Abstract
The ability of the retinoblastoma protein (RB) tumor suppressor to repress transcription stimulated by the E2 promoter binding factors (E2F) is integral to its biological functions. Our recent report described a conserved feedback mechanism mediated by the RNA-binding proteins Pumilio and Nanos that increases in importance following RB loss and helps cells to tolerate deregulated E2F.
Many of the genes that are needed for cell proliferation are not constitutively expressed but are transcribed in a controlled manner during discrete phases of the cell cycle. In mammals, plants, and insects, the E2 promoter binding factor (E2F) family of transcription factors has a central function in the temporal control of a large number of these genes. E2F, in turn, is regulated by the retinoblastoma (RB) family of transcriptional repressors, a class of proteins that are named after the retinoblastoma susceptibility gene, Rb1. pRB is one of the best-known tumor suppressor genes and tumor studies have shown that RB function is compromised in most cancers. Loss of pRB activity, and the subsequent deregulation of E2F, places tumor cells in a state that is permissive for cell proliferation (for review).Citation1
The transcriptional program controlled by E2F is large and diverse. One curious finding from studies of mutant animals is that, in many developmental contexts, Rb1 loss has only minimal effects on the normal pattern of cell proliferation.Citation2 There are multiple explanations for this, including the expression of related family members and the presence of redundant pathways.Citation3 Here, by comparing the classes of genes that are directly bound by RB/E2F proteins in fliesCitation4 and humans, and by examining the lists of genes that are deregulated by the loss of RBCitation5 or retinoblastoma-family protein 1 (Rbf1) (its Drosophila ortholog), we recently discovered an additional regulatory loop that helps cells cope with transcriptional changes associated with RB loss.Citation6
Our results show that, in addition to the standard E2F target genes involved in cell cycle progression, members of the RB family and E2F family also control the expression of components of the Pumilio (Pum) post-transcriptional repressor complex. Pum proteins repress the translation of their substrates by binding to a Pumilio regulatory element (PRE: UGUAXAUA) within the 3′ untranslated region (UTR) of mRNAs.Citation7 The Pum complex contains several subunits—Pumilio (Pum), Nanos, and Brain tumor (Brat)—and uses multiple mechanisms to suppress the expression of its targets. Pum proteins have been shown to act by promoting ribosome stalling,Citation8 decapping or de-adenylation of mRNAs,Citation9 and by facilitating the recruitment of microRNAs (miRNAs).Citation10 In both flies and human cells, inactivation of RB/Rbf1 increases the expression of the components of the Pum complex, most notably Nanos1/Nanos, and this increases the post-transcriptional repression mediated via PRE elements.Citation6
Informatics analysis of retinoblastoma tumors revealed that approximately 20% of the transcripts that have altered gene expression contain putative PREs in their 3′UTRs. Most of these upregulated genes are transcribed from E2F-responsive loci. This suggests that the increase in E2F-dependent transcription may be counterbalanced, at least at some targets, by increased post-transcriptional regulation (). PREs are also enriched in many transcripts that are downregulated in retinoblastomas. Whether this is a consequence of the change in PUM/NANOS levels remains unclear. Nevertheless, reducing transcript levels and increasing post-transcriptional regulation should synergistically affect protein synthesis. Interestingly, this class of transcripts includes components of stress-activated pro-apoptotic pathways, such as mitogen-activated protein kinase kinase 3 (MAP2K3) and mitogen-activated protein kinase kinase kinase 1, E3 ubiquitin protein ligase (MAP3K1) that signal to tumor protein p53 (TP53, best known as p53). Indeed, we were able to confirm that MAP2K3 and MAP3K1 are bona fide targets of the PUM complex, and that PRE-mediated regulation of their UTRs is enhanced in RB-deficient cells.
Figure 1. Increased PUMILIO/NANOS activity helps to balance the effects of retinoblastoma protein (RB) loss. RB inactivation leads to a strong upregulation of E2 promoter binding factor (E2F)-dependent transcription and increases cellular stress. In addition, RB loss increases expression of the NANOS RNA-binding protein. NANOS acts with its binding partner, Pumilio (PUM), to repress target mRNAs. PUM/NANOS complexes act on mRNAs of genes with important roles in both stress responses and cell proliferation pathways. In this way, enhanced post-transcriptional regulation by PUM/NANOS counterbalances some of the transcriptional changes associated with RB loss.
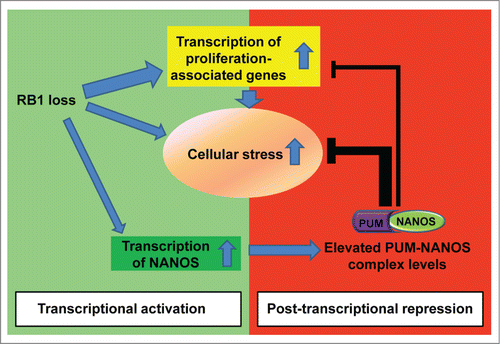
Collectively, these results suggest that RB loss increases PUM/NANOS levels and enhances regulation through PRE elements. This change has at least 2 consequences. First, PRE-mediated repression of E2F-induced transcripts may provide a buffer that reduces the functional consequences of elevated E2F activity. Second, PRE-mediated repression dampens stress-activated pathways that signal to p53. We hypothesize that deregulated E2F activity places multiple stresses on the cell (such as chromatin changes, replication stress, unbalanced changes in metabolic pathways). We propose that increased levels of PUM/NANOS help to promote homeostasis in RB-deficient cells by dampening stress-activated pathways that could potentially trigger p53-dependent apoptosis ().
