The editors are very grateful to Dr. Thomas E. Starzl for allowing us to publish his John E. Hoopes Lecture as a guest editorial in this inaugural issue of VCA.a It represents personal reflections of an exceptional transplantation journey by one of the foremost pioneers of this field.
It has been a particular pleasure to see the life-changing impact of the hand, face, and other composite allografts. Over the past decade, a rapidly growing number of this novel type of transplants have been performed worldwide with highly encouraging functional and immunological outcomes. Recipients of these grafts represent a new generation of transplant recipient pioneers. The uniqueness of their grafts, which include donor bone and its marrow, could help further elucidate the mechanisms by which transplanted organs and tissues are accepted. In turn, novel strategies to facilitate these mechanisms may be developed.
The title of my talk today could be improved. A more explanatory title would be “The missing half of the Billingham-Brent-Medawar discoveries." This informational void caused a pervasive early error that precluded the orderly development of transplantation immunology and limited clinical progress almost exclusively to the development of more potent immunosuppressive drugs. To understand how an error of this magnitude could have occurred, it is necessary to go back to the birth of modern day transplantation. The midwife was the English Zoologist, Peter Medawar.
The seed from which all else derived was Medawar's demonstration in 1943 that skin graft rejection is an immunologic event.Citation1 In the next 10 years, efforts to weaken the immune response with irradiation or steroids had little or no effect on experimental graft survival. During the same period, however, a study by Medawar's team of the natural tolerance in freemartin cattle revealed a chink in the immunologic armor. In freemartin cattle, fusion of their placentas allowed mixture of the 2 animal circulations during gestation. After birth and throughout life, the animals shared each others blood cells (blood chimerism). Moreover, the cattle were tolerant to each others tissues and organs.Citation2
Inspired by the freemartin findings, Medawar and his colleagues demonstrated in 1953 that similar chimerism-associated tolerance could be deliberately produced.Citation3 In their experimental model, splenic or bone marrow leukocytes were infused from adult mouse donors into newborn mouse recipients whose immune system was not developed enough to reject the cells. With leukocyte engraftment, the neonatal recipients had lifetime tolerance to skin (or other tissues) from the original leukocyte donor, but not to tissues from any other donor. These chimeric mice were analogs of future patients treated with bone marrow transplantation for immune deficiency diseases. In 1956, Main and Prehn at the NIH extended these observations to adult mouse recipients whose fully competent immune system had been weakened with high dose total body irradiation before the cell infusion.Citation4 These mouse chimeras were analogs of today's cytoablated human bone marrow recipients.
Stable donor leukocyte chimerism in both mouse models was achievable only when the donors and recipients had a good histocompatibility match. Otherwise, the donor leukocytes were either rejected, or they turned the tables and rejected the immunologically defenseless recipient—graft versus host disease (GVHD). Because human histocompatibility antigens were yet to be discovered, clinical bone marrow transplantation for the treatment of hematologic disorders and other indications was delayed until 1968. As in the mice, donor-specific tolerance was associated with the leukocyte chimerism. GVHD was the most common and specific complication that could be avoided or minimized only with a perfect HLA match. This was a beautiful story. The escalation of the mouse tolerance models to humans with parallel developments in histocompatibility research was heralded as a perfect example of bench-to-bedside research.
In contrast, kidney transplantation with survival of at least one year was precociously accomplished in 7 patients between 1959 and 1962 without a preceding animal model: 2 in Boston and 5 in Paris.Citation5 The first success was a fraternal twin recipient treated by the Boston plastic surgeon and future Nobel laureate, Joe Murray.Citation6 All of the first 6 patients were irradiated before transplantation but had limited therapy afterwards because drug immunosuppression was not yet available. The exceptional seventh patient (also a Brigham patient of Murray) was not irradiated but was treated daily with azathioprine throughout the 17 months of graft function. Although the 7 successes were isolated exceptions in more than 300 failures, they were hailed as a collective breakthrough. However, the accomplishments were inexplicable. Engraftment had been achieved without donor leukocyte infusion, without HLA matching, and with no hint of GVHD. If there was any connection with Medawar's mouse models or with the future human bone marrow transplant triumphs, it was not apparent.
