Abstract
Recently, we revealed that ubiquitination of MEKK2 and MEKK3 by inhibitor of apoptosis proteins (IAPs) directly disrupts MEK5/ERK5 interaction and subsequently attenuates ERK5 activation. In addition, loss of XIAP promotes human myogenic differentiation in an ERK5-dependent manner. These results reveal another layer of MAPK regulation and a novel role for XIAP in controlling myogenic differentiation.
Abbreviations
| cIAP1 | = | Cellular inhibitor of apoptosis protein 1 |
| DUB | = | Deubiquitinase |
| ERK5 | = | Extracellular signal-regulated kinase 5 |
| IAP | = | Inhibitor of apoptosis proteins |
| MAPK | = | Mitogen activated protein kinase |
| MEK5 | = | Mitogen activated protein kinase kinase 5 |
| MEKK2/3 | = | Mitogen-activated protein kinase kinase kinase |
| XIAP | = | X-linked inhibitor of apoptosis protein |
Commentary
Mitogen activated protein kinases (MAPKs) are evolutionarily conserved enzymes that regulate fundamental cellular processes such as proliferation, migration, differentiation, and survival.Citation1 Typically, MAPKs are activated in a 3-tier cascade, in which sequential phosphorylation and activation of the cascade components occurs in a highly regulated manner. Several scaffolding proteins control the activation kinetics and fidelity of the MAPK cascade. The spatiotemporal dynamics of MAPK activation has been extensively studied because deregulation of the fine-tuning of the cascade leads to the pathogenesis of various human diseases. Dephosphorylation of MAPK module components by phosphatases is known to be a prime mechanism for inactivation of the MAPK cascade. In this context, we have recently uncovered a role for K-63 linked ubiquitin chains in the functional inactivation of the extracellular signal-regulated kinase 5 (ERK5)-MAPK module.Citation2
ERK5, also called big MAPK 1 (BMK1), is activated in response to various physiological stimuli via MEKK2/3 and MEK5. Recent studies reveal a crucial role of this pathway in cardiovascular development and tumor metastasis.Citation3 We uncovered a role for X-linked inhibitor of apoptosis protein (XIAP) and cellular IAP1 (cIAP1) in regulating the activation dynamics of the MEKK2/3–MEK5–ERK5 signaling cascade (). XIAP and cIAP1 are evolutionarily conserved RING domain-containing E3 ubiquitin ligases belonging to the inhibitors of apoptosis protein (IAP) family. Although IAP family members are predominantly recognized for their role in regulating cell death and immune signaling, emerging evidence suggests a crucial role for IAPs in controlling cell shape, migration, and differentiation.Citation4,5 We have previously shown that XIAP and cIAP1 function as direct E3 ubiquitin ligases of the well-studied Rho GTPase Rac1, thus controlling the plasticity of cell migration and polarization of cerebellar granule neurons during vertebrate development.Citation6 Furthermore, XIAP and cIAPs are also implicated in the homeostasis of CRAF protein, a central member of the RAS-MAPK pathway, the first MAPK cascade to be characterizedCitation7 ().
Figure 1. XIAP–cIAP1 complex as a novel regulator of the ERK5 signaling pathway. X-linked inhibitor of apoptosis protein (IAP) and cIAP1 directly ubiquitinate Rac1 leading to proteasomal degradation and thus controlling cell shape and migration. XIAP also indirectly regulates CRAF stability by directly binding and promoting the CRAF–Hsp90–CHIP complex. XIAP and cIAP1 directly ubiquinitate MEKK2 and MEKK3, thus attenuating complex formation between MEK5 and ERK5. Ubiquitination of MEKK2 and MEKK3 does not impair their kinase activities. It is currently unclear whether ubiquitination of MEKK2 and MEKK3 influences the nuclear localization of ERK5 and its partners. Loss of XIAP promotes human myogenic differentiation in an ERK5-dependent manner (A). Non-ubiquitinated MEKK3 activates ERK5 via MEK5 phosphorylation (B), whereas ubiquitination of MEKK3 interferes with ERK5 phosphorylation (C). The ternary complex of the MEK5-PB1 domain (orange)–ERK5 kinase domain (gray)–MEKK3-PB1 (cyan) was constructed using the structures of the MEK5-PB1–ERK5-KD binary complex (PDB ID: 4IC7) and the MEKK3-PB1–MEK5-PB1 heterodimer (PDB ID: 2O2V) (as described in Glatz et al. 2013). Structural models of MEKK3 (cyan) and MEK5 (orange) kinase domains were created with the SwissModel homology modeling software using ASK1 (4BF2) and MKK1 (3VVH) crystal structures as starting models, respectively. The MEK5-KD–ERK5-KD heterodimer within the ternary complex is hypothetical and was generated from monomeric crystal structures such that the MEK5 kinase domain (KD) active site would face the phosphorylatable residues on the ERK5 activation loop in a logical catalytic arrangement. Kinase domains (KD) are shown in cartoon whereas PB1 domains and ubiquitin chains are shown by surface