Abstract
Melanoma cells that express metabotropic glutamate 1 (mGlu1) receptors depend on glutamate for their survival and proliferation. The dependence receptor properties of mGlu1 allow us to propose and justify three promising approaches for melanoma treatment: glutamate depletion, mGlu1 receptor antagonism, and targeting of mGlu1 receptor signaling.
Abbreviations
| BAY36-7620 | = | (3aS,6aS)-Hexahydro-5-methylene-6a-(2-naphthalenylmethyl)-1H-cyclopenta[c]furan-1-one |
| ERK | = | extracellular signal-regulated kinase |
| JNJ16259685 | = | (3,4-Dihydro-2H-pyrano[2,3-b]quinolin-7-yl)-(cis-4-methoxycyclohexyl)-methanone |
| MAPK | = | mitogen-activated protein kinase |
| mGlu1 | = | metabotropic glutamate 1 |
| PARP | = | poly ADP-ribose polymerase |
| PI | = | phosphoinositide |
Metastatic melanoma is an aggressive disease with a poor prognosis and a high rate of resistance to current therapies. The development of targeted and combinatorial therapies has shown promise for extending patient survival,Citation1 but the discovery of novel drug targets and mechanisms is required to improve therapeutic outcomes. Metabotropic glutamate 1 (mGlu1) receptors are one such novel drug target.Citation2-5 Others have shown that blocking or decreasing the expression of mGlu1 receptors can slow the growth of melanomas that express these receptors.Citation3-5 In a recent report, we confirm that activation of mGlu1 receptors is proliferative, and additionally demonstrate and explain why glutamate depletion induces melanoma cell death.Citation2 On the basis of this evidence, mGlu1 can be defined as a dependence receptor: a receptor whose expression makes cells dependent on its agonist for survival.Citation6 These findings show how targeting mGlu1 receptor signaling can be cytostatic, and also highlight a new avenue for the cytotoxic treatment of mGlu1 receptor-expressing melanomas.
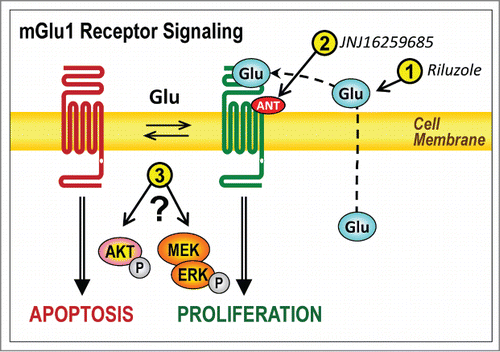
Glutamate stimulation of mGlu1 receptors in melanoma cells activates a proliferative signaling cascade resulting in increased DNA synthesis and cell growth. In the absence of glutamate, this proliferative signaling is inactive as DNA synthesis and melanoma cell growth is halted instead, mGlu1 receptor-expressing melanomas undergo agonist-independent, proapoptotic signaling resulting in melanoma cell death.Citation2 Through our studies we have identified 3 different mechanisms of potential treatment for mGlu1 receptor-positive melanoma: (1) glutamate depletion, (2) mGlu1 receptor antagonism, and (3) targeting downstream proteins of mGlu1 receptors (). In this commentary, we categorize these 3 mechanisms of treatment as either cytostatic or cytotoxic.
Figure 1. Three potential therapeutic approaches to activate apoptosis and prevent proliferation in metabotropic glutamate 1 (mGlu1) receptor-expressing melanomas. Simplified scheme of the actions of mGlu1 as a dependence receptor in melanoma cells. In the presence of the agonist glutamate (Glu) mGlu1 receptors promote proliferation, whereas the absence of glutamate leads to apoptosis. The numbers in yellow circles indicated the 3 putative targets for melanoma therapy: (1) depletion of extracellular glutamate, (2) pharmacological antagonism of the receptor, and (3) interference with downstream mechanisms of signal transduction. ANT, antagonist; P in gray circle, phosphate; MEK, mitogen-activated protein kinase kinase; ERK, extracellular signal-regulated kinase.
Glutamate Depletion
We showed that enzymatic depletion of glutamate inactivated the mGlu1 receptor proliferative signaling cascade, preventing DNA synthesis and cellular proliferation.Citation2 Supporting our data, others have shown that an FDA-approved glutamate release inhibitor, riluzole, blocks anchorage-dependentCitation5 and anchorage-independentCitation7 melanoma cell growth only in mGlu1 receptor-expressing melanoma cells. These studies support our hypothesis that the mGlu1 receptor proliferative signaling cascade is inactive in the absence of extracellular glutamate.
Our recent report also shows that only melanoma cells that express mGlu1 receptors are apoptotic when cultured in the absence of glutamate.Citation2 This proapoptotic signaling has been confirmed by others using the glutamate release inhibitor riluzole. This inhibitor not only blocked melanoma cell growth, but also activated proapoptotic signaling as measured by an increase in poly ADP-ribose polymerase (PARP) cleavage and the fraction of cells in the sub-G1 phase of the cell cycle.Citation5 Glutamate depletion blocks growth and activates apoptosis in mGlu1 receptor-expressing melanoma cells (), making this mechanism a possible cytostatic and cytotoxic therapy for the treatment of metastatic melanoma.
mGlu1 Receptor Antagonism
Our study demonstrated that the non-competitive mGlu1 receptor antagonist JNJ16259685 [(3,4-dihydro-2H-pyrano[2,3-b]quinolin-7-yl)-(cis-4-methoxycyclohexyl)-methanone] selectively blocked the growth of mGlu1 receptor-expressing melanoma cells both in vitro and in a melanoma cell xenograft model.Citation2 Another non-competitive mGlu1 receptor antagonist BAY36-7620 [(3aS,6aS)-hexahydro-5-methylene-6a-(2-naphthalenylmethyl)-1H-cyclopenta[c]furan-1-one] blocked both anchorage-dependentCitation5 and anchorage-independentCitation7 growth of mGlu1 receptor-expressing melanoma cells. BAY36-7620 also increased PARP cleavage and the fraction of cells in sub-G phase in mouse melanocytes transformed by the expression of mGlu1 receptors.Citation3 These experiments examining cell death in the presence of mGlu1 receptor antagonists should be validated using native human melanoma cells both in vitro and in vivo. Taken together, these results demonstrate that mGlu1 receptor antagonists block proliferation and increase apoptosis (), demonstrating both cytostatic and cytotoxic mechanisms of action.