At this critical juncture, the over-arching error that I described in my title was introduced. Based largely on the handful of successful human kidney transplant cases, consensus was reached by 1962 that organ engraftment (exemplified by the kidney) did not depend on the donor leukocyte chimerism-associated mechanisms of the mouse tolerance models. Thus, organ transplantation was disconnected from the scientific base soon to be occupied by human bone marrow transplantation. Parenthetically, I was not involved in the consensus. Between 1957 and 1961, I had been preoccupied with the development of canine liver replacement and multivisceral transplant procedures as tools for the study of metabolic interactions between visceral organs. With the advent of azathioprine, however, the potential human use of the visceral transplant operations became obvious. A prerequisite would be a clinical track record with this drug for kidney transplantation. The anticipated track record failed to materialize. When the early results of kidney transplantation with azathioprine in Boston and in London were no better than with irradiation, I obtained a supply of azathioprine at the University of Colorado and combined its use with prednisone in dog models. Based on our observations in dogs, we launched a clinical kidney transplant program in the autumn of 1962 with an unprecedented one-year survival of 75%. The early clinical results were reported in 1963.Citation7 The title described the 2 features of the alloimmune response that provided an empirical foundation for the development of all kinds of organ transplantation: first, the reversibility of rejection and second the tolerogenic quality of organs.
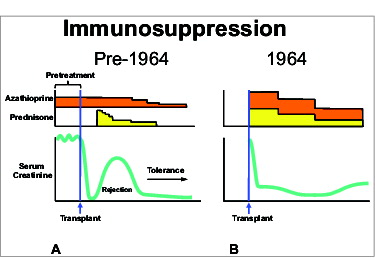
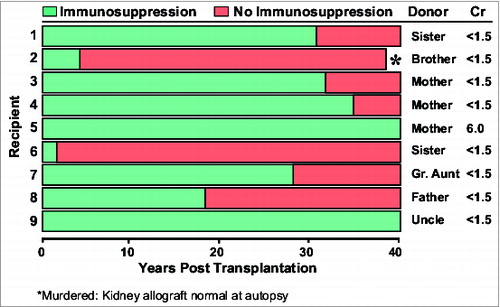
The features were dramatically exposed by the use of the treatment algorithm shown in . Azathioprine was started one to 4 weeks before transplantation of kidneys from mostly familial live donors. Large doses of prednisone were added to the post-transplant azathioprine monotherapy only to treat the breakthrough rejections that occurred in almost every patient. In about 85% of cases, the rejections were responsive to prednisone as indicated here by the rise of serum creatinine and its subsequent fall. Variable tolerance was inferred from the rapidly declining need for immunosuppression after rejection reversal. Since none of the patients were completely off drugs, the most compelling argument that these patients were tolerant required the passage of time. Nine (19%) of the 46 renal allografts transplanted from genetically-related donors functioned continuously for the next 4 decades, each depicted in as a horizontal bar. In 7 of these patients, immunosuppression eventually was stopped with subsequent drug-free intervals of 13 to 47 years. Now after 46 to 49 post-transplant years, these patients currently bear the longest functioning organ allografts in the world. However, no comparable cohort of drug-free kidney recipients was ever produced again, anywhere in the world, during the next 40 years.
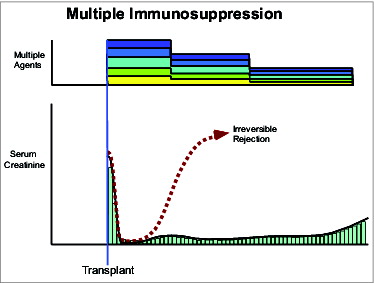
The probable reason was not recognized until the 1990s: namely alteration of the timing and dosage of immunosuppression. At the end of 1963, the pretreatment with azathioprine was abandoned, in part because it would not be feasible using cadaveric organs. The second change was administration of large doses of prednisone from the time of surgery instead of being added “as needed." This modification was made to avoid the 15 to 20% loss of grafts whose rejection could not be reversed (). With the revised use of azathioprine and prednisone, a budding industry of clinical renal transplantation was formed. However, further advances were driven almost exclusively by more versatile or more potent immunosuppressive drugs. Beginning in 1966, antilymphocyte globulin (ALG) extracted from the serum of horses immunized with human lymphoid tissue was added in Colorado to the original combination of azathioprine and prednisone. The ALG was the grandfather of the monoclonal antibodies made possible by the evolution of the hybridoma technology.
Using the triple drug immunosuppression, my original objective of human liver transplantation was finally accomplished in 1967, 10 years after the first models were invented in dogs. This was followed by the first successful heart transplantations in 1968, and in 1969 by the first one-year survival after pancreas transplantation—all with the triple drug strategy.Citation8 As more potent drugs became available, they were folded into the preemptive treatment formula in place by 1964. Azathioprine was simply replaced as the baseline drug by cyclosporine, which was replaced in turn by tacrolimus. By the 1990's a bewildering array of stacked drugs, begun at the time of transplantation, had become the worldwide standard, with the stipulated purpose of reducing the incidence of acute rejection to zero (). The preemptive strategy allowed better mid-range patient and graft survival with all organs. With development of increasingly potent baseline drugs, the history of clinical organ transplantation came to be written in terms of 3 eras defined by azathioprine, cyclosporine, and tacrolimus-based immunosuppression.