representation. The active sites of MEKK3 and MEK5 are highlighted, showing the catalytic aspartate residues with red spheres. Dashed lines indicate structurally unknown “linker” regions between kinase domains and PB1 domains from MEKK3 and MEK5. On panel D, K63-linked diubiquitin (in magenta, PDB ID: 3H7P) is attached to MEKK3 on K456, an important ubiquitination site that plays a major role in blocking ERK5 activation by MEK5 (Takeda et al, 2014). Panel C shows the similarity between the PB1 domain of MEK5 and ubiquitin. Main chain atoms superimpose remarkably well around the ERK5 binding surface while covalent linkage between K63 and the C-terminus of ubiquitin leaves this surface accessible. This suggests that K63-linked ubiquitin chains on MEKK3 may potentially compete with the PB1 domain of MEK5 and that this may indirectly block the formation of an MEK5–ERK5 catalytic complex. Alternatively, growing K63-linked ubiquitin chains on MEKK3 may somehow also directly block the catalytic MEK5-KD–ERK5-KD interface. The exact mechanism by which MEKK3 K63-linked ubiquitination within the ternary complex inhibits MEK5→ERK5 signaling remains to be discovered.
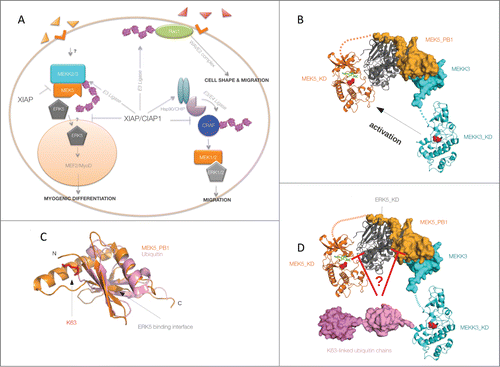
To investigate whether IAPs also regulate related MAP kinases, we screened for the activation of other MAPKs in IAP-depleted cells. We found that loss of XIAP and cIAP1 leads to an increase in basal and growth factor-mediated activation of ERK5. XIAP and cIAP1 directly bind to upstream MEKK2 and MEKK3 and conjugate K-63–linked ubiquitin chains in a RING domain-dependent manner. In vitro reconstitution of the entire kinase module (MEKK2/3-MEK5 and ERK5) revealed a direct role for ubiquitin conjugated to MEKK2 or MEKK3 in displacing MEK5 from ERK5 and thus blocking phosphorylation and subsequent activation of ERK5. MEKK2 and MEKK3 kinases each possess a PB1 domain in their N-termini that mediates an electrostatic interaction with the PB1 domain of MEK5 (). Although ERK5 does not possess a PB1 domain, it can still interact with the PB1 domain of the MEK5 kinase through its N-terminus.Citation8 As ubiquitin and the PB1 domain are structurally similar () it is plausible that there is physical competition, by which ubiquitin displaces the PB1-dependent interaction between MEK5 and ERK5 (). However, we cannot rule out the possibility that the growing ubiquitin chains on MEKK2/3 indirectly impede complex formation between MEK5 and ERK5 by inducing conformational changes (). Furthermore, it is worth mentioning that loss of XIAP also promotes the basal interaction between MEKK2 and MEK5, as XIAP directly binds to the PB1 domain of MEKK2 and MEKK3. This led to the proposal that IAPs might regulate this pathway in a 2-step process: under basal conditions XIAP might impede the interaction between MEKK2 and MEK5, whereas when MEKK2 and MEKK3 are activated, ubiquitination of MEKK2 might lead to the displacement of MEK5 from ERK5, thus inactivating the kinase cascade.Citation2
Ubiquitination of substrate proteins has many consequences, ranging from proteostasis, to translocation of proteins to different subcellular compartments, to formation of signaling complexes.Citation9 Here, we reveal a direct role of ubiquitin in the physical disassembly of a MAPK cascade, thus adding another layer of MAPK regulation. Intriguingly, loss of either MEKK2 or MEKK3 prevented the activation of ERK5, suggesting that these 2 kinases could potentially function as heteromers. Furthermore, we also identified both MEKK2 and MEKK3 as ubiquitin-binding proteins, suggesting the possible presence of ubiquitin-binding domains in these kinases. In fact, K63-ubiquitination of MEKK2 and MEKK3 does not impair their kinase activity; in fact, it promotes homo- and heterodimerization between the kinases although we could not detect any enhancement in their in vitro kinase activity. However, further studies are required to test whether deubiquitinases (DUBs) regulate the activation dynamics of ERK5 cascade in response to growth factors. Apart from the physical disruption of the complex, ubiquitination of these kinases might have implications for their intracellular localization. Currently, it is unclear whether the loss of IAPs has any effects on the nuclear localization of the ERK5 kinase components (). Although we have identified several ubiquitination sites in both MEKK2 and MEKK3, further studies are needed to decipher whether ubiquitination at any of these lysine residues is physiologically significant. Initial screening of these lysines revealed that MEKK2-K450 or MEKK3-K456 might be relevant in this regard as mutation of these sites led to constitutive activation of ERK5. Structural studies are required to evaluate whether these lysine residues play any role in maintaining the kinase-competent MEKK2/3 complex.