Targeting mGlu1 Receptor Signaling Pathways
We have previously characterized mGlu1 as a dependence receptor in other cell types,Citation8 but the concept of this receptor having dual and opposite signal transduction cascades in melanoma is novel. These dependence receptor properties give a new perspective to the current literature and enrich the analysis of mGlu1 receptor signaling in melanoma. In a heterologous expression system, mGlu1 receptors can signal through 2 independent cascades. In the first cascade, mGlu1 receptors signal through the Gαq protein and stimulate phosphoinositide (PI) hydrolysis.Citation9 In mGlu1 receptor-expressing mouse melanoma cells, the mGlu1 receptor agonist quisqualate stimulated PI hydrolysis, which was blocked by BAY36-7620.Citation3 In the second cascade, mGlu1 receptors activate a G protein-independent pathway involving the mitogen-activated protein kinase (MAPK) cascade, resulting in long-term extracellular signal-regulated kinase (ERK) phosphorylation and an increase in cell viability.Citation9 In melanoma cells expressing mGlu1 receptors, glutamate depletion using riluzole selectively decreased ERK phosphorylation. Riluzole also decreased AKT phosphorylation in these same cells.Citation7 Finally, targeted knockdown of mGlu1 receptors decreased AKT and ERK phosphorylation,Citation4 as well as melanoma cell and tumor growth.Citation2,4 Based on this evidence, mGlu1 receptors in melanoma may regulate both the MAPK cascade and the AK tcascade, although the degree to which AKT and ERK contribute to proliferative and/or pro-apoptotic signaling mediated by mGlu1 receptors remains unclear (). Future experiments in our laboratory will be aimed at determining the mGlu1 receptor-dependent signal transduction mechanisms regulating proliferation and cell death in melanoma.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was partially supported by the Pharmaceutical Research and Manufacturers of America Foundation Predoctoral Fellowship in Pharmacology/Toxicology to T.G. and The National Institutes of Health Grant NS37436 to J.T.W.
References
- Flaherty KT, Infante JR, Daud A, Gonzalez R, Kefford RF, Sosman J, Hamid O, Schuchter L, Cebon J, Ibrahim N, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med 2012; 367:1694-703; PMID:23020132; http://dx.doi.org/10.1056/NEJMoa1210093
- Gelb T, Pshenichkin S, Rodriguez OC, Hathaway HA, Grajkowska E, DiRaddo JO, Wroblewska B, Yasuda RP, Albanese C, Wolfe BB, et al. Metabotropic glutamate receptor 1 acts as a dependence receptor creating a requirement for glutamate to sustain the viability and growth of human melanomas. Oncogene 2014; 1-10; PMID:25065592; http://dx.doi.org/10.1038/onc.2014.231
- Shin S, Namkoong J, Wall BA, Gleason R, Lee HJ, Chen S. Oncogenic activities of metabotropic glutamate receptor 1 (Grm1) in melanocyte transformation. Pigment Cell Melanoma Res 2008; 21:368-78; PMID:18435704; http://dx.doi.org/10.1111/j.1755-148X.2008.00452.x
- Wangari-Talbot J, Wall BA, Goydos JS, Chen S. Functional effects of GRM1 suppression in human melanoma cells. Mol Cancer Res 2012; 10:1440-50; PMID:22798429; http://dx.doi.org/10.1158/1541-7786.MCR-12-0158
- Namkoong J, Shin SS, Lee HJ, Marín YE, Wall BA, Goydos JS, Chen S. Metabotropic glutamate receptor 1 and glutamate signaling in human melanoma. Cancer Res 2007; 67:2298-305; PMID:17332361; http://dx.doi.org/10.1158/0008-5472.CAN-06-3665
- Goldschneider D, Mehlen P. Dependence receptors: a new paradigm in cell signaling and cancer therapy. Oncogene 2010; 29:1865-82; PMID:20173780; http://dx.doi.org/10.1038/onc.2010.13
- Le MN, Chan JL, Rosenberg SA, Nabatian AS, Merrigan KT, Cohen-Solal KA, Goydos JS. The glutamate release inhibitor Riluzole decreases migration, invasion, and proliferation of melanoma cells. J Invest Dermatol 2010; 130:2240-9; PMID:20505744; http://dx.doi.org/10.1038/jid.2010.126
- Pshenichkin S, Dolińska M, Klauzińska M, Luchenko V, Grajkowska E, Wroblewski JT. Dual neurotoxic and neuroprotective role of metabotropic glutamate receptor 1 in conditions of trophic deprivation- possible role as a dependence receptor. Neuropharmacology 2008; 55:500-8; PMID:18619982; http://dx.doi.org/10.1016/j.neuropharm.2008.06.039
- Emery AC, Pshenichkin S, Takoudjou GR, Grajkowska E, Wolfe BB, Wroblewski JT. The protective signaling of metabotropic glutamate receptor 1 is mediated by sustained, beta-arrestin-1-dependent ERK phosphorylation. J Biol Chem 2010; 285:26041-8; PMID:20566651; http://dx.doi.org/10.1074/jbc.M110.139899