The golden age of transplantation had arrived, but with a dark side. Chronic rejection and the devastating morbidity and mortality of long-term immunosuppression had now become non-resolvable problems. Moreover, the anticipated increase in drug-free kidney recipients that had not been rare in the pioneer experience was almost never seen again. By 1992, the field of organ transplantation had reached the position of an unlucky mountaineer—unable to reach the top, but long since too far committed to go back down. However, during the 30 year climb, tantalizing clues about the mechanisms of alloengraftment had been encountered that could now be reassessed.
One clue was in studies that had been done in our 1963 kidney transplant cases and reported in the Journal of Experimental Medicine.Citation9 It was found that tuberculin, histoplasmin, and other positive skin tests in the donors were systematically transferred after transplantation to their previously skin test negative kidney recipients. This evidence of adoptive transfer by migratory donor leukocytes was not correctly interpreted until 30 years later. Although tolerant kidney recipients had all but disappeared, a trickle of drug-free liver recipients continued to be seen. At my 80th birthday party in March 2006, the kidney longevity winner was a patient 49 years post-transplantation joined by drug-free liver recipients who survived from infancy to adult life and currently have follow-ups of 31 to 42 years. All of these liver recipients now have been off immunosuppression between 15 to 37 years. The continued production of such liver recipients was not surprising. The unusual ability of the liver to self-induce tolerance with the aid of a short course of azathioprine was first recognized in our earliest dog experiments in which animals lived for 10 drug-free years following 100 days of azathioprine treatment in 1963.Citation10
Moreover, permanent liver engraftment without any treatment at all was reported in France and England in the mid-1960s in about 20% of outbred pig recipients. Moreover, such spontaneous liver tolerance is reliably induced in about 10% of rat strain combinations, and in 80% of mouse strain combinations. Importantly, heart and kidney allografts also can induce such spontaneous engraftment, although in much fewer strain combinations. In short, all kinds of organs are potentially tolerogenic without treatment.
These human and experimental exceptions to the usual outcome of rejection in the absence of treatment were dismissed for 30 years as something other than tolerance, given descriptive names, and ascribed to various mechanisms. The list of possibilities involved various tolerogenic cells, antibodies, molecules, and other factors. However, the experimental evidence for these theories was almost always model-specific. This reservation applies to the so-called tolerogenic suppressor or regulatory cells of today. In contrast, my contention throughout was that clonal exhaustion-deletion—not of a cell, but of a cell population—was the seminal mechanism of organ alloengraftment. This view was depicted graphically in my 1969 textbook on liver transplantation, as follows “Special susceptibility to these agents of a fraction of the lymphoid population could lead to exhaustion of a clone and, hence, tolerance. Since maintenance of such cell lines even in adult life is apparently thymic dependent in experimental animals, thymectomy would be expected to aid the process; this appears to be true in rodents, but such an effect of thymus removal has not been detected in dogs or humans."Citation11 However, neither the existence nor the importance of clonal exhaustion-deletion was formally proved until the early 1990's. Consequently, the hypothesis was difficult to defend.
Ideas advanced in the late 1960s by Clyde Barker also were ahead of their time. Working with Rupert Billingham at the University of Pennsylvania, Barker demonstrated in 1967 that skin grafts were not normally rejected if they were placed on an island of recipient skin that had been detached from lymphatic drainage and nourished by a vascular pedicle.Citation12 This simple experiment exposed the fundamental principle that the immune system does not recognize the presence of donor antigen that fails to reach host lymphoid organs. The current term for this is “immune ignorance." Alloengraftment by immune ignorance was diametrically opposite to alloengraftment by clonal exhaustion-deletion. Between 1967 and 1975, Barker identified other privileged sites that had in common the absence or deficiency of lymphatic drainage.Citation13 His rodent experiments and confirmatory studies elsewhere established the scientific foundation for transplantation of pancreatic islets and bits of other endocrine tissues: e.g. parathyroid and thyroid. As I will discuss later, however, Barker's experiments had broader implications than those immediate objectives.
