T, Lyons B, et al. The Banff 2007 working classification of skin-containing compsoite tissue allograft pathology. Am J Transplant 2008; 8:1396-400; PMID:18444912; http://dx.doi.org/10.1111/j.1600-6143.2008.02243.x
- Kumnig M, Jowsey SG, DiMartini AF. Psychological aspects of hand transplantation. Curr Opin Organ Transplant 2014; 19:188-95; PMID:24503494; http://dx.doi.org/10.1097/MOT.0000000000000047
- Kumnig M, Jowsey SG, Moreno E, Brandacher G, Azari K, Rumpold G. An overview of psychosocial assessment procedures in reconstructive hand transplantation. Transpl Int 2014; 27:417-27; PMID:24164333; http://dx.doi.org/10.1111/tri.12220
- Kumnig M, Jowsey SG, Rumpold G, Weissenbacher A, Hautz T, Engelhardt TO, Brandacher G, Gabl M, Ninkovic M, Rieger M, et al. The sychological assessment of candidates for reconstructive hand transplantation. Transpl Int 2012; 25:573-85; PMID:22448727; http://dx.doi.org/10.1111/j.1432-2277.2012.01463.x
- Weissenbacher A, Hautz T, Zelger B, Mueller H, Zelger BG, Margreiter R, Pratschke J, Schneeberger S. Bullous pemphigoid eleven years after bilateral hand transplantation. Am J Transplant 2012; 12:1064-5; PMID:22221867; http://dx.doi.org/10.1111/j.1600-6143.2011.03912.x
- Weissenbacher A, Hautz T, Zelger B, Zelger BG, Mayr V, Brandacher G, Pratschke J, Schneeberger S. Antibody-mediated rejection in hand transplantation. Transpl Int 2014; 27:E13-17; PMID:24266875; http://dx.doi.org/10.1111/tri.12233
- Gander B, Brown CS, Vasilic D, Furr A, Banis JC Jr, Cunningham M, Wiggins O, Maldonado C, Whitaker I, Perez-Abadia G, et al. Composite tissue allotransplantation of the hand and face: a new frontier in transplant and reconstructive surgery. Transpl Int 2006; 19:868-80; PMID:17018121; http://dx.doi.org/10.1111/j.1432-2277.2006.00371.x
- Hettiaratchy S, Buttler PE. Extending the boundaries of transplantation. BMJ 2003; 326:1226-7; PMID:12791714; http://dx.doi.org/10.1136/bmj.326.7401.1226
- Hautz T, Engelhardt TO, Weissenbacher A, Kumnig M, Zelger B, Rieger M, Rumpold G, Pierer G, Ninkovic M, Gabl M, et al. World experience after more than a decade of clinical hand transplantation: Update on the Innsbruck program. Hand Clin 27:423-31; PMID:22051384; http://dx.doi.org/10.1016/j.hcl.2011.07.004
- Landin L, Bonastre J, Casado-Sanchez C, Diez J, Ninkovic M, Lanzetta M, del Bene M, Schneeberger S, Hautz T, Lovic A, et al. Outcomes with respect to disabilities of the upper limb after hand allograft transplantation: A systematic review. Transpl Int 2012; 25:424-32; PMID:22332605; http://dx.doi.org/10.1111/j.1432-2277.2012.01433.x
- Mengel M, Sis B, Haas M, Colvin RB, Halloran PF, Racusen LC, Solez K, Cendales L, Demetris AJ, Drachenberg CB, et al. Banff 2011 Meeting report: new concepts in antibody-mediated rejection. Am J Transplant 2012; 12:563-70; PMID:22300494; http://dx.doi.org/10.1111/j.1600-6143.2011.03926.x
- Haas M, Sis B, Racusen LC, Solez K, Glotz D, Colvin RB, Castro MC, David DS, David-Neto E, Bagnasco SM, et al. Banff 2013 meeting report: inclusion of c4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant 2014; 14:272-83; PMID:24472190; http://dx.doi.org/10.1111/ajt.12590
- Schneeberger S, Gorantla VS, Brandacher G, Zeevi A, Demetris AJ, Lunz JG, Metes DM, Donnenberg AD, Shores JT, Dimartini AF, et al. Upper-extremity transplantation using a cell-based protocol to minimize immunosuppression. Ann Surg 2013; 257:345-51; PMID:23001085; http://dx.doi.org/10.1097/SLA.0b013e31826d90bb
- Kawai T, Cosimi AB, Spitzer TR, Tolkoff-Rubin N, Suthanthiran M, Saidman SL, Shaffer J, Preffer FI, Ding R, Sharma V, et al. HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med 2008; 358:353-61; PMID:18216355; http://dx.doi.org/10.1056/NEJMoa071074
- Leventhal J, Abecassis M, Miller J, Gallon L, Ravindra K, Tollerud DJ, King B, Elliott MJ, Herzig G, Herzig R, et al. Chimerism and tolerance without GVHD or engraftment syndrome in HLA-mismatched combined kidney and hematopoietic stem cell transplantation. Sci Transl Med 2012; 4:124ra28; PMID:22399264; http://dx.doi.org/10.1126/scitranslmed.3003509
- Scandling JD, Busque S, Dejbakhsh-Jones S, Benike C, Millan MT, Shizuru JA, Hoppe RT, Lowsky R, Engleman EG, Strober S. Tolerance and chimerism after renal and hematopoietic-cell transplantation. N Engl. J Med 2008; 358:362-8; PMID:18216356; http://dx.doi.org/10.1056/NEJMoa074191
- Kawai T, Sachs DH, Sykes M, Cosimi AB. HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med 2013; 368:1850-2; PMID:23656665; http://dx.doi.org/10.1056/NEJMc1213779

![Figure 4. Compound Motor Action Potentials (CMAP) Amplitudes of compound motor action potentials recorded from left [▴] and right [♦] m. abductor pollicis brevis, and left [x] and right [▪] m. abductor digiti minimi; abscissa, time after transplantation in years; ordinate, amplitude in mV.](/cms/asset/6781a077-8ec2-44b0-ad5a-32e2dc89a812/kvca_a_973798_f0004_c.jpg)