This idea is supported by experiments in both flies and human cells. In Drosophila, we found that RNAi transgenes that reduced the activity of Pum, Nanos, or Brat had minimal effects on wing development on their own. However, when these RNAi lines were combined with transgenes that reduce the levels of Rbf or E2f/Rbf repressor complexes, normal developmental patterns were disrupted. Similarly, depletion of Nanos1/NANOS1 from mammalian cells had little effect on its own, but strikingly reduced cell number when co-depleted with RB, or when assayed in Rb1 mutant mouse embryonic fibroblasts (MEFs) and retinoblastoma cells. We then tested the effect of depleting NANOS1 in a panel of cancer cell lines, and found that only cell lines that retained wild-type p53 were sensitive to NANOS1 loss. These results are consistent with the idea that elevated levels of NANOS help to suppress p53-dependent signaling in the absence of normal RB function.
Transcriptome studies have been used extensively to classify tumor cells and to provide clues about changes in gene expression programs. One of the appealing features of the model outlined in is the concept that feedback loops that increase translational (or post-transcriptional) regulation can protect cells from abnormal patterns of transcription. We note that PRE-mediated regulation is clearly only a modulator of expression levels, since increased transcription from E2F-dependent genes is able to overwhelm NANOS/PUM-mediated regulation in RB-deficient tumors. We also note that, since PREs are found in many 3′UTRs, changes in PRE-mediated regulation may have indirect effects on many diverse processes in RB-deficient cells. Finally, it is worth noting that it is not currently pharmacologically possible to antagonize PUM/NANOS-mediated regulation. However, if such technologies did exist, they might preferentially reduce the viability of RB-/p53+ tumor cells.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Di Fiore R, D'Anneo A, Tesoriere G, Vento R. RB1 in cancer: different mechanisms of RB1 inactivation and alterations of pRb pathway in tumorigenesis. J Cell Physiol 2013; 228:1676-87; PMID: 23359405; http://dx.doi.org/10.1002/jcp.24329
- Maandag EC, van der Valk M, Vlaar M, Feltkamp C, O'Brien J, van Roon M, van der Lugt N, Berns A, te Riele H. Developmental rescue of an embryonic-lethal mutation in the retinoblastoma gene in chimeric mice. EMBO J 1994; 13:4260-8; PMID:7925271
- Bremner R, Chen D, Pacal M, Livne-Bar I, Agochiya M. The RB protein family in retinal development and retinoblastoma: new insights from new mouse models. Dev Neurosci 2004; 26:417-34; PMID:15855771; http://dx.doi.org/10.1159/000082284
- Korenjak M, Anderssen E, Ramaswamy S, Whetstine JR, Dyson NJ. RBF binding to both canonical E2F targets and noncanonical targets depends on functional dE2F/dDP complexes. Mol Cell Biol 2012; 32:4375-87; PMID:22927638; http://dx.doi.org/10.1128/MCB.00536-12
- Ganguly A, Shields CL. Differential gene expression profile of retinoblastoma compared to normal retina. Mol Vis 2010; 16:1292-303; PMID:20664703
- Miles WO, Korenjak M, Griffiths LM, Dyer MA, Provero P, Dyson NJ. Post-transcriptional gene expression control by NANOS is up-regulated and functionally important in pRb-deficient cells. EMBO J 2014; 33(16):2201-15; PMID:25100735
- Sonoda J, Wharton RP. Recruitment of Nanos to hunchback mRNA by Pumilio. Genes Dev 1999; 13:2704-12; PMID:10541556; http://dx.doi.org/10.1101/gad.13.20.2704
- Friend K, Campbell ZT, Cooke A, Kroll-Conner P, Wickens MP, Kimble J. A conserved PUF-Ago-eEF1A complex attenuates translation elongation. Nat Struct Mol Biol 2012; 19:176-83; PMID:22231398; http://dx.doi.org/10.1038/nsmb.2214
- Van Etten J, Schagat TL, Hrit J, Weidmann CA, Brumbaugh J, Coon JJ, Goldstrohm AC. Human Pumilio proteins recruit multiple deadenylases to efficiently repress messenger RNAs. J Biol Chem 2012; 287:36370-83; PMID:22955276; http://dx.doi.org/10.1074/jbc.M112.373522
- Miles WO, Tschop K, Herr A, Ji JY, Dyson NJ. Pumilio facilitates miRNA regulation of the E2F3 oncogene. Genes Dev 2012; 26:356-68; PMID:22345517; http://dx.doi.org/10.1101/gad.182568.111