During the same period as Barker was doing his experiments, there was another finding in the clinics that was equally slow to be understood. In 1967 and 1968, karyotyping studies were done in human female recipients of livers from male donors. These revealed that the hepatocytes and other parenchymal components retained their donor sex. However, the Kupffer cells and most of the transplanted livers’ other bone marrow-derived leukocytes disappeared and were replaced with the recipient female cells of the same lineages. Twenty-5 years passed before it was recognized that the resulting composite structure (part donor – part recipient) was a feature of all other successfully engrafted organs. The obvious question was whether the missing donor cells had migrated into the recipient and survived. Studies of serial blood samples in rat and human recipients showed that donor cells in the early days after organ transplantation accounted for between 1% and 20% of the recipients’ circulating mononuclear leukocytes. In human intestine recipients, the circulating donor cells quickly rose to a peak, and then diminished steadily until they were undetectable with flow cytometry after 30-60 days. The blood findings coincided with the disappearance of the passenger leukocytes from the graft.
A pivotal clarifying step finally was taken in 1992 with the study of 30 liver or kidney recipients whose allografts had been functioning for up to 3 decades.Citation14,15 Biopsies were obtained from various sites and studied with sensitive immunocytochemical and molecular methods. In all 30 patients, small numbers of multilineage donor cells were detected in one or more of the sampled sites.
The reports in 1992 and 1993 of these microchimerism discoveries provoked a firestorm.Citation14-17 Donor leukocyte chimerism had not, to my knowledge, been proposed to be a factor in organ engraftment a single time in the 30 years of scientific literature between 1962 and 1992. Moreover, if our interpretation of the findings was valid, the conceptual base of transplantation immunology had crumbled.
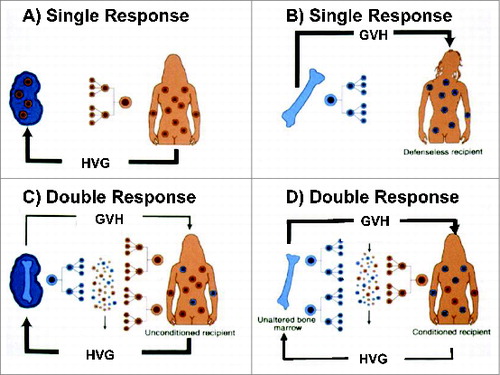
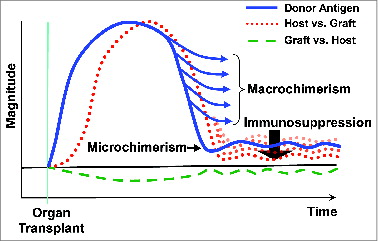
An engrafted organ had been viewed previously as an island in a hostile sea inhabited solely by leukocytes of the recipient (). The revised view with the discovery of microchimerism in various nonlymphoid and lymphoid recipient sites is shown in . In the reverse image of bone marrow transplantation, the perfect result had been considered to be complete replacement of all hematolymphopoietic cells (). However, when Donna Przepiorka and Donnall Thomas in Seattle detected a trace population of recipient leukocytes in essentially all such "perfect" bone marrow recipients (), it was evident that organ recipients () and bone marrow cell recipients () were mirror image chimeras. In principle, they differed fundamentally only in the reverse proportions of donor and recipient cells. The surviving cells of the minority populations in both kinds of recipient obviously were progeny of lymphopoietic stem cells that had survived a violent double immune reaction in the first few days or weeks after transplantation. Alloengraftment was explained in our report by "…responses of co-existing donor and recipient cells, each to the other, resulting in reciprocal clonal exhaustion, followed by peripheral clonal deletion."Citation15 These mechanisms coincided with the reversal of rejection, and the development of variable tolerance first observed in kidney and liver recipients 30 years earlier.
The host response was the dominant one in most cases of organ transplantation. But there also was a graft vs. host reaction that in exceptional cases was expressed as clinical GVHD. The GVHD complication usually was in recipients of a leukocyte-rich organ (a liver or intestine), but it also has been seen, albeit rarely, in kidney recipients.Citation15 In bone marrow recipients, the naturally weak or deliberately enfeebled immune system inverted the scale, explaining all of the major differences between bone marrow and organ transplantation.
After 30 years of estrangement, bone marrow and organ transplantation were united. However, a fundamental question remained about both kinds of transplantation. "Why was the leukocyte the indispensable tolerogenic cell?." And how could such a small minority cell population survive, much less be the key factor in long term graft survival? The answers could be found in the studies of Barker and Billingham described above.