Loss of XIAP enhances human myogenic differentiation in an ERK5-dependent manner. We also showed that loss of XIAP leads to an increase in cIAP1 levels in human myoblasts, and the cross regulation between these 2 IAPs requires further characterization. Similarly, it would be highly interesting to investigate whether ubiquitination of upstream MAP2Ks also fine-tunes the activation dynamics of other MAPKs. These studies also open up an avenue for targeting XIAP in muscle-related disorders.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was partially supported by the Lowe ubiquitin-network grant as well as the CRC-128 grant from the DFG to KR. KR is a PLUS3 fellow of the Boehringer Ingelheim Foundation and a Heisenberg professor of the DFG (RA1739/4-1). AR is an International Senior Research Fellow of the Wellcome Trust. This work was also supported by the “Lendület” grants (LP-2013-57/2013) from the Hungarian Academy of Sciences (to AR).
References
- Raman M, Chen W, Cobb MH. Differential regulation and properties of MAPKs. Oncogene 2007; 26:3100-12; PMID:17496909; http://dx.doi.org/10.1038/sj.onc.1210392
- Takeda AN, Oberoi-Khanuja TK, Glatz G, Schulenburg K, Scholz RP, Carpy A, Macek B, Remenyi A, Rajalingam K. Ubiquitin-dependent regulation of MEKK23-MEK5-ERK5 signaling module by XIAP and cIAP1. EMBO J 2014; 33:1784-801; PMID:24975362; http://dx.doi.org/10.15252/embj.201487808
- Nithianandarajah-Jones GN, Wilm B, Goldring CE, Muller J, Cross MJ. ERK5: structure, regulation and function. Cell Signalling 2012; 24:2187-196; PMID:22800864; http://dx.doi.org/10.1016/j.cellsig.2012.07.007
- Kenneth NS, Duckett CS. IAP proteins: regulators of cell migration and development. Curr Opin Cell Biol 2012; 24:871-5; PMID:23219152; http://dx.doi.org/10.1016/j.ceb.2012.11.004
- Vucic D, Dixit VM, Wertz IE. Ubiquitylation in apoptosis: a post-translational modification at the edge of life and death. Nat Rev Mol Cell Biol 2011; 12:439-52; PMID:21697901; http://dx.doi.org/10.1038/nrm3143
- Oberoi TK, Dogan T, Hocking JC, Scholz RP, Mooz J, Anderson CL, Karreman C, Meyer Zu Heringdorf D, Schmidt G, Ruonala M, Namikawa K, Harms GS, Carpy A, Macek B, Koster RW, Rajalingam K. IAPs regulate the plasticity of cell migration by directly targeting Rac1 for degradation. EMBO J 2011; 31:14-28; PMID:22117219; http://dx.doi.org/10.1038/emboj.2011.423
- Dogan T, Harms GS, Hekman M, Karreman C, Oberoi TK, Alnemri ES, Rapp UR, Rajalingam K. X-linked and cellular IAPs modulate the stability of C-RAF kinase and cell motility. Nat Cell Biol 2008; 10:1447-55; PMID:19011619; http://dx.doi.org/10.1038/ncb1804
- Glatz G, Gogl G, Alexa A, Remenyi A. Structural mechanism for the specific assembly and activation of the extracellular signal regulated kinase 5 (ERK5) module. J Biol Chem 2013; 288:8596-609; PMID:23382384; http://dx.doi.org/10.1074/jbc.M113.452235
- Fulda S, Rajalingam K, Dikic I. Ubiquitylation in immune disorders and cancer: from molecular mechanisms to therapeutic implications. EMBO Mol Med 2012; 4:545-56; PMID:22730341; http://dx.doi.org/10.1002/emmm.201100707