The fundamental principle demonstrated by the Barker-Billingham experiments was that antigen which does not reach host lymphoid organs is not recognized to be present (immune ignorance). The only mobile antigen in organs consists of passenger leukocytes. The en masse migration of these leukocytes to host lymphoid organs was a prerequisite for the seminal tolerance mechanism of clonal activation, exhaustion, and deletion.
Our studies in rodents and humans showed that the cell migration occurs in 2 stages and by the same hematogenous pathways as those of the infused cells of bone marrow transplantation. In stage one, the donor leukocytes go selectively to host lymphoid destinations, where immune activation occurs. The second stage begins after 1-3 weeks. Cells that have escaped initial immune destruction move on to the skin and other non-lymphoid destinations that are relatively inaccessible to humoral and cellular effector mechanisms. Thus, thousands of tiny islands of multi lineage donor leukocytes are established body-wide in protected locations—analogous to the privileged sites studied by Barker and his associates. The colonialization of the donor leukocytes occurs from the organ via blood to the lymphoid compartment. However, some of the donor cells escape to non-lymphoid areas. We postulated that donor leukocytes percolate back from these protected sites to host lymphoid organs and maintain the clonal exhaustion-deletion achieved at the outset. Despite much supporting evidence, and no evidence to the contrary, our tolerance paradigm was viewed skeptically or repudiated outright by many critics.
However, immediate support was provided by Bob Good, who described the new paradigm as clairvoyant in a March 1993 NEJM editorial.Citation18 Another advocate was Nobel Laureate Rolf Zinkernagel of Zurich. In the 1970s, Zinkernagel and Doherty had elucidated the mechanisms of the MHC-restricted T-cell immunity induced by non-cytopathic microorganisms—and by inference by allografts. However, the opposite outcome of tolerance, had remained enigmatic. In 1993, and unaware of our 1992 publications, Zinkernagel independently proposed a paradigm of acquired tolerance to pathogens that was almost identical to our organ tolerance paradigm.Citation19
With the mutual recognition that the Pittsburgh and Zurich investigations were on parallel pathways, a crossover review was published in the December 1998 issue of the New England Journal of Medicine.Citation20 Equivalent roles were attributed to allogeneic leukocytes and non-cytopathic micro-organisms. Consequently, much of the article consisted of descriptions of a range of transplant outcomes and their infection analogs. However, our main purpose was to propose 2 generalizable rules of immunology: The first and most fundamental rule is that the immune response is regulated (that is, governed) by the migration and localization of antigen. The very simplicity of this rule had artfully concealed it for more than 100 years—ever since the individual humoral and effector mechanisms of the immune system were discovered by von Behring, Metchnikoff, Bordet, and Erlich. What were the crucial “localization” sites? These were the host lymphoid organs with their rich milieu of cytokines, growth factors, different interacting cell types, and other microenvironmental features required for immune activation.
As an example of migration and localization, we were comparing acute viral hepatitis and its analog acute liver allograft rejection. With hepatitis, the tropism of the hepatitis virus makes the liver the primary immune target. However, that is not where the immune response is generated. Instead, small numbers of virus travel to host lymphoid organs where a virus-specific clonal T-cell response is induced that attacks the infected cells in the liver and elsewhere. In the transplant analog, most of the passenger leukocytes of the allograft migrate directly to host lymphoid organs and induce a specific response against all donor cells, most of which are of course in the outlying organ graft.
The second rule was equally simple: the outcome of an immune response, if it occurs, is determined by balances between antigen with access to host lymphoid organs and the number of cytolytic T-cells induced by the antigen at these lymphoid sites. All infection and transplantation outcomes—no matter what the pathogen or what kind of allograft—could be reduced to these basic rules.
If antigen specific T cells completely eliminate a virus, or the analogous donor leukocytes, the antigen-specific immune response is terminated without memory. In other words, neither the infected patient nor the recipient of the failed allograft have been immunized. However, with the much more common usual outcome the virus or the analogous donor leukocytes that have survived in protected sites can leak back to host lymphoid organs and perpetuate cellular plus antibody memory. These are the presensitized organ transplant candidates for whom a crossmatch negative donor cannot be found. I would be delinquent if I did not acknowledge here the monumental contributions to solution of this problem—made at Hopkins by Bob Montgomery and his colleagues.
The necessary condition for alloengraftment is a quantity of mobile donor leukocytes that is persistently greater than the number of anti-donor T-cells. Of course, this balance favoring antigen supremacy also defines the carrier disease states in the infection analogs. It goes without saying that stable antigen dominance without a need for maintenance immunosuppression is more likely with higher percentages of donor cells, but with the very real risk of GVHD. However, donor leukocyte dominance also is possible with microchimerism (). This microchimerism is exemplified by spontaneous organ tolerance models and the anecdotal drug-free human organ recipients I have been describing throughout this lecture. In the vast majority of patients, however, maintenance of such a slender leukocyte advantage requires the aid of maintenance immunosuppression that is pushing down the T-cell response (). Finding just the right amount of immunosuppression is the art of organ transplant medicine practiced all over the world. In this view, it is axiomatic that long-term organ engraftment means that the recipient has developed some degree of chimerism-dependent donor specific tolerance—without or with the aid of on-going immunosuppression. And that the completeness of the tolerance can be inferred from the amount of immunosuppression required to maintain stable function and structure of the graft.
However, the immunosuppression without which human transplantation would not be possible is a 2-edged sword. In this second review with Zinkernagel in 2001, our most important conclusion was that the widespread practice of heavy multiple drug immunosuppression during the early days and weeks after organ transplantation is anti-tolerogenic.Citation21
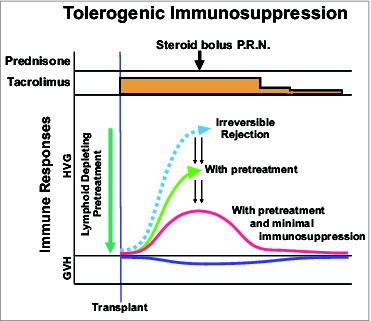
Ever since the switch to such heavy prophylactic treatment in 1964, the extent to which acute rejection could be prevented had been used to judge the quality of immunosuppression regimens. The counter argument in our 2001 article was that this policy systematically undermines the clonal exhaustion and deletion induced by the massive migration of the graft's passenger leukocytes during the first 30 or 60 post-operative days. To the extent this one-time-only window of opportunity is closed by overtreatment, patients are unnecessarily committed at the outset to permanent dependence on heavy immunosuppression. We proposed that immunosuppression could be made more tolerance-friendly by applying 2 therapeutic principles, singly or together. The first principle was recipient pretreatment to weaken the immune response in advance of transplantation, making clonal deletion easier of the impending donor-specific response. The second was the use of as little post-transplant immunosuppression as possible.Citation21
It will not have escaped your notice that these recommendations turned the clock back 40 years to the first ever series of successful kidney transplantations of 1962-63 that were described above. The only difference was that the availability of more potent drugs could make this strategy safe and practical. Immune enfeeblement in advance of transplantation could now be done with a single large dose of a lymphoid depleting agent such as anti-thymocyte globulin (ATG) or Campath-1H, followed by tacrolimus monotherapy that could be subsequently weaned—either by reducing the amount of daily doses or by increasing the intervals between doses ().
Both components of the strategy were implemented on the Pittsburgh organ transplant service in July 2001. The results and quality of life of all kinds of organ recipients were improved. The greatest impact was on the procedures with the most troubled histories. Intestinal and lung transplantation had been targets of criticism for years because of their high mortality. With a 25% gain in survival and greatly improved quality of life, both of these operations became reliable clinical services.Citation22 The extent to which long-term immunosuppression could be minimized by the improved management is epitomized by an intestinal recipient who was 68 years old at the time of her transplantation on August 5, 2001. Now in her 10th post-transplant year, she has been on 2 tacrolimus doses per week with no added immunosuppression from the second year onward.
The elucidation of engraftment mechanisms and recognition of the stultifying effect of immune suppression on these mechanisms had other therapeutic implications. The obvious next step was to tip the antigen/T-cell balance that defines outcome toward antigen dominance. This could be readily done in organ recipients with a properly timed adjunct infusion of donor leukocytes. The objective was to begin the donor-specific tolerization well before arrival of the transplanted organ. Followed by a second cell dose of the organ's passenger leukocytes post-transplant.
The optimal use of this strategy would be in recipients whose organs are obtained from live donors. In such a protocol, the recipient will be lymphoid-depleted with Campath-1H 3 weeks before the organ transplantation. A day or 2 later, cells are obtained from the donor with leukopheresis and infused into the recipient. At the time of organ transplantation 20 days later, a second surge of donor cells arrives as the passenger leukocytes migrate from the organ allograft. Immune suppression throughout is with tacrolimus from which weaning is considered after about 4 post-transplant months.
For recipient of cadaveric organs or of composite grafts, it is necessary for logistic reasons to deliver the 2 boluses of donor leukocytes in reverse order. Following lymphoid depletion, transplantation of the cadaveric organ provides the first cell dose, which consists of the allografts passenger leukocytes. Two or 3 weeks later, the second bolus consists of stored leukocytes obtained from the deceased donor at the time of the original tissue and organ retrieval.
Such a protocol was originally developed for solid organs. However, the leadership for its use was taken by Andy Lee and his associates Gerald Brandacher and Stefan Schneeberger who had a long-standing interest in hand transplantation. The pure technical challenge of hand transplantation makes organ transplantation, even of the liver, look like child's play. Suffice it to say, the challenge was met by Dr. Lee and his associates in 6 recipients of bilateral or unilateral hand transplants all but one of whom have been on decremental single-drug therapy with tacrolimus.Citation23
On that high note, I will close with a short synopsis of the history of clinical transplantation and then with a conclusion. The history can be summarized by a short list of empirical steps, almost all taken by clinicians. Bone marrow transplantation was conceptually anchored by mouse models that revealed the essential but unexplained association of donor leukocyte chimerism and tolerance. In contrast, the evolution of organ transplantation resembled the piecemeal construction of the floors of a house without an architectural blueprint. Both kinds of engraftment were made incrementally more practical as the years passed by the immunosuppressive agents developed. These drugs were used like scalpels in scrupulously HLA-matched bone marrow recipients and like sledge hammers in organ recipients. During all this time, the self-defeating anti-tolerogenic effects of immunosuppression were not appreciated. Nevertheless, bone marrow and organ transplantation became 2 of medicine's great triumphs of all time. They also generated a supporting literature so vast and complicated that it resembles an assembly of all of the phone books in the world, each in its own language.
So much for this history. I believe that the mystical and incomprehensible transplant literature can reach consilience—the unity of knowledge described by the Harvard biologist E. O. Wilson, in his 1998 book.Citation24 With unity of knowledge (consilience), a small number of simple natural laws accommodate observations, principles, and facts in all models, in all disciplines and in all domains of knowledge.
Our observations in transplantation and Zinkernagel's studies of the infection analogs exposed 2 such natural laws of immunology. First, the immune responsiveness or non-responsiveness to antigen is governed by the migration and localization of the antigen. The second law is that the outcome if immune activation occurs is determined by the balance reached between antigen and the antigen-reactive host cells.
With these 2 laws, consilience can be reached for all of the diverse observations in all of the tolerance and alloengraftment circumstances I have discussed today, and for that matter in all circumstances of transplantation failures. The linkage throughout the tolerance and alloengraftment spectrum is, of course, donor leukocyte chimerism. Start with the naturally tolerant freemartin cattle of Ray Owen that inspired the mouse models of Billingham, Brent, and Medawar, and parabiosis experiments of Bob Good. Then, continue with human bone marrow, organ, hand and ultimately face transplantation. They are all variations of the same theme.
Note
aThis Hoopes Lecture was delivered on November 17, 2011 at Johns Hopkins Hospital, Baltimore, MD.
References
- Gibson T, Medawar PB. The fate of skin homografts in man. J Anat 1943 Jul; 77(Pt 4):299-310.4; PMID:17104936
- Owen RD. Immunogenetic consequences of vascular anastomoses between bovine twins. Science 1945; 102:400; PMID:17755278; http://dx.doi.org/10.1126/science.102.2651.400
- Billingham RE, Brent L, Medawar PB. Actively acquired tolerance to foreign cells. Nature 1953; 172:603; PMID:13099277; http://dx.doi.org/10.1038/172603a0
- Main JM, Prehn RT. Fate of skin homografts in x-irradiated mice treated with homologous marrow. J Natl Cancer Inst 1957 Dec; 19(6):1053-64; PMID:13502760
- Murray JE, Merrill JP, Dammin GJ, Dealy JB Jr, Alexandre GW, Harrison JH. Kidney transplantation in modified recipients. Ann Surg 1962 Sep; 156:337-55; PMID:14477464; http://dx.doi.org/10.1097/00000658-196209000-00002
- Merrill JP, Murray JE, Harrison JH, Guild WR. Successful homotransplantation of the human kidney between identical twins. J Am Med Assoc 1956 Jan 28; 160(4):277-82; PMID:13278189; http://dx.doi.org/10.1001/jama.1956.02960390027008
- Starzl TE, Marchioro TL, Waddell WR. The reversal of rejection in human renal homografts with sugsequent development of homograft tolerance. Surg Gynecol Obstet 1963 Oct; 117:385-95; PMID:14065716
- Starzl TE, Porter KA, Putnam CW, Schroter GPJ, Halgrimson CG, Weil R, III, Hoelscher M, Reid HAS. Orthotopic liver transplantation in ninety-three patients. Surg Gynecol Obstet 1976; 142:487-505; PMID:176741
- Billingham RE, Silvers WK, Wilson DB. Further studies on adoptive transfer of sensitivity to skin homografts. J Exp Med 1963 Sep 1; 118:397-420; PMID:14078000; http://dx.doi.org/10.1084/jem.118.3.397
- Starzl TE, Marchioro TL, Porter KA, Taylor PD, Faris TD, Herrmann TJ, Hlad CJ, Waddell WR. Factors determining short- and long-term survival after orthotopic liver homotransplantation in the dog. Surgery 1965 Jul; 58:131-55; PMID:14305148
- Starzl TE: Experience in Hepatic Transplantation. WB Saunders Co., 1969.
- Barker CF, Billingham RE. The role of afferent lymphatics in the rejection of skin homografts. J Exp Med 1968 Jul 1; 128(1):197-221; PMID:4873840; http://dx.doi.org/10.1084/jem.128.1.197
- Barker CF, Billingham RE. Immunologically privileged sites. Adv Immunol 1977; 25:1-54; PMID:345773; http://dx.doi.org/10.1016/S0065-2776(08)60930-X
- Starzl TE, Demetris AJ, Trucco M, Ramos H, Zeevi A, Rudert WA, Kocova M, Ricordi C, Ildstad S, Murase N. Systemic chimerism in human female recipients of male livers. Lancet 1992 Oct 10; 340(8824):876-7; PMID:1357298; http://dx.doi.org/10.1016/0140-6736(92)93286-V
- Starzl TE, Demetris AJ, Murase N, Ildstad S, Ricordi C, Trucco M. Cell migration, chimerism, and graft acceptance. Lancet 1992 Jun 27; 339(8809):1579-82; PMID:1351558; http://dx.doi.org/10.1016/0140-6736(92)91840-5
- Starzl TE, Demetris AJ, Trucco M, Ricordi C, Ildstad S, Terasaki PI, Murase N, Kendall RS, Kocova M, Rudert WA, et al. Chimerism after liver transplantation for type IV glycogen storage disease and type 1 Gaucher's disease. N Engl J Med 1993 Mar 18; 328(11):745-9; PMID:8437594; http://dx.doi.org/10.1056/NEJM199303183281101
- Starzl TE, Demetris AJ, Trucco M, Murase N, Ricordi C, Ildstad S, Ramos H, Todo S, Tzakis A, Fung JJ, et al. Cell migration and chimerism after whole-organ transplantation: the basis of graft acceptance. Hepatology 1993 Jun; 17(6):1127-52; PMID:8514264; http://dx.doi.org/10.1002/hep.1840170629
- Good RA. Mixed chimerism and immunologic tolerance. N Engl J Med 1993 Mar 18; 328(11):801-2; PMID:8437600; http://dx.doi.org/10.1056/NEJM199303183281111
- Moskophidis D, Lechner F, Pircher H, Zinkernagel RM. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature 1993 Apr 22; 362(6422):758-61; PMID:8469287; http://dx.doi.org/10.1038/362758a0
- Starzl TE, Zinkernagel RM. Antigen localization and migration in immunity and tolerance. N Engl J Med 1998 Dec 24; 339(26):1905-13; PMID:9862947; http://dx.doi.org/10.1056/NEJM199812243392607
- Starzl TE, Zinkernagel RM. Transplantation tolerance from a historical perspective. Nat Rev Immunol 2001 Dec; 1(3):233-9; PMID:11905833; http://dx.doi.org/10.1038/35105088
- Starzl TE, Murase N, Abu-Elmagd K, Gray EA, Shapiro R, Eghtesad B, Corry RJ, Jordan ML, Fontes P, Gayowski T, et al. Tolerogenic immunosuppression for organ transplantation. Lancet. 2003 May 3; 361(9368):1502-10; PMID:12737859; http://dx.doi.org/10.1016/S0140-6736(03)13175-3
- Schneeberger S, Gorantla VS, Brandacher G, Zeevi A, Demetris AJ, Lunz JG, Metes DM, Donnenberg AD, Shores JT, Dimartini AF, et al. Upper-extremity transplantation using a cell-based protocol to minimize immunosuppression. Ann Surg 2013 Feb; 257(2):345-51; PMID:23001085; http://dx.doi.org/10.1097/SLA.0b013e31826d90bb
- Wilson EO. Consilience: The Unity of Knowledge 1998; ISBN 9780679450771.